Introduction
Tissue kallikrein 1 (TK1) cleaves
low-molecular-weight kininogen to release bradykinin and
Lys-bradykinin (kallidin), which exert biological functions via
kinin receptor signaling (1,2). All
components of the tissue kallikrein-kinin system have been
identified in the cardiovascular system. Using various delivery
approaches of TK1 protein, numerous studies have demonstrated that
TK1 exhibits a wide spectrum of beneficial effects through kinin B2
receptor signaling by reducing cardiac remodeling, renal injury and
ischemic stroke, and preventing long-term in-stent restenosis
(3–6). Our previous studies in vitro
and in vivo, revealed that the effects of human TK1 (hTK1)
gene delivery inhibited vascular smooth muscle cell (VSMC)
proliferation and partially inhibited neointima formation following
carotid artery injury in rats (7,8).
The extracellular matrix (ECM) is responsible for
the three-dimension spatial arrangement and structural integrity of
the arterial wall, and the metabolic function of intracellular
components. Alterations have been reported in the density,
architecture and composition of the ECM in vessels as a result of
hypertension (9,10). Matrix metalloproteinases (MMPs) and
tissue inhibitors of metalloproteinases (TIMPs) are vital in the
regulation of ECM metabolism in normal and pathological conditions
(11). MMP-9 digests gelatin,
elastin, fibronectin, laminin, and types IV and V collagen, which
are found in the subendothelial basement membranes (11,12).
TIMP-1 blocks the activation of MMPs, preventing their proteolytic
activity. MMPs and TIMPs regulate the metabolism of collagen and
elastin and are, therefore, responsible for structural and
functional alterations in the arterial wall during vascular
remodeling (11,12). TIMP-1 has been demonstrated to
inhibit the process of vascular remodeling in vitro, and
overexpression of this gene demonstrates potential for
destabilizing vessel differentiation (13). Delivery of the adenovirus vector
cDNA encoding TIMP-1 could partly restrain VSMC proliferation and
migration, and therefore reduce neointimal hyperplasia in a rat
model of vascular balloon injury (14).
At present, there are two main methods for multigene
therapy. The first method involves the target cells being
transfected with multiple independent vectors carrying different
genes simultaneously (15,16). The second method involves the
co-expression of multiple genes in one identical vector (17). Compared with the first method, the
use of a multigene co-expression vector may increase the efficiency
of transfection and expression. The low efficiency of gene transfer
is the bottleneck in gene therapy at present. In theory, a
combination of two or more anti-restenosis genes carried by a
single vector could improve treatment efficacy, reduce the side
effects associated with vectors, and have potential for clinical
application (18). However, these
methods have seldom been investigated in the context of
cardiovascular disease.
In previous studies, TK1 and TIMP1 have been
observed to have numerous biological effects on vascular
remodeling. The synergistic suppression of a conjunction of TK1 and
TIMP1 for VSMC proliferation remains to be elucidated. The aim of
the present study was to construct an adenovirus vector containing
human TK1 and TIMP1 genes. The vector would be used for the
co-expression of TK1 and TIMP1 proteins, and provide a novel
strategy for inhibiting VSMC proliferation.
Materials and methods
Plasmid and recombinant adenovirus
Plasmid pDC316, adenoviral skeleton plasmid
pBHGloxE1, 3Cre and DH5-α were purchased from Mixcrobix-Biosystems
(Toronto, ON, Canada). Plasmid pDC316-hTIMP1-enhanced green
fluorescent protein (EGFP), which contains the mCMV promoter and
hTIMP1 cDNA, pDC316-hTK1, which contains hTK1 cDNA, and recombinant
adenovirus Ad5-hTK1-IRES-EGFP (Ad-hTK1) and Ad-hTIMP1-EGFP
(Ad-hTK1), as well as control vector Ad-EGFP were constructed and
maintained in our laboratory (Fujian Provincial Hospital Key
Laboratory of Geriatrics, Fuzhou, China). Rabbit anti-hTK1
monoclonal antibodies and rabbit anti-hTIMP1 polyclonal antibodies
were purchased from Abcam (Cambridge, MA, USA). UV transilluminator
was purchased from Jingke Scientific Instrument Co., Ltd.
(Shanghai, China). AdMax system was obtained from Microbix
Biosystems, Inc. (Mississauga, Canada).
Construction of recombinant plasmid
containing hTK1 and hTIMP1 genes
The mCMV-hTIMP1 fragment from constructed
pDC316-mCMV-hTIMP1 was amplified using polymerase chain reaction
(PCR) with the following primers: mCMV-Bg1II, forward
5′-GCCAGATCTGTTGACATTGATTATTGA-3′, hTIMP1-SalI and reverse
5′-GCCGTCGACTCAGGCTATCTGGGACCG-3′. This pair of primers contained
restriction sites for Bg1II and SalI at the 5′
terminal, respectively. The PCR reaction system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) contained primer 1, primer
2, dNTP and pyrobest Taq DNA polymerase (Takara Bio, Inc., Otsu,
Japan). The PCR reaction program was accomplished as follows:
Initial denaturation at 94°C for 5 min followed by 94°C for 30 sec,
57°C for 40 sec for annealing and 72°C for 80 sec for extension,
which was repeated for 30 cycles, with an extra final 5 min
incubation at 72°C to complete all extensions.
The loxP-containing shuttle vector pDC316-hTK1
contains hTK1 cDNA driven by the mCMV promoter, which was verified
and maintained in our lab (7). The
mCMV-hTIMP1 PCR fragment was purified using a PCR purification kit
(Takara Bio, Inc.) and then double-digested using Bg1II and
SalI restriction enzymes (Takara Bio, Inc.). Subsequently,
the mCMV-hTIMP1 fragment was cloned into pDC316-hTK1, the digestion
products of which were identified by gel electrophoresis, prior to
being recycled and ligated by T4 DNA ligase (Takara, Bio, Inc.).
The ligation products underwent a heat shock reaction in a 42°C
water bath for 90 sec, and were quickly transferred onto ice for 2
min. The ligation products were then transformed into competent
Escherichia coli DH5-α (American Type Culture Collection,
Manassas, VA, USA) for 24 h. Various clones were screened using the
colony PCR method. The positive clones and extracted plasmids were
analyzed using PCR, digestion using Bg1II and SalI
and sequencing (Invitrogen Life Technologies, Carlsbad, CA, USA)
using an mCMV-hTIMP1-F primer.
