Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide. Non-small-cell lung cancer (NSCLC) accounts
for ~85% all cases of lung cancer (1,2), and
commonly develops resistance to chemotherapy and radiotherapy,
often presents at stages too late for surgical intervention,
resulting in a five-year survival rate of <15% (3).
In the past three decades, several chemotherapeutic
agents (such as platinum, gemcitabine and pemetrexed) and
molecular-targeted agents (such as erlotinib and bevacizumab) have
been put into clinical trials. Combination chemotherapy is the
preferred standard care of patients with advanced NSCLC, as it can
improve patient survival (4).
Gemcitabine (2′,2′-difluorodeoxycytidine) is one of
the most effective chemotherapeutic agents against NSCLC (5–8). It
has been used as a single antitumor agent or in combination with
other cytotoxic agents for solid tumor types, including ovarian and
pancreatic cancer (9,10).
Inula britannica is a commonly used Chinese
traditional herbal medicines and its extracts have been reported to
have immunomodulatory (11,12),
anti-inflammatory (13) and
antitumor activities (14–16). 1-O-acetylbritannilactone (ABL) is
an effective chemical component extracted from inula
britannica. Previous studies have suggested that ABL has
anti-inflammatory (13) and
anticancer (17–19) activities, and therefore has the
potential to be developed as a chemotherapeutic agent. ABL is able
to elicit apoptosis of human breast cancer cells and inhibit the
growth of human leukemia cell lines in vitro (17–19).
However, the effectiveness of ABL alone or in combination with
gemcitabine, one of the first-line chemotherapeutic agents for lung
cancer, remains unknown. The present study was conducted to
investigate the effect and mechanism of ABL combined with
gemcitabine on cell growth and apoptosis in human NSCLC A549 cells
in vitro.
Materials and methods
ABL
ABL (purity, >99%) was isolated from Inula
britannica-F. var chinensis Regel and the structure of
ABL was determined spectroscopically, as has previously been
reported (20).
Cell culture
The A549 human NSCLC cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640
containing 10% fetal calf serum (FCS), 100 U/ml penicillin and 100
µg/ml streptomycin, and maintained in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. The cells were
passaged to ensure exponential growth.
Cell proliferation assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyltetrazolium bromide (MTT; Sigma Chemicals Co., St Louis,
MO, USA) assays were performed to evaluate cell growth and
viability following treatment with ABL and/or gemcitabine in
RPMI-1640 medium containing 10% FCS. The cells were seeded
(1×104 cells/well) in 96-well plates at 37°C with 5%
CO2 for 72 h. MTT reagent (5 mg/ml) was added to each
well and the cells were incubated for an additional 4 h. The
reaction was terminated with 150 µl dimethylsulfoxide (Sigma
Chemicals Co.,) per well. Absorbance values at 490 nm were
determined by using an ELISA reader (Model 680; Bio-Rad, Hercules,
CA, USA).
Analysis of cell apoptosis by flow
cytometry
The cells were seeded (4×105 cells per
well) in 6-well plates in RPMI-1640 medium for 24 h. The medium was
removed and cells were washed with phosphate-buffered saline (PBS);
ABL and/or gemcitabine was then added. Following 72 h, the cells
were detached by 0.02% EDTA and fixed overnight in 70% ice-cold
ethanol at 4°C. Prior to flow cytometric analysis, the fixed cells
were centrifuged at 1,800 × g for 10 min, washed twice with PBS and
resuspended in propidium iodide (PI) staining solution containing 5
µg/ml PI and 200 µg/ml RNase A (Sigma Chemicals Co.).
Using a FACScan flow cytometer (FCM-500; Beckman Coulter Inc., Los
Angeles, CA, USA), cell apoptosis analysis was performed on 10,000
cells for each sample.
