Introduction
Diseases resulting from the degeneration of the
intervertebral discs have become increasing threats to quality of
life due to a rapidly aging society (1). The abnormal apoptosis or
age-associated apoptosis of nucleus pulposus (NP) cells is
suggested to serve a key role in the development of degenerative
disc diseases (2–4). High levels of interleukin-1β (IL-1β)
are present in degenerating intervertebral discs and there is a
positive response of intervertebral disc cells to IL-1β inhibition
(5,6). This indicates that the inflammatory
process, in particular the IL-1β-induced degradation of
proteoglycans and type II collagen, results in damage to and
accelerates the apoptosis of NP cells and annulus fibrosus cells,
thereby leading to the development of degenerative disc diseases
(5,7–10).
Reducing inflammation and the deleterious impact of apoptosis is
therefore critical for the treatment of degenerative disc
diseases.
Due to their numerous advantages, including their
abundance, high activity, low immunogenicity, marked proliferation,
differentiation potential and nutrient secretion, mesenchymal stem
cells (MSCs) have been widely used in general transplantation
research, with positive effects (11–13).
MSC transplantation, which exhibits beneficial effects in the
treatment of degenerative disc diseases, including the inhibition
of intervertebral disc degeneration (IVDD), has been reported to
increase the number of cells in intervertebral discs and results in
the partial recovery of intervertebral disc height (14–16).
However, the specific effects and mechanisms of MSCs on NP cells
remain to be fully elucidated. Although certain previous studies
have used green fluorescent protein (GFP)-transfected cells as a
means of distinguishing between cell types, it remains challenging
to accurately separate transfected cells when assessing
intercellular interactions (17).
The complication of in vivo studies is whether the observed
therapeutic effect arises from in situ cells being
'nourished' by BMSCs (12,18,19),
or is instead an artifact of BMSCs, which exhibit high activity and
differentiation potential (13).
In vivo studies are therefore, inherently limited. In order
to further investigate the mechanisms underlying MSC therapy at the
cellular level, the present study used a Transwell assay involving
non-contacting and contacting co-culture systems to simulate the
in vivo paracrine interactions between cells and directed
migration (20,21). Unlike previous studies, the
anti-apoptotic and migratory capabilities, in addition to
mitochondrial transfer through tunneling nanotube (TnT) formation
of BMSCs were directly assessed in vitro. The present study
was able to measure specific alterations in NP cells via paracrine
mechanisms and mitochondrial transfer resulting from MSCs.
Materials and methods
Ethics statement
The male Sprague-Dawley rats (age, 3 months; weight,
250–300 g) used in the current study were provided by the Second
Military Medical University Laboratory Animal Center (Shanghai,
China). The rats were housed under a 12 h light/dark cycle at
constant temperature (25°C) and humidity, with ad libitum
access to food and water. All experiments were approved by the
Animal Ethical Committee of the Second Military Medical University
(no. 13071002114).
Isolation and culture of BMSCs and NP
cells from Sprague-Dawley rats
Primary BMSCs were isolated and cultured, as
described previously (16). The
harvested cells were centrifuged at 500 × g for 10 min at 4°C and
then resuspended in complete Dulbecco's modified Eagle's medium
(DMEM)/F-12 with 10% fetal bovine serum (FBS), 100 µg/ml
streptomycin and 100 U/ml penicillin (all purchased from Gibco Life
Technologies, Carlsbad, CA, USA). Cells at passage 3 were subjected
to flow cytometric analysis (Cytomics FC500; Beckman Coulter, Brea,
CA, USA) to assess the expression of the surface markers, cluster
of differentiation 29 (CD29), CD90, CD31 and CD45. The osteogenic
and adipogenic differentiation potentials of the cells were also
assessed by Alizarin red staining (Beyotime Institute of
Biotechnology, Haimen, China) and Oil Red O staining (Beyotime
Institute of Biotechnology).
The rat NP cells were isolated, as described
previously (22). The NP cells
were seeded into 60 mm tissue culture dishes and grown in complete
culture medium at 37°C with 5% CO2. The culture medium
was replaced every 2–3 days. The cells were passaged by digestion
with 0.25% trypsin (Gibco Life Technologies) once they reached
80–90% confluence. The NP cells at passage 3 in culture medium were
used for subsequent experiments.
Establishment of a co-culture system and
cell treatment
In order to construct the co-culture system, NP
cells were plated at a constant density of 2×104
cells/cm2 into certain culture plates and were
subsequently divided into different groups based on specific
treatments. Two steps were included and each lasted 24 h. In the
first step, serum-free medium with or without IL-1β (PeproTech,
Inc., Rocky Hill, NJ, USA) was used for stimulation and
subsequently, complete medium with or without BMSCs (the ratio was
1:2 to the NP cells) was used for co-culture intervention. The
groups used were as follows: i) Control group, the cells were
treated with serum-free medium, which was then replaced with
complete medium; ii) No-culture group, the cells were subjected to
intervention treatment with IL-1β (20 ng/ml), which was then
replaced with complete medium; iii) Indirect co-culture group,
following stimulation with IL-1β (20 ng/ml), the BMSCs were
co-cultured using a Transwell (pore size 0.4 µm; EMD
Millipore, Billerica, MA, USA) in complete medium; iv) direct
co-culture group, following stimulation with IL-1β (20 ng/ml), an
identical quanitity of GFP BMSCs were directly added to the NP
cells with complete medium.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated from the NP cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
and the RNA concentration was measured photometrically (NanoDrop
2000/2000c; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
Synthesis of the cDNA was performed using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA,
USA), according to the manufacturer's instructions. PCR analysis
was performed using gene-specific primers (Table I) for GAPDH, a disintegrin and
metalloproteinase with thrombospondin motifs 4 (ADAMTS-4),
ADAMTS-5, matrix metallopro-teinase 13 (MMP-13), tissue inhibitor
of metalloproteinases 1 (TIMP-1) and caspase-3 (Sangon Biotech Co.,
Ltd., Shanghai, China). RT-qPCR was performed using a MyiQ™
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with SYBR Green Real-time PCR Master mix (Toyobo Co., Ltd., Osaka,
Japan). Amplification of the target cDNA was normalized against the
expression of GAPDH. The relative levels of the target mRNA
expression were calculated using the 2−ΔΔCt method
(23).