Packaging and amplification of
recombinant adenovirus
Briefly, HEK293A cells (American Type Culture
Collection) at a confluence of 40–60% were co-transfected with a
recombinant plasmid containing hTK1 and hTIMP1 cDNA and pBHGlox
DE1, 3Cre with the aid of Lipofectamine 2000 (Invitrogen Life
Technologies) according to standard methods, as described
previously (7,19). High titer adenovirus was obtained
by repeated infection in HEK293A cells. The recombinant adenoviral
vectors containing target cDNA were screened and verified using PCR
with the two primers. The adenovirus was amplified and purified
using standard viral amplification and cesium chloride purification
methods (7,20).
The viral titer, expressed as transducing units per
milliliter, was determined using a dilution assay in HEK293A cells
that were transduced with a serial dilution of concentrated
adenovirus. The viral titer was detected by a TCID50 assay provided
by Obiogene, Inc. (Carlsbad, CA, USA). Following purification, the
virus was suspended in phosphate-buffered saline (PBS) with 3%
sucrose and preserved at −80°C until use.
VSMC culture and transfection with
co-expression vector
Rat primary VSMCs were isolated from the thoracic
aorta of Sprague-Dawley (SD) rats (2–3-month-old) using the explant
method as described previously (21), and then cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco Life Technologies, Carlsbad,
CA, USA) containing 10% fetal bovine serum (FBS; Gibco Life
Technologies), 100 U/ml penicillin, and 100 U/ml streptomycin
(Gibco Life Technologies). The cells were incubated at 37°C in a
humidified atmosphere of 5% CO2. The cells exhibited a
spindle-shaped appearance, and confluent VSMCs in culture exhibited
the characteristic 'hill and valley' growth pattern (7). The cell morphology was examined in
detail under an inverted phase contrast microscope (DMI4000B; Leica
Microsystems, Wetzlar, Germany) at 100× magnification. VSMCs were
used for experiments between the 3rd and 7th passages. SD rats were
obtained from the Experimental Animal Center of the Fujian Medical
University (Fuzhou, China). The study was approved by the
Institutional Ethics Committee for Laboratory Animal Care of Fujian
Provincial Hospital (Fuzhou, China).
VSMCs were seeded in 6-well culture plates at a
confluence of 30–40%, and the adenovirus of Ad-hTK1, Ad-hTIMP1,
Ad-hTIMP1-hTK1 and Ad-EGFP at different multiplicities of infection
(MOI) were added to the medium. The adenovirus was incubated with
cells in a small volume of serum-free medium at 37°C. After
absorption for 2 h, fresh complete growth medium was added and the
cells were further incubated for the following experiments. After
72 h of infection, adenovirus infectivity was measured using EGFP
with a fluorescent microscope (DMI4000B; Leica Microsystems) at
100× magnification. Reverse transcription-quantitative (RT-q) PCR
and western blot analyses were performed to detect the expression
of hTK1 and hTIMP1 mRNA and protein.
RT-qPCR
Total RNA was extracted using RNAiso Plus (Takara
Bio, Inc.) and stored at −80°C until further use. The concentration
and purity of RNA was measured under a UV spectrophotometer
(ND-2000; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
Reverse transcription was conducted to synthesize first-strand cDNA
from 1 µg total RNA using the PrimeScript RT Reagent kit
with gDNA Eraser (Takara Bio, Inc.), according to the
manufacturer's instructions. The resulting cDNA was then subjected
to RT-qPCR, in order to evaluate the relative mRNA expression
levels of hTK1 and hTIMP1. β-actin was used as an internal control.
Gene-specific amplification was performed using a Lightcycler 480
Real Time PCR system (Bio-Rad Laboratories, Inc.) with 12.5
µl SYBR PremixEx Taq II, 1.0 µl PCR forward (10
µM) and 1.0 µl PCR reverse primer (10 µM;
Invitrogen Life Technologies), 2.0 µl cDNA, 8.5 µl
raze-free water. The PCR reaction mix was pre-heated to 95°C for 30
sec, and then amplified at 95°C for 5 sec and 60°C for 30 sec for
40 cycles. The resolution curve was measured at 95°C for 15 sec,
60°C for 15 sec and 95°C for 15 sec. The Ct (threshold cycle) value
of each sample was calculated using the instrument's software, and
the relative mRNA expression levels of total hTK1 and hTIMP1 mRNA
were normalized to those of β-actin. The data were analyzed using
the 2−ΔΔCt method. The following primer sequences were
used: hTK1, forward 5′-GTCCAGAACAATCCGCCTCA-3′, reverse
5′-CAGACCACCATGCCCAGTTAGA-3′; hTIMP1, forward
5′-CCTTATACCAGCGTTATGAGATCAA-3′, reverse
5′-AGTGATGTGCAAGAGTCCATCC-3′; and β-actin, forward
5′-TGGCACCCAGCACAATGAA-3′, and reverse
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Western blot analysis
Cells were lysed in a buffer containing Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), separated by 12% SDS-PAGE (Beyotime Institute of
Biotechnology, Jiangsu, China), and blotted onto nitrocellulose
membranes (Merck Millipore, Carrigtwohill, Ireland). To detect hTK1
and hTIMP1, the membranes were blocked with 5% non-fat dry milk in
tris-buffered saline (TBS) containing 0.1% Tween-20 (Beyotime
Institute of Biotechnology) at room temperature for 2 h. The
membranes were then incubated overnight at 4°C with monoclonal
rabbit anti-rat hTK1 (1:5,000; cat. no. ab76495; Abcam), polyclonal
rabbit anti-rat hTIMP1 (1:5,000; cat. no. ab61224; Abcam) or
monoclonal rabbit anti-rat β-actin (1:1,000; cat. no. 4970; Cell
Signaling Biotechnology, Inc., Danvers, MA, USA). Following
washing, the membranes were incubated for 2 h at room temperature
with an anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. no. sc2030; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Subsequently, the membranes were washed five
times in TBS with 0.1% Tween-20, and target proteins bands were
detected using a horseradish-linked immunoassay with an enhanced
chemiluminescence western blotting system (CWBIO, Beijing, China).