Western blot analysis
The cellular lysates were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
electro-transferred onto nitrocellulose membranes. Following
blocking with 5% non-fat milk in TBST (20 mm Tris-buffered saline,
150 mm NaCl, 0.2% Tween-20, pH 7.6), the membranes were incubated
with specific anti-nuclear factor (NF)-κB p65, anti-Bcl-2,
anti-Bax, anti-inhibitor nuclear factor κBα (IκBα) or anti-β-actin
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibodies at
room temperature for 2 h, and subsequently with 1:4,000 horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (Santa
Cruz Biotechnology, Inc.) for 1 h, immunoreactive bands were
visualized by enhanced chemiluminescence (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) kit. β-actin (BD Biosciences, San Diego,
CA, USA) was used to normalize the quantity of the protein on the
blot.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences among the groups were identified by analysis of
variance for multiple comparisons, followed by Bonferroni post hoc
analysis for the least significant difference. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of ABL, gemcitabine and their
combination on cell growth in A549 cells
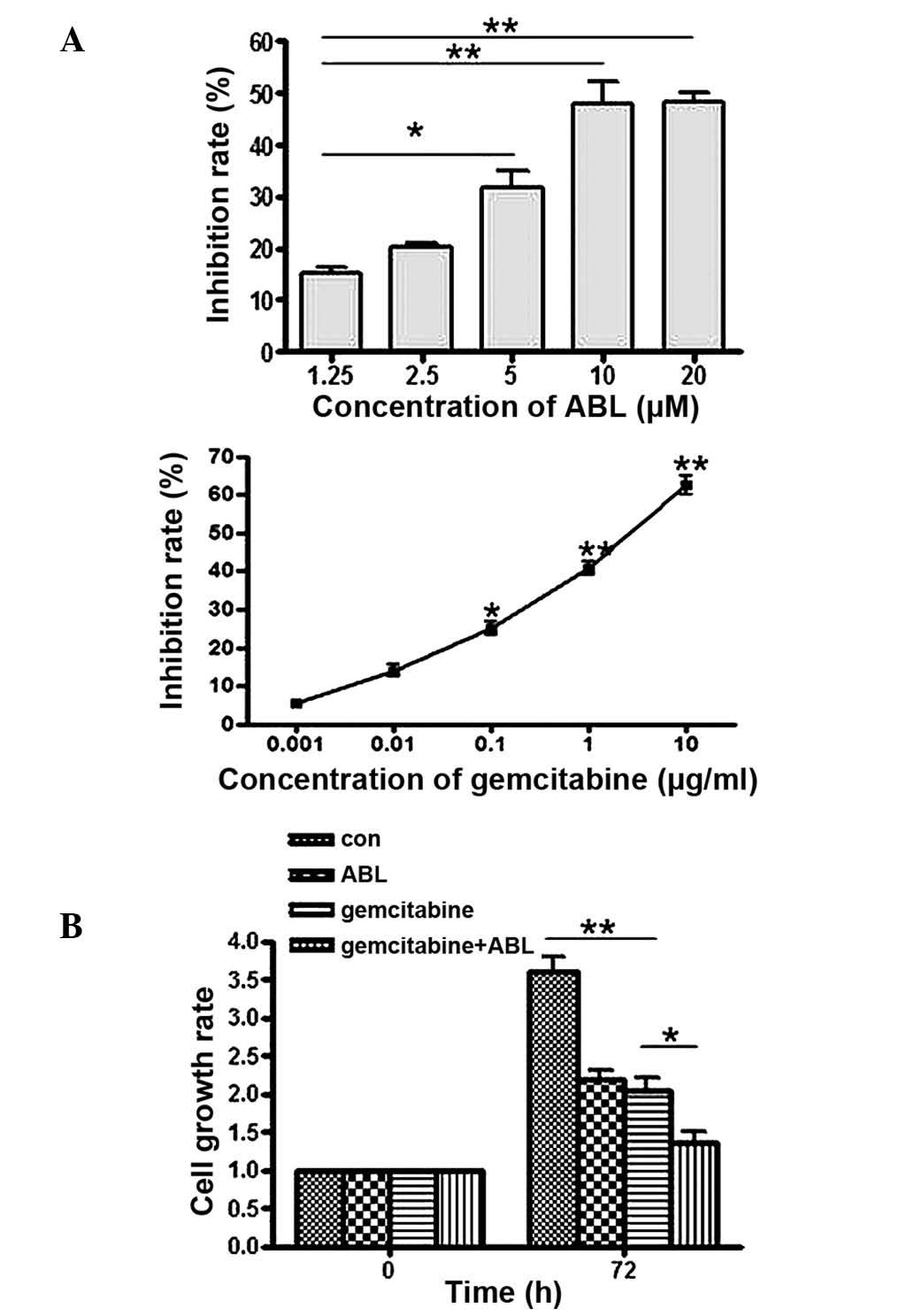
To examine the effect of ABL on cell growth, the
A549 human non-small-cell lung cancer cell line was treated with
increasing concentrations of ABL (1.25–20 µM) for 72 h. As
demonstrated in Fig. 1A, the cell
growth inhibitory effect of ABL was observed in a dose-dependent
manner. The effect on proliferation inhibition reached plateau at
10 µM. The inhibitory effect at 20 µM, 49.5%
(P<0.001) was comparable to that at 10 µM. This result
indicated that ABL is an effective inhibitor of non-small-cell lung
cancer cell growth as a single agent. In another experiment,
treatment with ABL was set at 10 µM to ensure the maximal
inhibitory effect of this compound. In addition, the effect of
gemcitabine (0.001–10 µg/ml) on cell growth was examined
in vitro and it was identified that gemcitabine inhibited
the proliferation of A549 cells with a strong potency (the
inhibitory rate was 62.6% at 10 µM of gemcitabine).