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer name | Sequence
(5′-3′) |
|---|
| GAPDH | GAPDH-F |
CCATCAACGACCCCTTCATT |
| GAPDH-R |
ATTCTCAGCCTTGACTGTGC |
| ADAMTS-4 | ADAMTS-F |
ACAATGGCTATGGACACTGCCTCT |
| ADAMTS-R |
TGTGGACAATGGCTTGAGTCAGGA |
| ADAMTS-5 | ADAMTS-F |
GTCCAAATGCACTTCAGCCACGAT |
| ADAMTS-R |
AATGTCAAGTTGCACTGCTGGGTG |
| MMP-13 | MMP-13-F |
CCCTGGAGCCCTGATGTTT |
| MMP-13-R |
CTCTGGTGTTTTGGGGTGCT |
| TIMP-1 | TIMP-F |
ATAGTGCTGGCTGTGGGGTGTG |
| TIMP-R |
TGATCGCTCTGGTAGCCCTTCTC |
| Caspase-3 | Caspase-3-F |
ACAGAGCTGGACTGCGGTAT |
| Caspase-3-R |
TGCGGTAGAGTAAGCATACAGG |
Terminal deoxynucleotide transferase dUTP
nick end labeling (TUNEL) apoptosis analysis
Following fixation in freshly prepared 4%
paraformaldehyde (Roche Diagnostics, Basel, Switzerland) for 1 h at
4°C, the cells were incubated with 3% H2O2
and 0.1% Triton X-100 for 10 min and washed three times with
phosphate-buffered saline (PBS) at each step. The cells were
stained using the In Situ Cell Death Detection kit (Roche
Diagnostics) and counterstained with Hoechst 33258 (Beyotime
Institute of Biotechnology), according to the manufacturer's
instructions. Apoptotic alterations were measured by fluorescence
microscopy (BX51; Olympus, Tokyo, Japan).
Caspase-3 activity assay
Caspase-3 activity was determined using a Caspase-3
Activity kit (Beyotime Institute of Biotechnology), which is based
on the caspase-3-mediated conversion of acetyl-Asp-Glu-Val-Asp
p-nitroanilide into the yellow formazan product, p-nitroaniline,
according to the manufacturer's instructions. The activity of
caspase-3 was quantified on a microplate spectrophotometer (Biotek
Instruments, Inc., Winooski, VT, USA) at 405 nm. Caspase-3 activity
was expressed as the fold-change in enzyme activity compared with
that of synchronized cells.
Detection of apoptotic incidence by flow
cytometry
Apoptotic incidence was detected using the Annexin
V-Fluorescein Isothiocyanate (FITC) [Phycoerythrin (PE) for direct
co-culture]/propidium iodide (PI) Apoptosis Detection kit I (BD
Pharmingen, San Diego, CA, USA), according to the manufacturer's
instructions. The samples were analyzed on a fluorescence activated
cell sorter (Cytomics FC500; Beckman Coulter) within 1 h. Apoptotic
cells, including annexin-positive/PI-negative in addition to
double-positive cells, were counted and represented as a percentage
of the total cell count.
Detection of migration of BMSCs
The migratory ability of BMSCs was assessed using
Transwell plates (Corning Inc., Corning, NY, USA), which were 6.5
mm in diameter with 8 µm pore filters. The BMSCs were
harvested and equal numbers of cells were added to each group of
Transwell plates. Following incubation for 24 h at 37°C, the cells
that had not migrated from the upper side of the filter were
scraped off with a cotton swab and the filters were stained with
Coomassie Blue (Beyotime Institute of Biotechnology). The number of
cells that had migrated to the lower side of the filter were
counted under a light microscope at a magnification of ×400 in five
randomly selected fields.
Staining cells and confocal
microscopy
To trace inter-cellular exchange of mitochondria,
GFP BMSCs were separately labeled with MitoTracker® Deep
Red (Invitrogen Life Technologies), according to the manufacturer's
instructions. Briefly, the cells were resuspended in pre-warmed
(37°C) staining solution, containing the MitoTracker®
probe (50 nM) for 30 min in complete medium. Following staining,
the cells were washed three times in PBS and resuspended in fresh
pre-warmed medium and were directly added into IL-1β stimulated NP
cells on glass slides for 24 h. Following the addition of the
cells, the glass slides with two types of cells were washed with
PBS and were labeled with DAPI (10 µg/ml, 5 min; Beyotime
Institute of Biotechnology). Images were captured by confocal
microscopy (TCS SP5; Leica Microsystems GmbH, Wetzlar, Germany) for
1 h and were subsequently analyzed by Leica LAS AF Lite_2.5.0_7266
software.
Statistical analysis
All statistical analyses were performed in
triplicate. All data are presented as the mean ± standard error.