The relative intensity of the bands of interest were analyzed with
relative protein analytic software (Imaje J 1.32; National
Institutes of Health, Bethesda, MD, USA) (n=3).
Cell proliferation assay
To determine whether the adenovirus Ad-hTIMP1-hTK1
is biologically active, two different methods were used, cell
counting and a
3-[4,5-dimethy1-2-thiazolyl]-2,5-dipheny1-2-tetrazolium-bromide
(MTT) assay. For the cell counting assay, cells were seeded in a
24-well plate at a density of 2×104 cells/well in DMEM
supplemented with 10% FBS. VSMCs were then infected with a
different adenovirus at an MOI of 20–200 for 72 h, or with various
infection incubation times (1–6 days), and then synchronized in
DMEM containing 0.2% FBS for 24 h and followed by adding 10 ng/ml
human recombinant platelet-derived growth factor (PDGF)-BB
(Peprotech Inc., Rocky Hill, NJ, USA) for 24 h. Following
treatment, cells were obtained by trypsinization (Gibco Life
Technologies), and cell numbers were counted using a hemacytometer
(Thermo Fisher Scientific, Waltham, MA, USA) with an inverted
microscope, assessing three counts for each well and a mean value
was acquired. Triplicate wells were assessed for each treatment and
experiments were performed three times.
An MTT (Sigma-Aldrich, St. Louis, MO, USA) assay was
performed as described previously (7). Briefly, VSMCs were subcultured into a
96-well plate at 2×103 cells/well in DMEM alone and
treated with as the above description in the cell counting assay.
The absorbance value was measured at 570 nm using an enzyme-linked
immnunosorbent assay (ELISA) reader (ELX800; BioTek Instruments,
Inc., Winooski, VT, USA). Experiments were performed in triplicate
(n=3).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences were analyzed using SPSS version 15.0 software (SPSS
Inc., Chicago, IL, USA). All values were analyzed using a one-way
analysis of variance and the Q test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cloning and verifification of recombinant
plasmid pDC316-hTK1-hTIMP1
The mCMV-hTIMP1 PCR fragment from pDC316-hTIMP1-EGFP
was cloned to predigested plasmid pDC316-hTK1 by double-digestion
(Bg1II/SalI), and subjected to ligation. The positive
recombinant plasmid was extracted and a PCR reaction was performed
using the aforementioned primers. The 1,338 bp fragment was
observed on 1% agarose using a UV transilluminator (Fig. 1A). Subsequently, the recombinant
plasmid was analyzed by double-digestion (Bg1II/SalI,
Fig. 1B) and sequencing (Fig. 1C) providing the expected results.
The hTK1 and hTIMP1 cDNA sequences are shown in Fig. 1C. A comparison of the hTK1 and
hTIMP1 gene sequence with available sequences in gene bank revealed
that the obtained sequence had a 100% homology with hTK1 and
hTIMP1, and indicated that mMCV-hTIMP1 was correctly cloned into
the plasmid pDC316-hTK1.
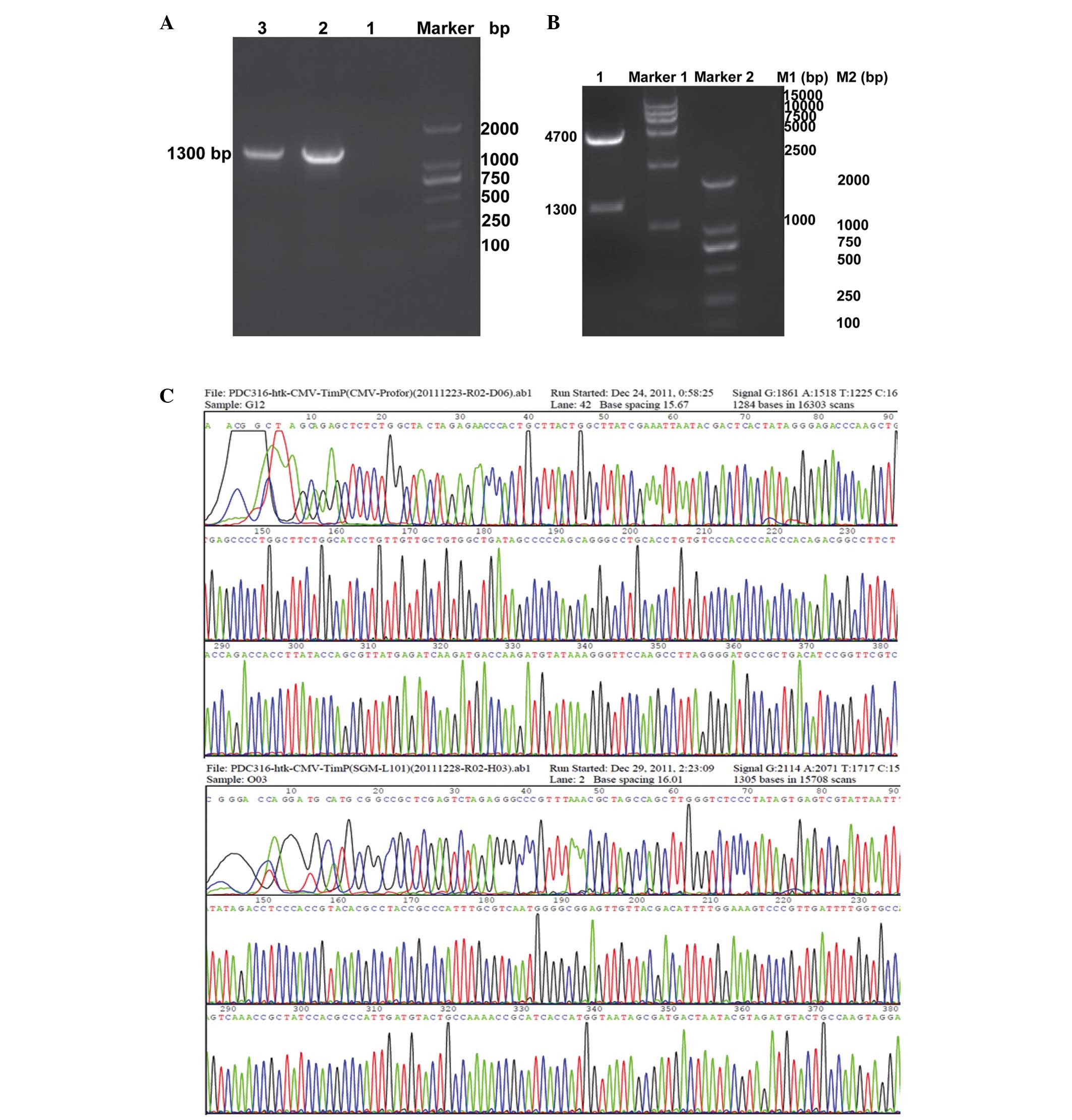 | Figure 1Identification of plasmid
pDC316-hTK1-hTIMP1. (A) PCR reaction detection assay. Lane 1,
negative control; Lane 2, mMCV-hTIMP1 (1,338 bp) gene obtained from
pDC316-hTIMP1-EGFP plasmid (positive control); Lane 3, mMCV-hTIMP1
(1,338 bp) obtained from recombinant plasmid pDC316-hTK1-hTIMP1.