Subsequently, the A549 cell lines were co-treated with ABL and
gemcitabine. The concentrations of ABL and gemcitabine were 10
µM and 10 µg/ml, respectively, due to the similar
inhibitory effect observed at these dosages in the experiment with
each individually. The effect of co-treatment with ABL and
gemcitabine on cell proliferation was measured by an MTT assay.
Following 72 h of treatment, the combination of the two agents
enhanced the efficacy significantly (cell survival rate, 30.2%) on
the suppression of cell growth compared with the control, ABL
alone, or gemcitabine alone (survival rates at 100, 59.1 and 49.7%,
respectively; Fig. 1B;
P<0.001). The above results demonstrate that ABL inhibited the
cell growth in the A549 cell line and this inhibitory effect was
further enhanced when used in combination with gemcitabine.
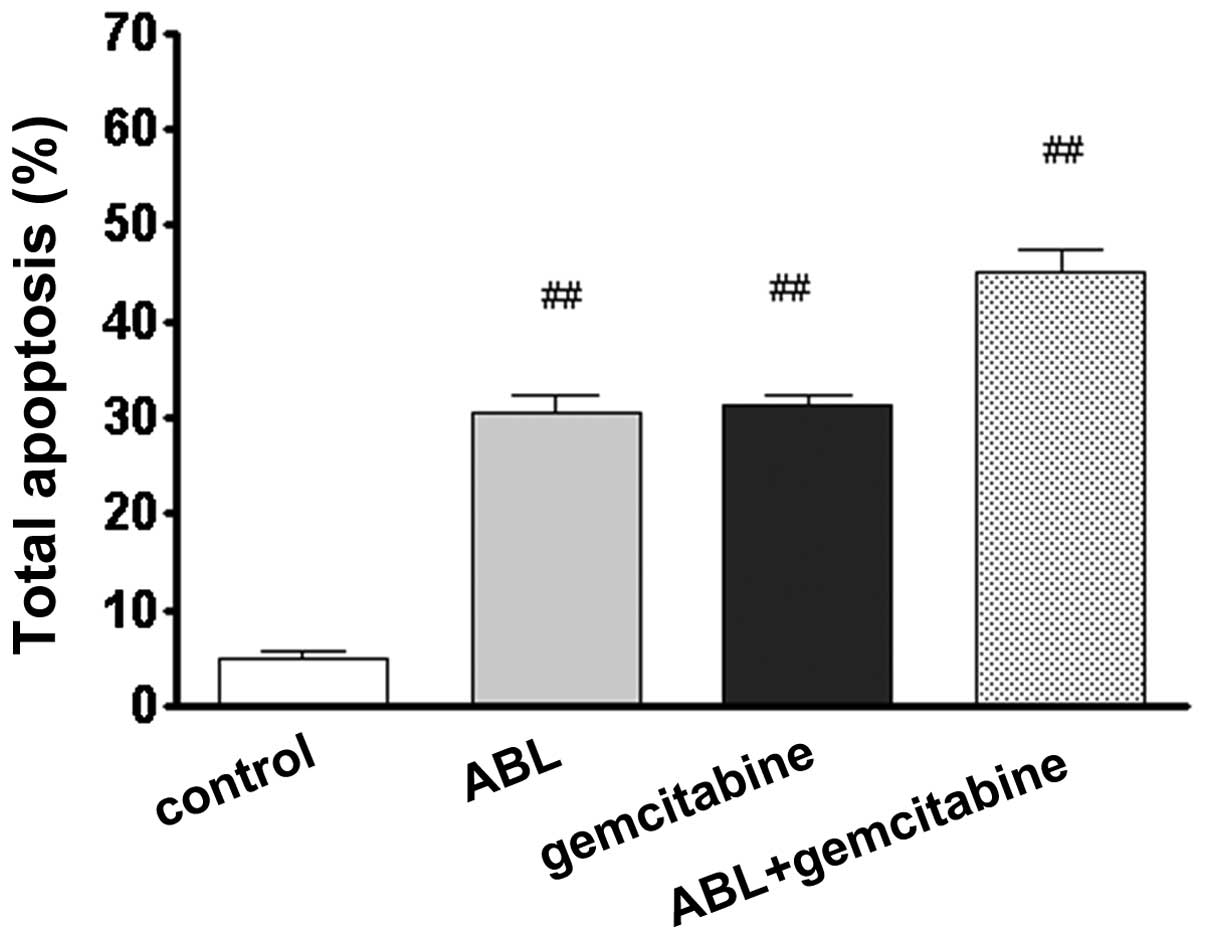
Effect of ABL alone or in combination
with gemcitabine, on A549 cell apoptosis in
The inhibitory effect of cell proliferation may be
caused by the induction of apoptotic cell death. To elucidate the
mechanism underlying this effect, it was investigated whether ABL
alone or in combination with gemcitabine induced cell apoptosis and
if so, whether the combination leads to an increase in the rate of
apoptosis. The A549 cells were treated with ABL (10 µM)
alone and in combination with gemcitabine (10 µg/ml). Cell
apoptosis was examined by PI staining and FACS analysis. As
demonstrated in Fig. 2, <5% of
the cells cultured with RPMI-1640 medium were apoptotic and
following ABL-treatment at a concentration of 10 µM for 72
h, 30.4% of cells were apop-totic (P<0.05), which was similar to
that observed following 10 µg/ml gemcitabine-treatment for