Differences between the groups were analyzed by one way analysis of
variance (*P<0.05, **P<0.01) using
GraphPad Prism software, version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
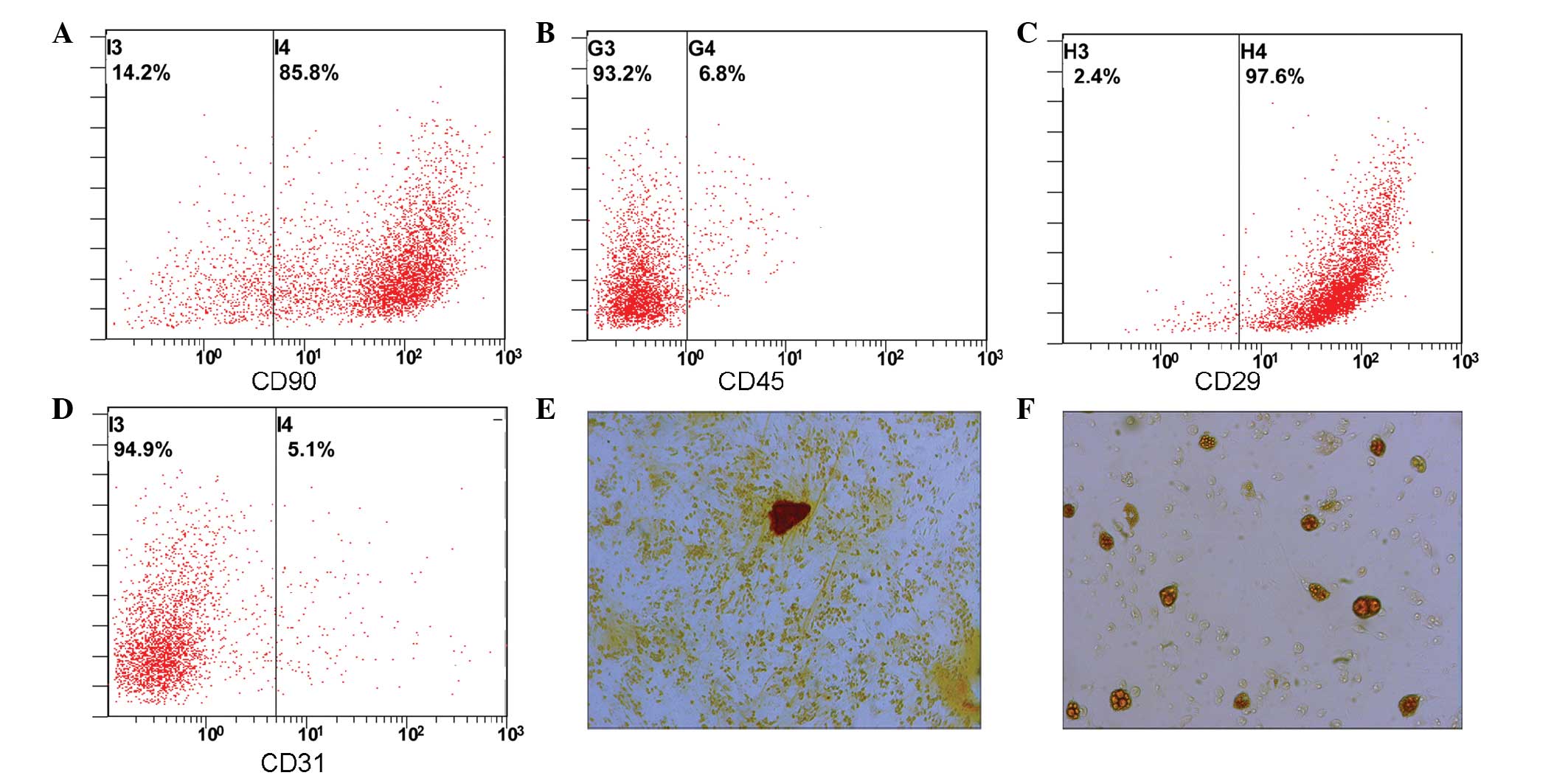
Identification of isolated BMSCs
Flow cytometric analysis was used to detect CD90
(Fig. 1A), CD45 (Fig. 1B), CD29 (Fig. 1C) and CD90 (Fig. 1D) expression. BMSCs (>85%) were
positive for CD31 and CD45, whereas <0.1% of BMSCs were positive
for CD31 and CD45. Following 2 weeks of induced differentiation,
osteogenic differentiation was examined by Alizarin red staining
(Fig. 1E), and adipogenic
differentiation was assessed by Oil Red O staining (Fig. 1F). The results suggest that cells
cultured in this manner can be induced to differntiate into both
osteogenic and adipogenic cells.