(B) Double digestion assay by Bg1II/SalI: Lane 1,
corresponding to double digested plasmid pDC316-hTK1-hTIMP1 (1,338
bp and 4.7 kb). (C) Sequence analysis of the cloned PCR products
confirmed hTK1 and hTIMP1 cDNA with primer pDC316-cexu-F. PCR,
polymerase chain reaction; TK1, tissue kallikrein 1; TIMP1, tissue
inhibitor of matrix metalloproteinase 1. |
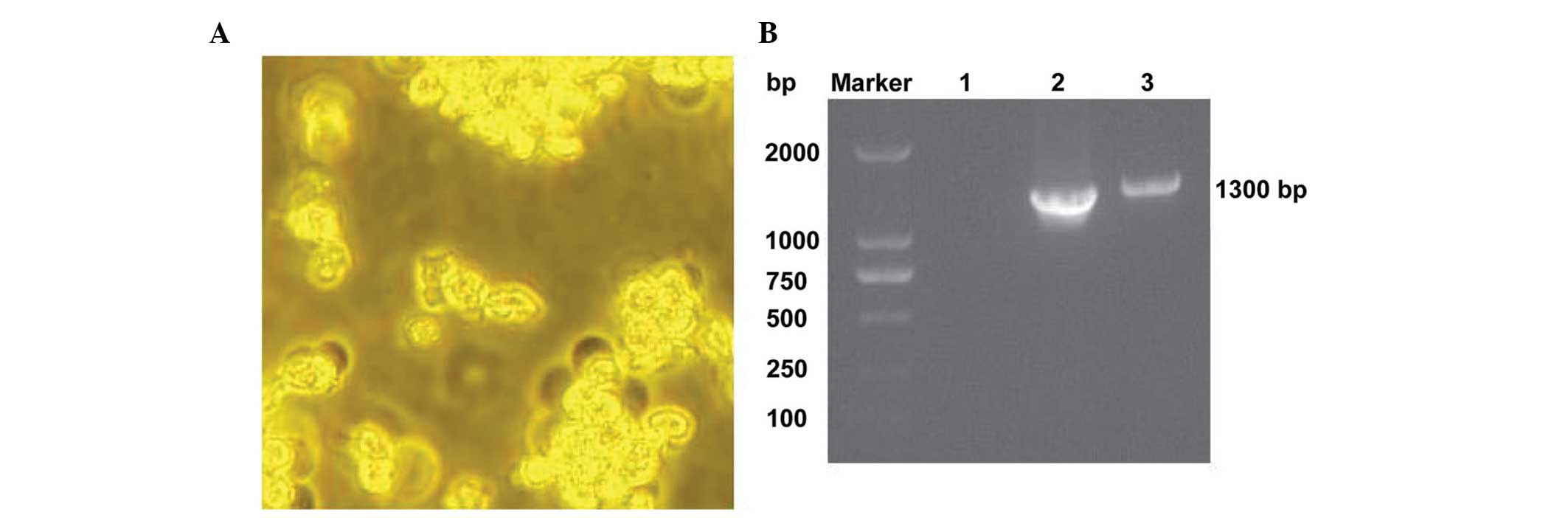
Packaging of recombinant adenovirus
With the aid of the AdMax system, the recombinant
adenovirus Ad-hTK1-hTIMP1 was prepared in HEK293A cells. A viral
plaque became visible at 9–11 days after transfection, as is shown
in Fig. 2A. The target gene was
confirmed to be correctly cloned in the adenovirus vector using a
PCR assay with corresponding primers of the hTK1 and hTIMP1 gene,
as shown in Fig. 2B. Following
plaque purification, the titration of the recombinant adenovirus
Ad-hTK1-hTIMP1 was achieved up to 1.26×1010 IU/ml using
a TCID assay.