72 h-treatment (31.4% of apoptotic cells). Compared with the single
treatment, the combination of ABL and gemcitabine increased the
percentage of apoptotic cells (P<0.01). These findings suggest
that ABL has the ability to induce cell apoptosis in vitro
and this pro-apoptotic effect may also be markedly increased
following the addition of gemcitabine.
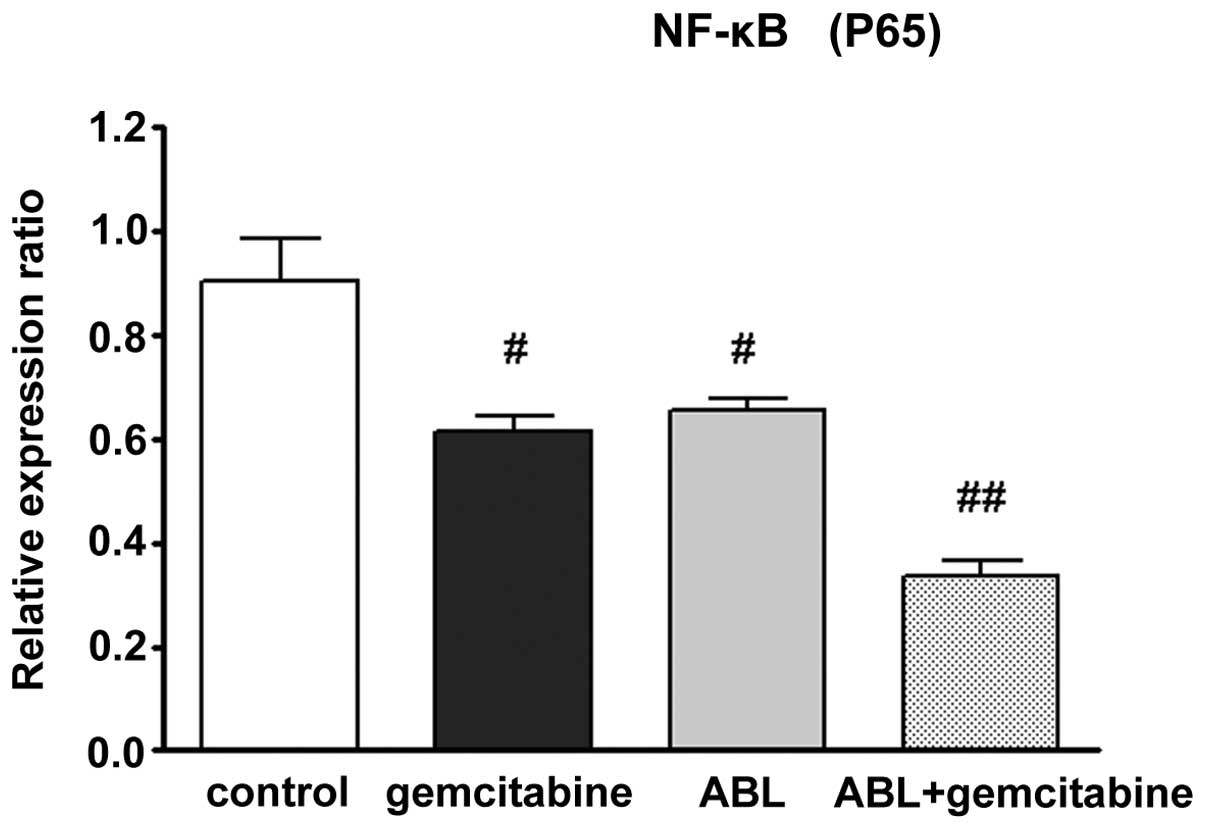
ABL alone and combined with gemcitabine
suppresses NF-κB activation
Next, the present study aimed to investigate the
change in the signaling pathway of NF-κB in lung cancer cells,
including nuclear p65 levels and transcription of all
NF-κB-regulated genes. It was identified that ABL alone resulted in
marginal suppression of NF-κB expression (NF-κB p65 protein level)
as determined by western blot analysis. When A549 cells were
co-cultured with ABL and gemcitabine, the NF-κB expression was also
inhibited and the inhibitory rate was notably more potent compared
with treatment with ABL alone (Fig.
3).
Treatment with ABL alone and combined
with gemcitabine downregulates Bcl-2, upregulates Bax and prevents
degradation of IκBα
To investigate the possible mechanism underlying the
enhanced cell apoptosis, the expression of IκBα and NF-κB
downstream molecules anti-apoptotic Bcl-2 and pro-apoptotic Bax was
determined. Western blot analysis (Fig. 4) demonstrated that the expression
of Bcl-2 was significantly downregulated in the combination group
compared with individual agent treatment group and RPMI-1640 medium
control group (P<0.01), while the expression of Bax markedly
increased. The expression of IκBα, which is the inhibitory protein