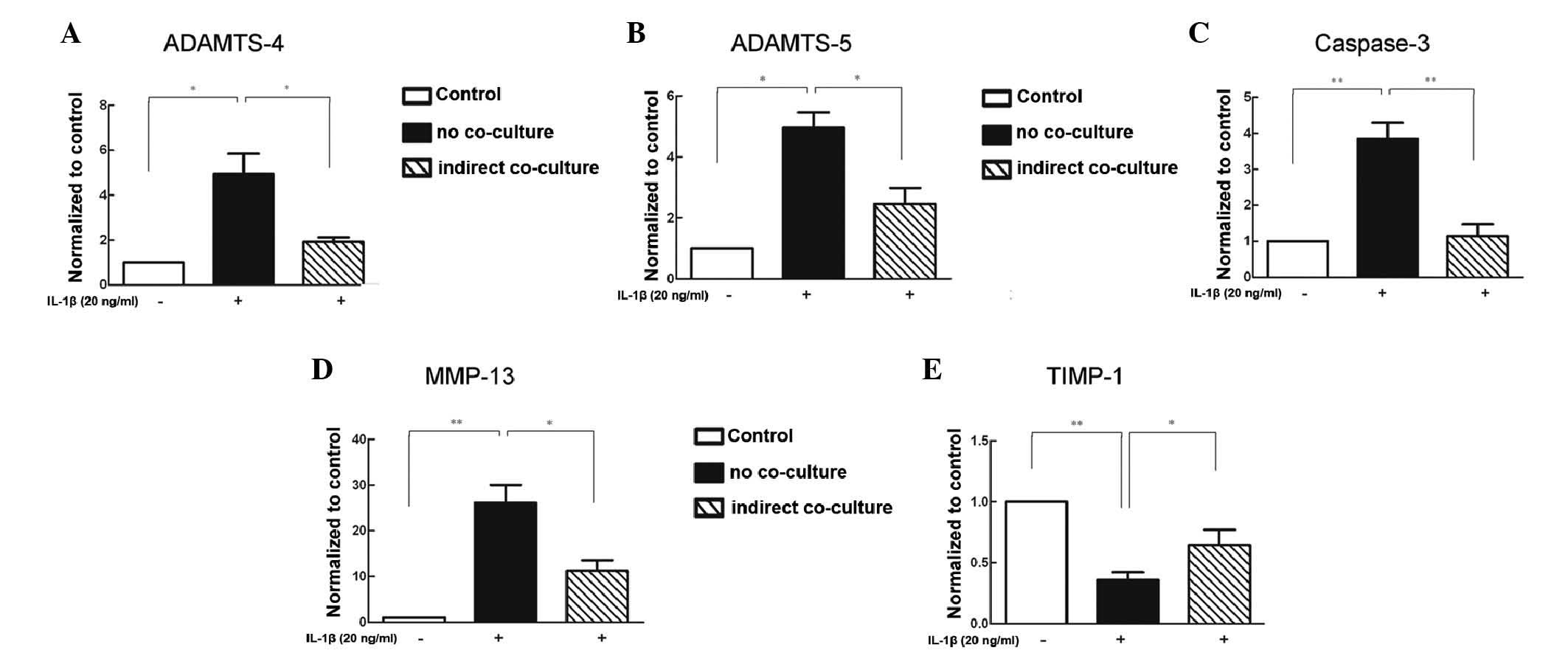
Co-culture of BMSCs with IL-1β-stimulated
NP cells reduces degenerative enzyme gene expression
Compared with the unstimulated NP cells, the NP
cells in the no co-culture groups exhibited markedly higher
expression levels of ADAMTS-4, ADAMTS-5, caspase-3 and MMP-13
following inflammatory stimulation (Fig. 2A–D), whereas the expression of
TIMP-1 was reduced (Fig. 2E).
Compared with the no co-culture group, various indexes were
significantly reduced in the co-culture group. In addition, the
expression of TIMP-1 was increased.
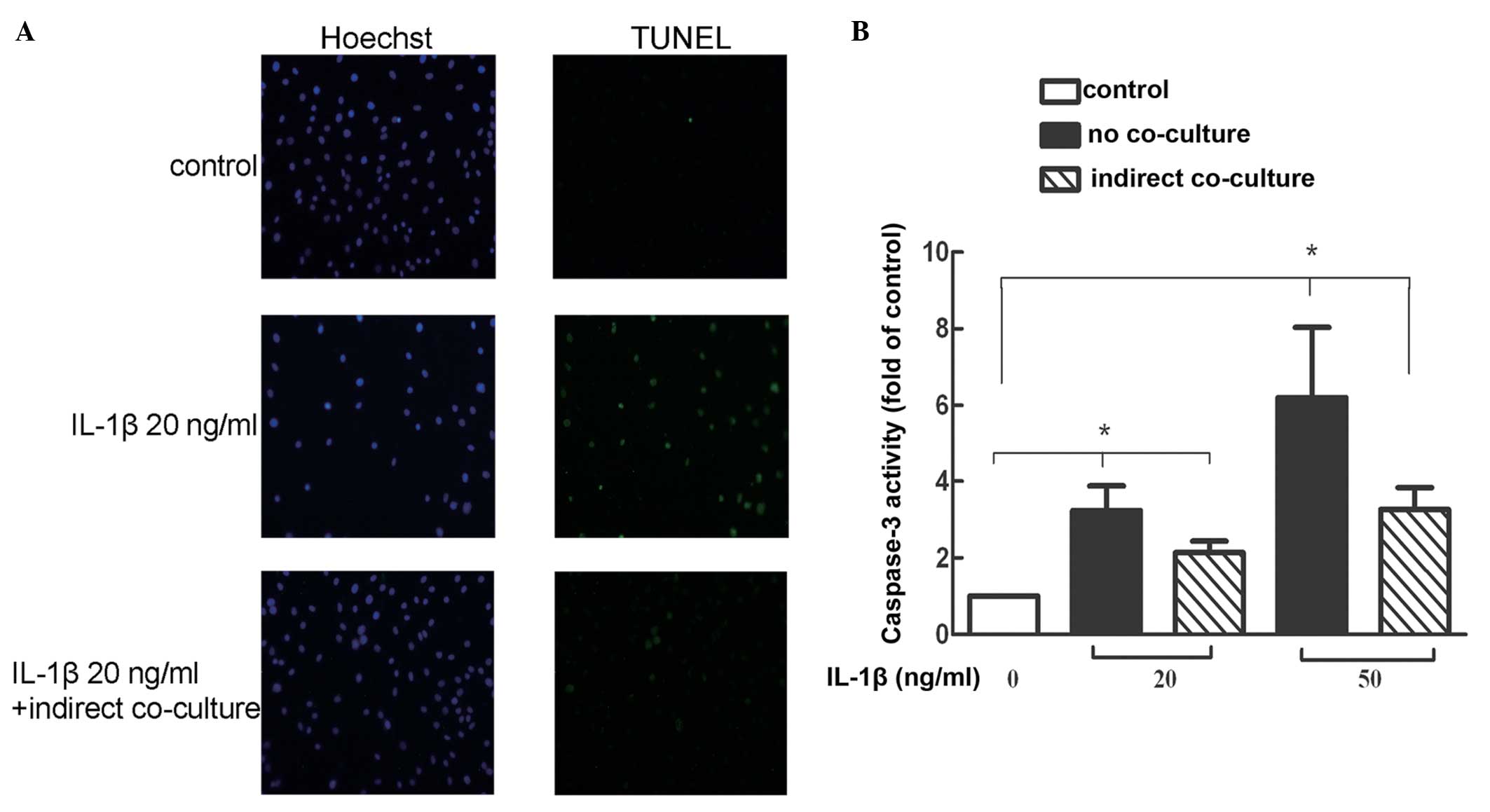
Co-culture of BMSCs with IL-1β-stimulated
NP cells reduces the number of TUNEL-stained cells
TUNEL staining allows for the detection and
quantification of apoptosis at the cellular level. The number of
TUNEL-positive cells increased with inflammatory factors (20
ng/ml), whereas the number of TUNEL-positive cells was reduced in
the co-culture group (Fig.
3A).
Co-culture of BMSCs with IL-1β-stimulated
NP cells reduces the activity of caspase-3
The results of the caspase-3 activity assay