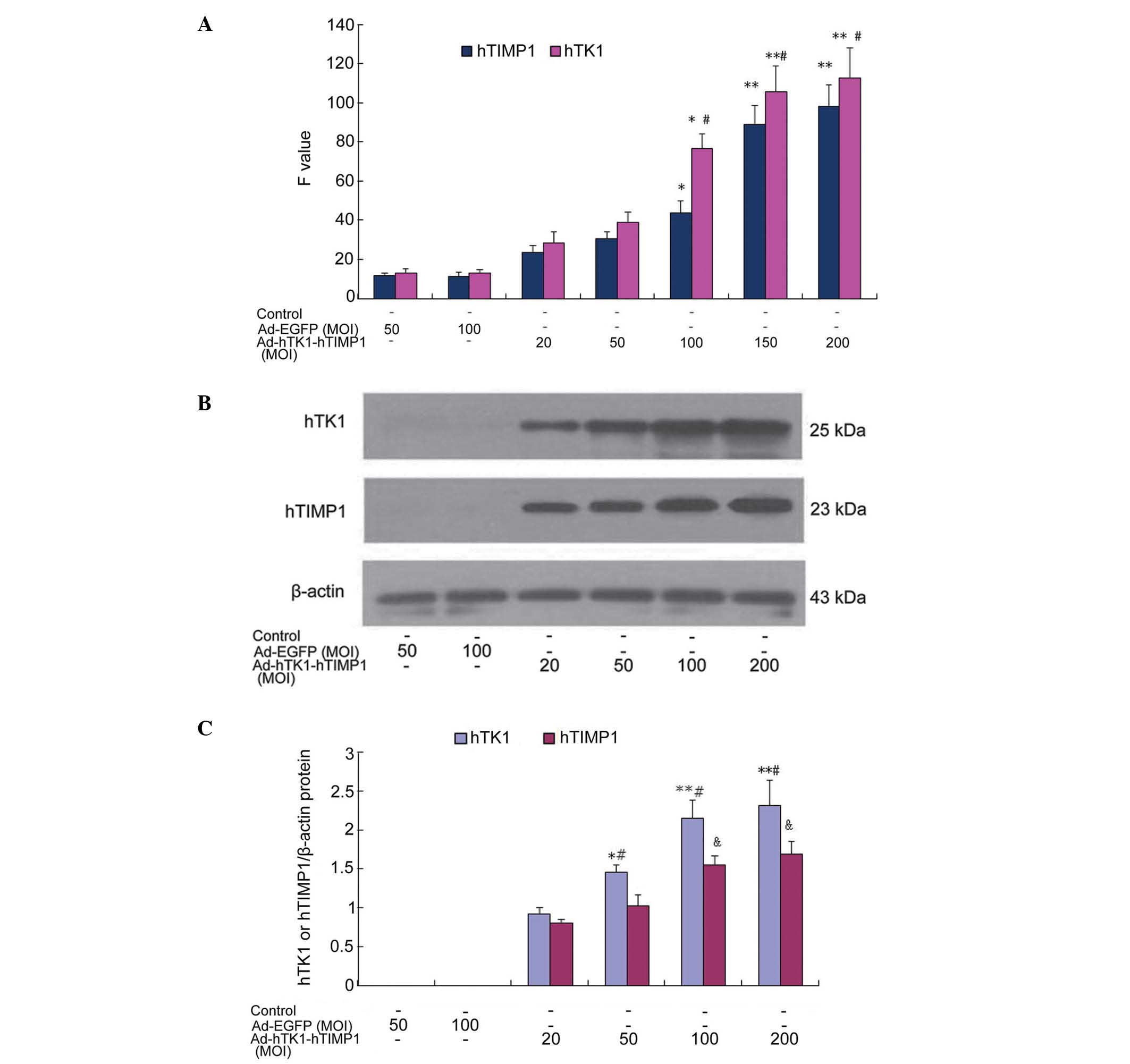
mRNA and protein expression in VSMCs
following transfection with Ad-hTK1-hTIMP1
VSMCs were infected with Ad-hTK1-hTIMP1 at an MOI of
20, 50, 100, 150 and 200, respectively, and mRNA expression was
estimated by indirect RT-qPCR. As is shown in Fig. 3A, MOI-dependent expression was
observed at 50–150 MOI at 72 h (P<0.05 50–100 MOI, P<0.01 150
MOI), respectively, whereas there was a lower level of expression
in adenovirus-infected cells at 20–50 MOI (P>0.05). However, no
significant difference was identified between 150 MOI and 200 MOI
(P>0.05).
The protein expression of Ad-hTK1-hTIMP1 was
assessed using western blot analysis. As expected, the protein
expression of hTK1 (33 kDa) and hTIMP1 (28.5 kDa) were detected in
VSMCs. Ad-hTK1-hTIMP1 at an MOI of 20, 50, 100 and 200, as is shown
in Fig. 3B and C, MOI-dependent
expression was observed at 20–200 MOI at 72 h after infection
(P<0.05 50 MOI, P<0.01 100–200 MOI), whereas there was a
lower level of expression of hTK1 and hTIMP1 in the
adenovirus-infected cells at 20 MOI (P>0.05). However, the mRNA
and protein expression of hTK1 were markedly higher than that of
hTIMP1 between 50 and 200 MOI in the co-expression vector
(P<0.05 50 MOI, P<0.01 100–200 MOI).
Therefore, 100–150 MOI of Ad-hTK1-hTIMP1 was
accepted as an ideal level for subsequent experiments based on the
evaluation of the transfection and expression efficiency assay.
Effects on cell growth and proliferation
of Ad-hTK1-hTIMP1
It was investigated whether the co-expression vector
of hTK1 and hTIMP1 genes had an effect on VSMC growth and
proliferation. Primary VSMCs were infected with Ad-hTK1-hTIMP1 or
Ad-EGFP, or left untreated as a control, and treated with or
without PDGF-BB. The cell growth was measured using a cell counting
assay. The number of cells induced by PDGF-BB was significantly
increased compared with the control. However, Ad-hTK1-hTIMP1
significantly decreased cell numbers as compared with Ad-EGFP
(P<0.01). As is shown in Fig.
4A, Ad-hTK1-hTIMP1 had minor inhibitory effects on VSMC
proliferation at lower concentrations (<20 MOI). Cell numbers
were significantly reduced at concentrations >50 MOI of
Ad-hTK1-hTIMP1 (P<0.05) as compared with 20 MOI. Compared with
100 MOI, the inhibition rate was 45.27% when VSMCs were treated
with 150 MOI (P<0.05). Cell number of cells infected with 200
MOI of Ad-hTK1-hTIMP1 were no further decreased than those infected
with 150 MOI (P>0.05). However, certain cells infected with
Ad-hTK1-hTIMP1 at 200 MOI appeared to shrink and float, suggesting
apoptosis and necrosis. Therefore, 150 MOI of Ad-hTK1-hTIMP1 was
the optimal dose for the present study.