of NF-κB activation, was subsequently examined by western blot
analysis. Following treatment by ABL alone and in combination with
gemcitabine for 72 h, total IκBα expression levels were increased
in the two groups.
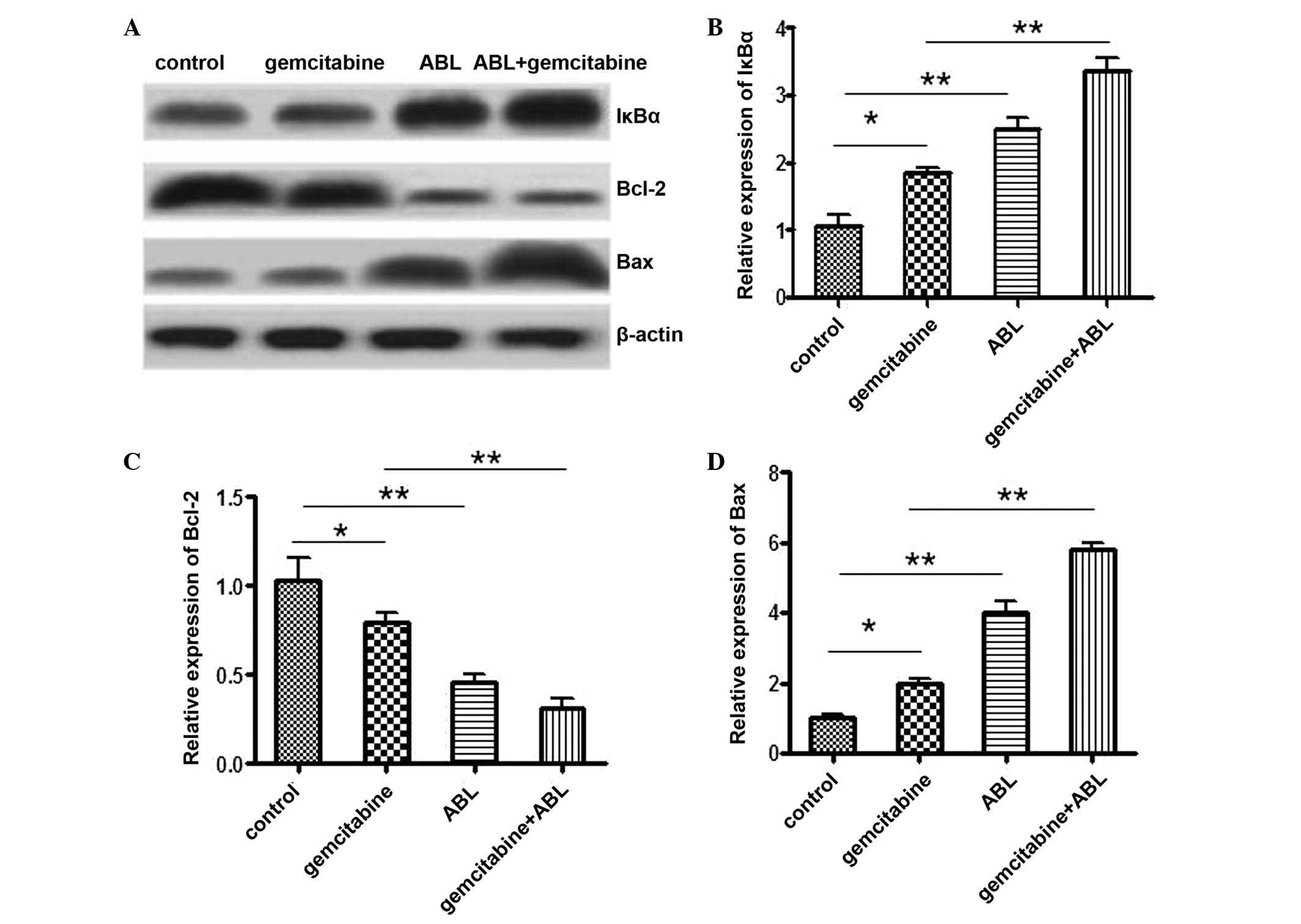 | Figure 4Expression of Bcl-2, Bax and IκBα
following ABL, gemcitabine and the combination treatments. Western
blot analysis of Bcl-2, Bax and IκBα expression in A549 cells
treated with cell medium, 10 µM ABL, 10 µg/ml
gemicitabine or their combination for 72 h. (A) The images are
representative of three different experiments and show the relative
intensities of Bcl-2, Bax and I-κBα proteins. β-actin protein was
used as an internal control. (B-D) Densitometric measurement for
these proteins levels was normalized to the internal control and
expressed as a relative number. *P<0.05,
**P<0.01. ABL, 1-O-acetylbritannilactone; Bcl-2, B
cell lymphoma gene-2; IκBα, inhibitor nuclear factor κBα. |
Discussion
In the last 25 years, there has been an advance in
the understanding of the characteristics of lung cancer. However,
progress in the treatment of this disease has been more limited and
novel therapies are required to reduce the effects of the
increasing incidence of lung cancer. In recent years, the natural
chemical compounds from various plants have been investigated as
potential antitumor drugs. As traditional Chinese medicine has been
used for hundreds of years, there has been increasing interest in
identifying novel antitumor agents derived from these herbs. ABL is
a natural product extracted from inula britannica, which is
a commonly used Chinese traditional medicine. This compound has
been reported to exhibit anticancer activities in various cell