demonstrated that compared with the normal group, the caspase-3
activities in the 20 and 50 ng/ml inflammatory factor treatment
groups were significantly increased. In each indirect co-culture
group, the caspase-3 activity was marginally lower compared with
the no co-culture group (Fig.
3B).
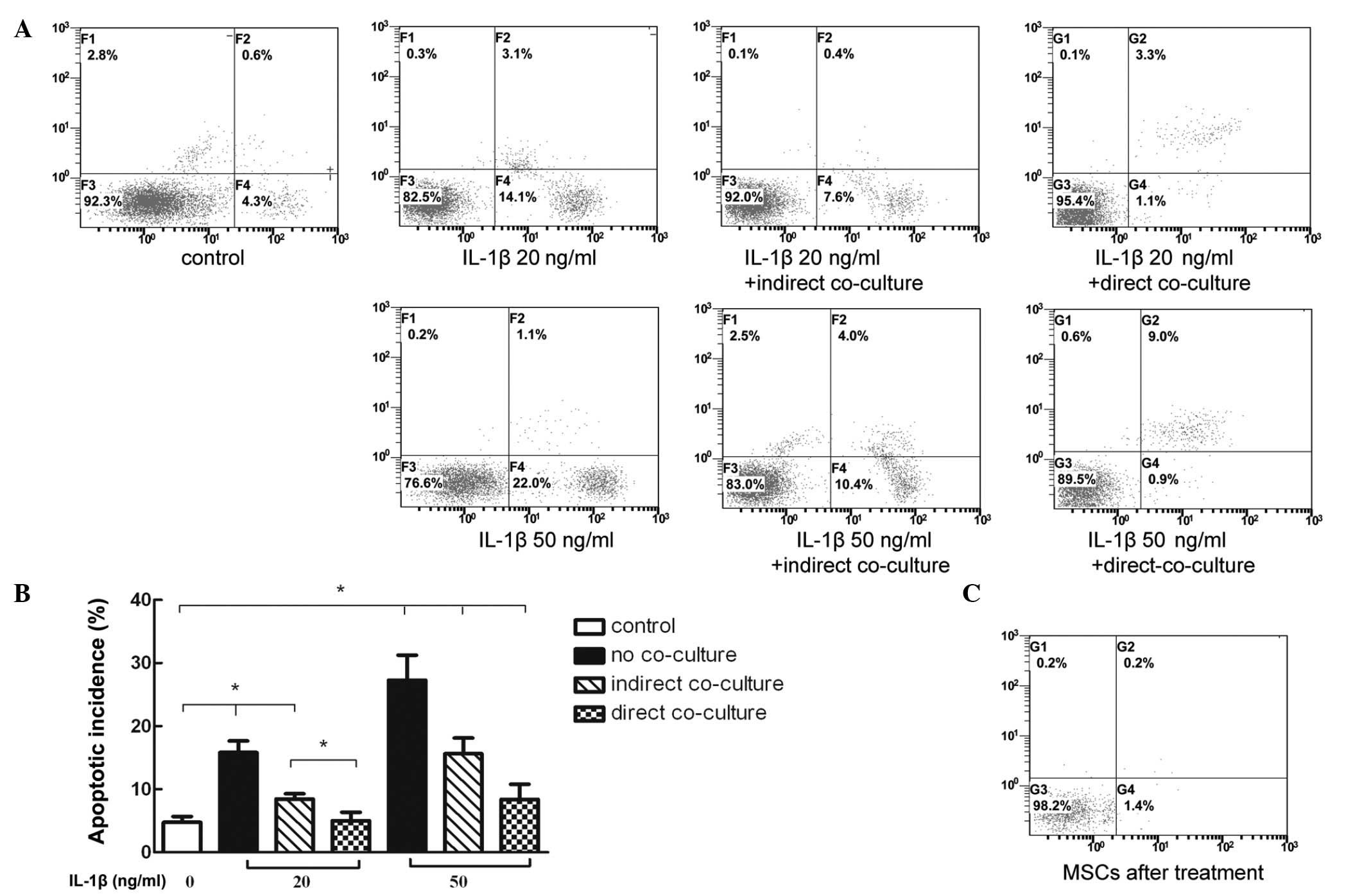
Co-culture of BMSCs with IL-1β-stimulated
NP cells reduces the apoptotic incidence
Flow cytometric analysis of annexin V-FITC PE/PI
staining demonstrated that the level of apoptosis was significantly
increased in the NP cells stimulated with inflammatory factors. In
addition, the apoptotic rate increased in line with the increase in
inflammatory factor concentration. These results indicated that
co-culturing NP cells with BMSCs significantly reduced the
apoptotic rate of different levels of IL-1β. This suggested that
direct co-culture may have improved the anti-apoptotic effects
compared with the indirect co-culture (Fig. 4A and B). Notably, the apoptotic
rate of the added BMSCs in the direct co-culture group was markedly
low (Fig. 4C).