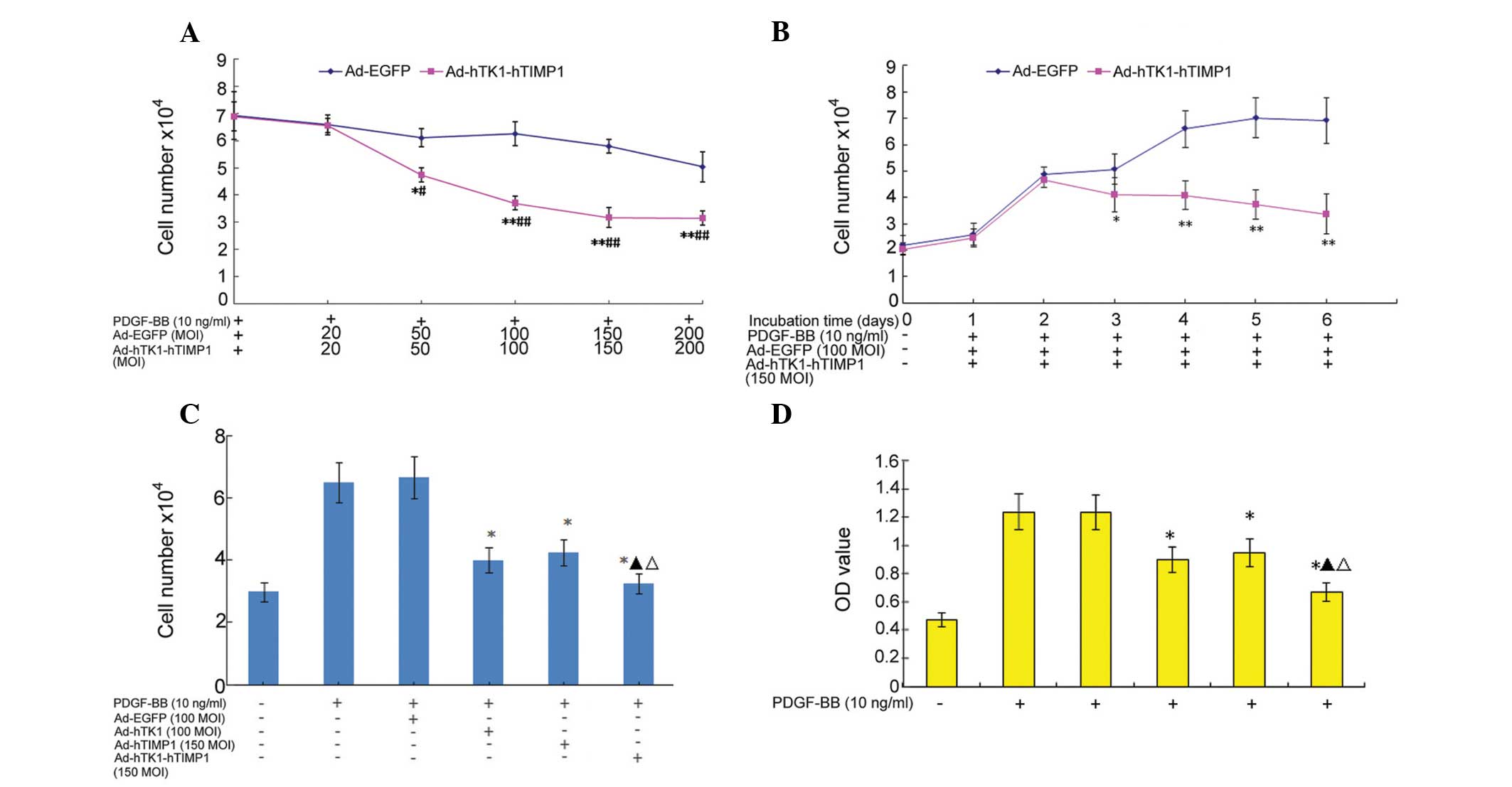 | Figure 4Inhibitory effects of Ad-hTK1-hTIMP1
on cell growth and proliferation in VSMCs. (A)
Concentration-dependent inhibitory effects of co-expression vector
on VSMC growth assessed by cell counting. Cells treated with 0.2%
fetal bovine serum were used as a control. *P<0.05
and **P<0.01, compared with cells infected with 20
MOI Ad-hTK1-hTIMP1; #P<0.05 and
##P<0.05, as compared with the cells infected with
Ad-EGFP. (B) Time-dependent (1–6 days) inhibitory effects of
co-expression vector on VSMC growth assessed by cell counting.
*P<0.05, **P<0.01, compared with
Ad-hTK1-hTIMP1 for 2 days. (C and D) Inhibitory proliferation
effects of co-expression vector evaluated using cell counting and a
3-[4,5-dimethy1-2-thiazolyl]-2,5-dipheny1-2-tetra-zoliumbromide
assay. *P<0.01, compared with cells stimulated with
platelet-derived growth factor-BB only; ▲P<0.05 as
compared with cells infected with Ad-hTK1; ΔP<0.05 as
compared with cells infected with Ad-hTIMP1. TK1, tissue kallikrein
1; TIMP1, tissue inhibitor of matrix metalloproteinase 1; VSMCs,
vascular smooth muscle cells. |
As is shown in Fig.
4B, 150 MOI of Ad-hTK1-hTIMP1 had minor effects on VSMC
proliferation following a short incubation time (<2 days). By
contrast, the cell numbers were significantly reduced after >3
days incubation in Ad-hTK1-hTIMP1-infected cells compared with 2
days (P<0.05). The peak inhibition rate was 46.6% when VSMCs
were infected for 5 days. No significant difference in the
inhibitory rate between the 6th and 7th day, as well as the 5th day
was observed (P>0.05). Certain cells infected with
Ad-hTK1-hTIMP1 for 6 and 7 days appeared to shrink and float,
suggesting apoptosis or necrosis. Therefore, it was found that 3
days after transfection was the optimal time for observation. These
experiments suggested that within a given MOI (50–150 MOI) and time
(2–5 days), the hTK1 and hTIMP1 co-expression inhibited
PDGF-BB-stimulated VSMC growth in an MOI-dependent and
time-dependent manner.
The synergistic inhibitory effects of the
co-expression vector was subsequenlty investigated on cell
proliferation. VSMCs were infected with Ad-hTK1-hTIMP1, Ad-hTK1,
Ad-hTIMP1, or Ad-EGFP, and PDGF-BB-mediated cell growth was
measured by cell count and MTT assay. PDGF induced significant
increases in cell numbers and optical density (OD)values in
PDGF-BB-stimulated and Ad-EGFP-infected VSMCs. As is shown in
Fig. 4C and D, it was detected
that the three recombinant adenoviruses significantly decreased
cell number and OD values as compared with cells treated with
PDGF-BB and Ad-EGFP (P<0.01). The peak inhibitory rates
(analyzed by the OD value) of Ad-hTK1, Ad-hTIMP1 and Ad-hTK1-hTIMP1
were 27.4, 23.3 and 45.7%, respectively. The inhibitory effect of
Ad-hTK1-hTIMP1 on cell growth and proliferation exhibited a greater
suppression than that of Ad-hTK1 and Ad-hTIMP1 (0.675±0.003 vs.