lines in vitro and is able to induce apoptosis of human
breast cancer cells and inhibit the growth of human leukemia cell
lines (17–19). However, to the best of our
knowledge, the effect of ABL in the A549 human non-small lung
cancer cell line has not previously been investigated.
Considering that gemcitabine is one of the
first-line chemotherapy drugs for NSCLC, the effects of ABL alone
and in combination with gemcitabine were investigated in in
vitro assays in the present study. The antiproliferative
activity was observed in A549 cells in vitro following
treatment with ABL alone, gemcitabine alone, and in ABL and
gemcitabine in combination. By comparing the results of these three
treatment groups, it was observed that there was a synergistic
effect on the suppression of proliferation with the combination of
ABL and gemcitabine. As it is well established that the inhibitory
effect of cell proliferation may be caused by the induction of cell
apoptosis, the percentage of apoptotic cells in the treatment
groups of ABL alone, gemcitabine alone and in combination was also
measured. A similar phenomenon to the cell growth suppression assay
was observed; treatment with ABL or gemcitabine alone induced cell
apoptosis and the effect was significantly enhanced when the cells
were treated by a combination of the two.
The NF-κB pathway is a key regulator of numerous
cellular events, including proliferation, differentiation and
apoptosis, and it is also associated with tumor development and
progression. It has been reported that NF-κB has bidirectional
roles (inhibit or promote) in cell apoptosis and the actual role
depends on the stimuli applied and the cell line used in the study.