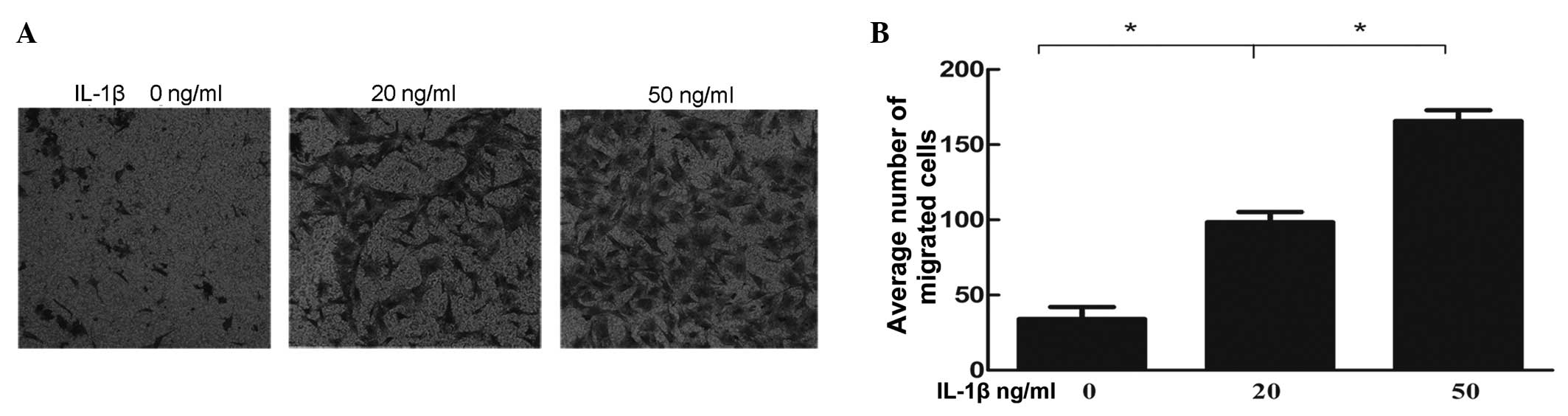
IL-1β stimulation of NP cells increases
the migratory ability of the BMSCs
The number of cells, which migrated through the
pores were counted under 10 random high power fields for each group
(Fig. 5A), which were then
compared (Fig. 5B). Amongst all
the groups, the differences between the negative control and IL-1β
pre-induced groups were statistically significant. Cell damage
resulting from IL-1β enhanced the migratory ability of BMSCs in a
dose-dependent manner.
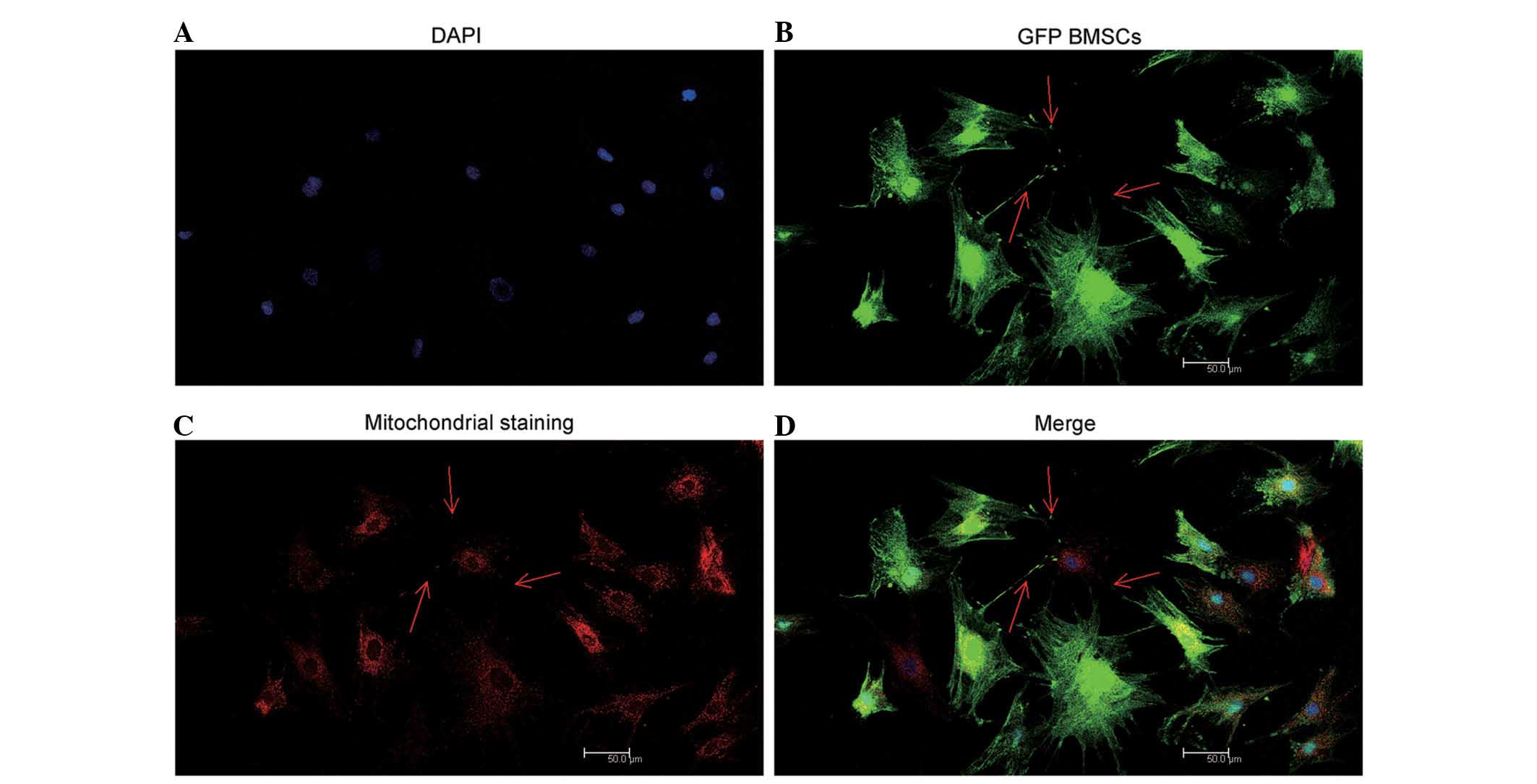
Mitochondrial transfer occurs between
direct co-cultured NP cells and BMSCs
The arrows in Fig.
6 indicate that TnT-like structures were observed between BMSCs
and NP cells by confocal microscopy. Mitochondria were clearly
observed in the red fluorescence channel, transferring from
relabeled GFP BMSCs to stimulated NP cells via TnTs (Fig. 6).
Discussion
In the present study, a novel model of BMSC
intervention was established in IVDD and the anti-apoptotic ability
of BMSCs in stimulated NP cells was observed to act via a paracrine
mechanism. In addition, the migration driven by IL-1β stimulation
and directed mitochondrial transfer was observed.
IL-1β has been used to induce inflammatory responses
in NP cells (6,9,24).
Following stimulation with IL-1β for 24 h, the majority of
degeneration indexes were increased and apoptosis was induced. To
simulate in vivo MSC-mediated damage repair processes
following inflammatory stimulation, Transwell chambers were used to
physically separate the two cell types. The use of a Transwell
chamber with a 0.4 µm pore size ensured that only secreted
factors were easily passed through the pores, while preventing
BMSCs from migrating through the pores via amoeboid-like formations
(20). Previous in vivo
studies have reported that these intercellular interactions involve
the indirect effects of cytokines, in addition to the influence of
cell migration and direct cell to cell contacts (25,26).
Through a series of experiments, the present study successfully
simulated and confirmed the directional migration of BMSCs toward
the inflammatory factor-stimulated cells. However, the model for
BMSC migration failed to completely mimic MSC action in vivo