0.912±0.008, P<0.05; 0.675±0.003 vs. 0.951±0.005, P<0.05).
This result suggests a possible association of the synergistic
suppression of proliferation by co-expression of the hTK1 and
hTIMP1 genes. No significant differences were observed between
cells treated with Ad-hTK1 and Ad-hTIMP1, and Ad-EGFP and PDGF-BB
only.
Discussion
Vascular remodeling occurs following the abnormal
migration and proliferation of VSMCs, as well as the ECM
rearrangement (22). Numerous risk
factors are involved in the development of the pathological
process. Multigene-based combination therapy represents an
effective practice in atherosclerosis, which may achieve greater
therapeutic benefits (23). Recent
studies have reported that TK1 is important in reducing blood
pressure, improving cardiac remodeling, renal injury and ischemic
stroke, and preventing the long-term in-stent restenosis (1–8).
Subsequent studies have demonstrated that TIMP1, as a regulator of
MMPs, exhibits a number of beneficial effects on the inhibition of
vascular remodeling by blocking the activation of MMPs, supressing
VSMC migration and destabilizing vessel differentiation (14,24).
However, the therapeutic effect of the combination treatment of TK1
and TIMP1 on VSMC proliferation remains to be investigated. Based
on the anti-restenosis properties of TK1 and TIMP1, it was
hypothesized that the combination of TK1 and TIMP1 would exert an
enhanced or synergistic effect on inhibiting VSMC
proliferation.
Adenoviral vectors harboring therapeutic genes have
been used successfully for gene transfer in vitro and in
vivo (16,17). In the present study, the
recombinant plasmid, pDC316-hTK1-hTIMP1 was successfully
constructed and verified using PCR, double digestion and sequencing
assays. The recombinant adenovirus, Ad-hTK1-hTIMP1 was successfully
packaged into HEK293A cells with the aid of a highly efficient
Cre/loxP-based system (7,18,25).
The co-expression vector contains hTK1 and hTIMP1 double stranded
cDNA by means of a double mCMV promoter, and avoids the production
of a fusion protein. The titer of the co-expression vector was
1.26×1010 IU/ml as assessed using the TCID50 method.
Furthermore, it was demonstrated that there was an increase in hTK1
and hTIMP1 transcription and protein when VSMCs were infected with
the co-expression vector, which occurred in a dose (50–150 MOI) and
time-dependent (2–5 day) manner. No significant differences were
identified in the expression levels of hTK1 and hTIMP1 in a 150 MOI
co-expression vector compared with a 200 MOI co-expression vector.
However, the expression level of hTK1 was greater compared with
that of hTIMP1 in the co-expression vector transfected with VSMCs.
The present study confirmed that the co-expression vector carrying
full length hTK1 and hTIMP1 cDNA could successfully co-express hTK1
and hTIMP1 protein in VSMCs.
Our previous study also demonstrated that the
over-expression of the hTK1 and hTIMP1 genes mediated by Ad-hTK1
and Ad-hTIMP1 could inhibit the proliferation of VSMCs induced by
PDGF-BB, where the peak inhibitory rate was 30.2 and 23.2%,
respectively (7). TIMP1 has been
observed to mediate the inhibitory effect of interleukin (IL)-6 on
the proliferation of a hepatocellular carcinoma cell line (26). To further examine the synergistic
effects of these genes and whether the co-expression vector
inhibits the proliferation of VSMCs, PDGF-BB-stimulated cells were
treated with Ad-hTK1-hTIMP1, Ad-hTK1 and Ad-hTIMP1, and were
assessed using cell counting and an MTT assay. It was demonstrated
that the co-expression vector supressed cell growth and
proliferation in an MOI-dependent (50–150 MOI) and time-dependent
manner (2–5 days), with a peak inhibitory rate of 48.2% (150 MOI)
and 39.7% (5 days). It was also demonstrated that the co-expression
vector synergistically inhibited VSMC growth and proliferation.
Zhao et al (27) also
demonstrated that the adenovirus-mediated inhibitor of growth
family, member 4 and IL-24 double tumor suppressor gene
co-expression enhances the antitumoral activity in human breast
cancer cells, reduces side effects from vectors, and has
satisfactory prospects for clinical application. The study of Deng
et al (28) implied that a
molecular therapy combining two or more functionally synergistic
tumor suppressors may constitute a novel and effective strategy for
cancer treatment. However, how protected effective function
contributed to improve vascular remodeling by means of a
co-expression vector remains to be further examined and
elucidated.
In conclusion, the present data demonstrated that
hTK1 and hTIMP1 full-length cDNA in a single adenovirus vector may
be successfully constructed and achieve co-expression of hTK1 and
hTIMP1 proteins, independently and abundantly. There was a
synergistic inhibitory proliferative effect on VSMCs infected with
the co-expression vector, suggesting that it may be an effective
therapeutic strategy for vascular remodeling disease.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of China (grant no. 81373785), the
Natural Science Foundation of Fujian Province (grant nos.
2011J01134 and 2010J01127) and the Projection of Excellent Yang
Doctor training plan in Fujian Provincial Health System (grant no.
2013-ZQN-ZD-2).
References
|
1
|
Ashley PL and MacDonald RJ:
Tissue-specific expression of kallikrein-related genes in the rat.
Biochemistry. 24:4520–4527. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerald WL, Chao J and Chao L: Sex
dimorphism and hormonal regulation of rat tissue kallikrein mRNA.
Biochim Biophys Acta. 867:16–23. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao Y, Sheng Z, Li Y, Yan F, Fu C, Li Y,
Ma G, Liu N, Chao J and Chao L: Tissue kallikrein promotes cardiac
neovascularization by enhancing endothelial progenitor cell
functional capacity. Hum Gene Ther. 23:859–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin H, Chao L and Chao J: Nitric oxide
mediates cardiac protection of tissue kallikrein by reducing
inflammation and ventricular remodeling after myocardial
ishchemia/reperfusion. Life Sci. 82:156–165. 2008. View Article : Google Scholar
|
|
5
|
Liu Y, Bledsoe G, Hagiwara M, Yang ZR,
Shen B, Chao L and Chao J: Blockade of endogenous tissue kallikrein
aggravates renal injury by enhancing oxidative stress and
inhibiting matrix degradation. Am J Physiol Renal Physiol.