Using western blot analysis, it was identified that the activation
of NF-κB in A549 cells was suppressed by treatment with ABL, which
may have promoted apoptosis in the tumor cells as was observed in
the present study. The effect following the combination of ABL and
gemcitabine was found to inhibit the activation of NF-κB, which
subsequently induced cell apoptosis. Further studies are required
to fully understand the underlying apoptotic mechanism of the
effects of ABL on A549 cells, including focusing on the apoptosis
execution molecules, such as caspase-3 or caspase-8 to determine
whether the caspase pathway is involved in this apoptotic
effect.
Mature NF-κB P65:P50 dimers are trapped in the
cytoplasm of unstimulated cells by interaction with the inhibitory
protein, termed IκBα. The IκB kinase (IKK) phosphorylates IκBα
proteins, thereby targeting them for rapid ubiquitin-dependent
proteolysis that initiates NF-κB activation (21,22).
The present study identified that all of the treatments (ABL alone,
gemcitabine alone and a combination of the two) were able to
upregulate the expression of IκBα in the A549 cells, which
maintained the NF-κB dimers sequestered in an inactive state in the
cytoplasm and protected the cell from apoptosis. The activation of
NF-κB may change the expression of a large number of target genes,
including Bcl-2. Bcl-2 family proteins are associated with signal
transduction in apoptosis and affect the chemosensitivity of tumor
cells to anticancer agents (23).
It has been revealed that Bcl-2 and Bcl-xL (anti-apoptotic protein)
protect numerous cell lines from apoptosis induction, while Bcl-xS
and Bax-α (pro-apoptotic protein) have the opposite effect
(24–26). In the present study, the levels of
NF-κB downstream molecules Bcl-2 and Bax were measured to examine
the possible mechanism underlying the induction of cell apoptosis.
The expression of Bcl-2 was significantly downregulated whereas Bax
was upregulated in all of the treatment groups compared with the
control group, and a synergistic effect was observed following
treatment with a combination of the two agents. Several studies
have reported that agents that treat lung cancer may induce
apoptosis via the Fas/FasL system (27–29).
In the present study, the expression of the Fas/FasL system (Fas,
soluble and membrane-bound Fas ligand) in A549 cells co-treated
with ABL and gemcitabine was also detected. However, there was no
significant difference prior to and following the combination
treatment. Therefore, it is hypothesized that ABL alone and in
combination with gemcitabine may inhibit the NF-κB signaling
pathway, which is involved in the cell growth and apoptosis of A549
cells. The increased levels of IκBα may inhibit NF-κB expression
and activation, and thus induce cell apoptosis. As a result the
anti-apoptotic Bcl-2 gene was downregulated and the proapoptotic
Bax protein was upregulated. Therefore, these results suggest that
the mechanism underlying the induction of apoptosis by ABL alone
and ABL combined with gemcitabine in A549 cells is by decreasing
antiapoptotic gene (Bcl-2) and increasing proapoptotic gene (Bax)
expression, respectively.
In conclusion, ABL specifically targets the NF-κB
pathway and significantly induces apoptosis in A549 human non-small
lung cells in vitro. This effect was significantly enhanced
when combined with gemcitabine. The present study merits the
further investigation of this compound, as it may represent a novel
chemotherapeutic agent in lung cancer treatment if this compound
has favorable in vivo pharmacokinetic properties.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81170028).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCracken M, Olsen M, Chen MS Jr, et al:
Cancer incidence, mortality, and associated risk factors among
Asian Americans of Chinese, Filipino, Vietnamese, Korean, and
Japanese ethnicities. CA Cancer J Clin. 57:190–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erridge SC, Moller H, Price A and Brewster
D: International comparisons of survival from lung cancer: pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: recent
advances and future directions. Oncologist. 13(Suppl 1): 5–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laskin JJ and Sandler AB: First-line
treatment for advanced non-small-cell lung cancer. Oncology
(Williston Park). 19:1671–1676. 2005.
|
|
6
|
Langer F, Helsberg K, Schütte WH and
Leschinger MI: Gemcitabine in the first line therapy of advanced
and metastatic non-small-cell lung carcinoma (NSCLC): review of the
results of phase III studies. Onkologie. 28(Suppl 1): 1–28.
2005.
|
|
7
|
Cucević B, Samarzija M, Baricević D, et
al: Gemcitabine in the first and second-line chemotherapy of
advanced non-small cell lung cancer. Coll Antropol. 29:583–588.