and was only suitable to separately investigate the effects of
BMSCs on damaged cells.
In addition to paracrine effects and migration,
direct cell to cell communication must be addressed. The
observation of TnTs between MSCs and other cell types has been
reported by numerous previous studies (27,28).
MSCs are capable of transferring mitochondria to cells with
severely compromised mitochondrial function via TnTs (29). In the present study, only GFP BMSCs
were pre-labeled with MitoTracker® Red following 24 h
direct co-culture, however, the pre-simulated NP cells were labeled
red. Due to the fact that mitochondrial transfer by TnTs was
commonly observed in the present study between GFP BMSCs and NP
cells, which had suffered cellular damage (identified by DAPI), it
was suggested that migration of BMSCs may be directed and BMSCs may
transfer mitochondria into cells with severe damage. Unfortunately,
quantifying this is challenging and further investigation is
required.
The co-culture model established in the present
study demonstrated that BMSCs exert beneficial anti-apoptotic
effects on inflammatory factor-stimulated NP cells via paracrine
mechanisms. The anti-inflammatory and anti-apoptotic capacities of
MSCs, in addition to their mechanisms of action, have been reported
in other fields. MSCs co-cultured in inflammatory medium have been
reported to inhibit the activation of nuclear factor κ-B and
thereby control a series of inflammatory reactions (30). It has been reported that MSCs
secrete factors, including transforming growth factor-β and
prostaglandin E2, which can inhibit the activation of lymphocytes,
thereby also contributing to the suppression of inflammation
(31–33). The paracrine-mediated
anti-apoptotic effects are likely to be based on the activity of
growth factors produced by MSCs, which are able to prevent
oxidative stress by increasing the activity of antioxidants and
normalizing mitochondrial function (34). Previous studies have demonstrated
with in vivo and in vitro models that protein kinase
B (Akt) was significantly increased in cells treated with MSCs and
MSC culture medium, and that Akt exerts an anti-apoptotic effect
through the phosphorylation of the BLC-associated cell death
promoter protein (12,35,36).