298:F1033–F1040. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan W, Yang F, Liu L, Yin Q, Li M, Li Z,
Sang H, Xu G, Ma M, Zhang Z, et al: Tissue kallikrein preventing
the restenosis after stenting of symptomatic MCA atherosclerotic
stenosis (KPRASS). Int J Stroke. 9:533–535. 2014. View Article : Google Scholar
|
|
7
|
Yu HZ, Xie LD, Zhu PL, Xu CS and Wang HJ:
Human tissue kallikrein 1 gene delivery inhibits PDGF-BB-induced
vascular smooth muscle cells proliferationand upregulates the
expressions of p27Kip1 and p2lCip1. Mol Cell Biochem. 360:363–371.
2012. View Article : Google Scholar
|
|
8
|
Yu HZ, Xie LD, Zhu PL, Xu CS, Wang HJ and
Li TY: Effects of human tissue kallikrein 1 gene delivery on
carotid artery neointima formation after balloon angioplasty in
spontaneously hypertensive rats. Zhonghua Xin Xue Guan Bing Za Zhi.
38:67–71. 2010.In Chinese. PubMed/NCBI
|
|
9
|
Heagerty AM, Heerkens EH and Izzard AS:
Small artery structure and function in hypertension. J Cell Mol
Med. 14:1037–1043. 2010.PubMed/NCBI
|
|
10
|
Intengan HD and Schiffrin EL: Vascular
remodeling in hypertension: Roles of apoptosis, inflammation and
fibrosis. Hypertension. 38:581–587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papazafiropoulou A and Tentolouris N:
Matrix metalloproteinases and cardiovascular diseases. Hippokratia.
13:76–82. 2009.PubMed/NCBI
|
|
13
|
Docherty AJ, Lyons A, Smith BJ, Wright EM,
Stephens PE, Harris TJ, Murphy G and Reynolds JJ: Sequence of human
tissue inhibitor of metalloproteinases and its identity to
erythroid-potentiating activity. Nature. 318:66–69. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dollery CM, Humphries ES, McClelland A,
Latchman DS and McEwan JR: Expression of tissue inhibitor of matrix
metal-loproteinases 1 by use of an adenoviral vector inhibits
smooth muscle cell migration and reduces neointimal hyperplasia in
the rat model of vascular balloon injury. Circulation.
99:3199–3205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R,
Gu J, Qian C and Liu X: An oncolytic adenoviral vector of Smac
increases antitumor activity of TRAIL against HCC in human cells
and in mice. Hepatology. 39:1371–1381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto
KI, Fan DS, Ogawa M, Mizukami H, Urabe M, Kume A, Nagatsu I, et al:
Triple transduction with adeno-associated virus vectors expressing
tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase and GTP
cyclohydrolase I for gene therapy of Parkinson's disease. Hum Gene
Ther. 11:1509–1519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ngoi SM, Chien AC and Lee CG: Exploiting
internal ribosome entry sites in gene therapy vector design. Curr
Gene Ther. 4:15–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denes L, Entz L and Jancsik V: Restenosis
and therapy. Int J Vasc Med. 4062362012.PubMed/NCBI
|
|
19
|
Ng P, Parks RJ, Cummings DT, Evelegh CM,
Sankar U and Graham FL: A high-efficiency Cre/loxP-based system for
construction of adenoviral vectors. Hum Gene Ther. 10:2667–2672.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda M, Namikawa K, Kobayashi I, Ohba N,
Takahara Y, Kadono C, Tanaka A and Kiyama H: Targeted gene therapy
toward astrocytoma using a Cre/loxP-based adenovirus system. Brain
Res. 1081:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HF, Xie LD and Xu CS: The signal
transduction pathways of heat shock protein 27 phosphorylation in
vascular smooth muscle cells. Mol Cell Biochem. 333:49–56. 2010.
View Article : Google Scholar
|
|
22
|
Gariepy J, Massonneau M, Levenson J,
Heudes D and Simon A: Evidence for in vivo carotid and femoral wall
thickening in human hypertension. Groupe de Prévention
Cardio-vasculaire en Médecine du Travail. Hypertension. 22:111–118.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilson DR: Viral-mediated gene transfer
for cancer treatment. Curr Pharm Biotechnol. 3:151–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eefting D, Seghers L, Grimbergen JM, de
Vries MR, de Boer HC, Lardenoye JW, Jukema JW, van Bockel JH and
Quax PH: A novel urokinase receptor-targeted inhibitor for plasmin
and matrix metalloproteinases suppresses vein graft disease.
Cardiovasc Res. 88:367–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang M, Shi W, Zhang Q, Wang X, Guo M,
Cui Z, Su C, Yang Q, Li Y, Sham J, et al: Gene therapy using
adenovirus-mediated full-length anti-HER-2 antibody for HER-2
overexpression cancers. Clin Cancer Res. 12:6179–6185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo SY, Shen X, Yang J, Yuan J, Yang RL,
Mao K, Zhao DH and Li CJ: TIMP-1 mediates the inhibitory effect of
interleukin-6 on the proliferation of a hepatocarcinoma cell line
in a STAT3-dependent manner. Braz J Med Biol Res. 40:621–631. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao YD, Li ZY, Sheng WH, Miao J and Yang
J: Adenovirus-mediated ING4/IL-24 double tumor suppressor gene
co-transfer enhances antitumor activity in human breast cancer
cells. Oncol Rep. 28:1315–1324. 2012.PubMed/NCBI
|
|
28
|
Deng WG, Kawashima H, Wu G, Jayachandran
G, Xu K, Minna JD, Roth JA and Ji L: Synergistic tumor suppression
by coexpression of FUS1 and p53 is associated with down-regulation
of murine double minute-2 and activation of the apoptotic
protease-activating factor 1-dependent apoptotic pathway in human
non-small cell lung cancer cells. Cancer Res. 67:709–717. 2007.
View Article : Google Scholar : PubMed/NCBI
|