2005.
|
|
8
|
Natale R: A ten-year review of progress in
the treatment of non-small-cell lung cancer with gemcitabine. Lung
Cancer. 50:S2–S4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lund B, Hansen OP, Theilade K, Hansen M
and Neijit JP: Phase II study of gemcitabine
(2′,2′-difluorodeoxycytidine) in previously treated ovarian cancer
patients. J Natl Cancer Inst. 86:1530–1533. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinemann V: Gemcitabine: progress in the
treatment of pancreatic cancer. Oncology. 60:8–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Tae N, Lee JJ, Kim T and Lee JH:
Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS
expression in RAW264.7 cells by inducing proteasomal degradation of
TRAF6. Eur J Pharmacol. 636:173–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin HZ, Lee D, Lee JH, et al: New
sesquiterpene dimers from Inula britannica inhibit NF-kappaB
activation and NO and TNF-alpha production in LPS-stimulated
RAW264.7 cells. Planta Med. 72:40–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YP, Wen JK, Zheng B, Zhang DQ and Han
M: Acetylbritannilactone suppresses lipopolysaccharide-induced
vascular smooth muscle cell inflammatory response. Eur J Pharmacol.
577:28–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rafi MM, Bai NS, Chi-Tang-Ho, et al: A
sesquiterpenelactone from Inula britannica induces anti-tumor
effects dependent on Bcl-2 phosphorylation. Anticancer Res.
25:313–318. 2005.PubMed/NCBI
|
|
15
|
Bai N, Lai CS, He K, et al: Sesquiterpene
lactones from Inula britannica and their cytotoxic and apoptotic
effects on human cancer cell lines. J Nat Prod. 69:531–535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan MH, Chiou YS, Cheng AC, et al:
Involvement of MAPK, Bcl-2 family, cytochrome c, and caspases in
induction of apoptosis by 1,6-O,O-diacetylbritannilactone in human
leukemia cells. Mol Nutr Food Res. 51:229–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Liu H, Yan W, et al: Studies on
1-O-acetylbritannilactone and its derivative,
(2-O-butyloxime-3-phenyl)-propionyl-1- O-acetylbritannilactone
ester. Bioorg Med Chem Lett. 14:1101–1104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Liu H, Yan W, et al: Design,
synthesis, and anti-tumor activity of
(2-O-alkyloxime-3-phenyl)-propionyl-1-O-acetylbritannilactone
esters. Bioorg Med Chem. 15:2783–2789. 2005. View Article : Google Scholar
|
|
19
|
Liu B, Han M, Sun RH, et al: ABL-N-induced
apoptosis in human breast cancer cells is partially mediated by
c-Jun NH2-terminal kinase activation. Breast Cancer Res. 12:R92010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Shi X, Fu Y, et al: Preparation
and determination of 1-O-acetylbritannilactone in Inula Britannica
L. Se Pu. 23:573–576. 2005.In Chinese.
|
|
21
|
Baltimore D and Beg AA: DNA-binding
proteins. A butterfly flutters by. Nature. 373:287–288. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilmore TD, Koedood M, Piffat KA and White
DW: Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene.
13:1367–1378. 1996.PubMed/NCBI
|
|
23
|
Boise LH, Gottschalk AR, Quintáns J and
Thompson CB: Bcl-2 and Bcl-2-related proteins in apoptosis
regulation. Curr Top Microbiol Immunol. 200:107–121.
1995.PubMed/NCBI
|
|
24
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: a rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
25
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fahy BN, Schlieman MG, Mortenson MM,
Virudachalam S and Bold RJ: Targeting BCL-2 overexpression in
various human malignancies through NF-κB inhibition by the
proteasome inhibitor bortezomib. Cancer Chemother Pharmacol.
56:46–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rho JK, Choi YJ, Ryoo BY, et al: p53
enhances gefitinib-induced growth inhibition and apoptosis by
regulation of Fas in non-small cell lung cancer. Cancer Res.
67:1163–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimizu M, Kondo M, Ito Y, et al: Soluble
Fas and Fas ligand provide new information on metastasis and
response to chemotherapy in SCLC patients. Cancer Detect Prev.
29:175–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun SY, Yue P, Hong WK and Lotan R:
Induction of Fas expression and augmentation of Fas/Fas
ligand-mediated apoptosis by the synthetic retinoid CD437 in human
lung cancer cells. Cancer Res. 60:6537–6543. 2000.PubMed/NCBI
|