In addition, serum may function as a positive factor, whcih reduces
the overall inflammatory and apoptotic indexes (9). Significant differences were detected
between the co-culture experiments with BMSCs and the monoculture
experiments lacking BMSCs. Through paracrine mechanisms, BMSCs were
suggested to exert an anti-apoptotic effect on the damaged cells,
migrating to the damaged cells and communicating with them via
TnTs. Therefore, in models of IVDD, MSC transplantation has been
reported to exert a significant therapeutic effect (15,17).
In conclusion, BMSCs actively migrate towards
inflammatory NP cells and effectively reduce the apoptotic rate in
NP cells following inflammatory stimulation via paracrine effects
and mitochondrial transfer through direct TnTs connections. The
in vitro model established in the present study simulates,
to a certain extent, these paracrine anti-apoptotic effects, in
addition to the migratory behavior of BMSCs. This model provided a
simple and accurate means to investigate the mechanisms underlying
the potentially beneficial therapeutic effects of BMSCs.
Acknowledgments
The present study was supported by the Shanghai
Science and Technology Committee Project of International
Cooperation (grant no. 13430721000) and the Joint Research Project
on Major Diseases of Shanghai Health System (grant no.
2013ZYJB0502).
References
|
1
|
Waddell G: Low back pain: A twentieth
century health care enigma. Spine (Phila Pa 1976). 21:2820–2825.
1996. View Article : Google Scholar
|
|
2
|
Ariga K, Miyamoto S, Nakase T, Okuda S,
Meng W, Yonenobu K and Yoshikawa H: The relationship between
apoptosis of endplate chondrocytes and aging and degeneration of
the intervertebral disc. Spine (Phila Pa 1976). 26:2414–2420. 2001.
View Article : Google Scholar
|
|
3
|
Lotz JC, Colliou OK, Chin JR, Duncan NA
and Liebenberg E: Compression-induced degeneration of the
intervertebral disc: An in vivo mouse model and finite-element
study. Spine (Phila Pa 1976). 23:2493–2506. 1998. View Article : Google Scholar
|
|
4
|
Yamada K, Sudo H, Iwasaki K, Sasaki N,
Higashi H, Kameda Y, Ito M, Takahata M, Abumi K, Minami A, et al:
Caspase 3 silencing inhibits biomechanical overload-induced
intervertebral disk degeneration. Am J Pathol. 184:753–764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF- κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts S, Caterson B, Menage J, Evans EH,
Jaffray DC and Eisenstein SM: Matrix metalloproteinases and
aggrecanase: Their role in disorders of the human intervertebral
disc. Spine (Phila Pa 1976). 25:3005–3013. 2000. View Article : Google Scholar
|
|
8
|
Zhang CC, Zhou JS, Hu JG, Wang X, Zhou XS,
Sun BA, Shao C and Lin Q: Effects of IGF-1 on IL-1β-induced
apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep.
7:441–444. 2013.
|
|
9
|
Zhao CQ, Liu D, Li H, Jiang LS and Dai LY:
Interleukin-1beta enhances the effect of serum deprivation on rat
annular cell apoptosis. Apoptosis. 12:2155–2161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi H, Suguro T, Okazima Y, Motegi
M, Okada Y and Kakiuchi T: Inflammatory cytokines in the herniated
disc of the lumbar spine. Spine (Phila Pa 1976). 21:218–224. 1996.
View Article : Google Scholar
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morigi M, Rota C, Montemurro T,
Montelatici E, Lo Cicero V, Imberti B, Abbate M, Zoja C, Cassis P,
Longaretti L, et al: Life-sparing effect of human cord
blood-mesenchymal stem cells in experimental acute kidney injury.
Stem Cells. 28:513–522. 2010.PubMed/NCBI
|
|
13
|
Richardson SM, Walker RV, Parker S, Rhodes
NP, Hunt JA, Freemont AJ and Hoyland JA: Intervertebral disc
cell-mediated mesenchymal stem cell differentiation. Stem Cells.
24:707–716. 2006. View Article : Google Scholar
|
|
14
|
Sakai D, Mochida J, Yamamoto Y, Nomura T,
Okuma M, Nishimura K, Nakai T, Ando K and Hotta T: Transplantation
of mesenchymal stem cells embedded in Atelocollagen gel to the
intervertebral disc: A potential therapeutic model for disc
degeneration. Biomaterials. 24:3531–3541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiyama A, Mochida J, Iwashina T, Omi H,
Watanabe T, Serigano K, Tamura F and Sakai D: Transplantation of
mesenchymal stem cells in a canine disc degeneration model. J
Orthop Res. 26:589–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crevensten G, Walsh AJ, Ananthakrishnan D,
Page P, Wahba GM, Lotz JC and Berven S: Intervertebral disc cell
therapy for regeneration: Mesenchymal stem cell implantation in rat
intervertebral discs. Ann Biomed Eng. 32:430–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakai D, Mochida J, Iwashina T, Watanabe
T, Nakai T, Ando K and Hotta T: Differentiation of mesenchymal stem
cells transplanted to a rabbit degenerative disc model: Potential
and limitations for stem cell therapy in disc regeneration. Spine
(Phila Pa 1976). 30:2379–2387. 2005. View Article : Google Scholar
|
|
18
|
Strassburg S, Richardson SM, Freemont AJ
and Hoyland JA: Co-culture induces mesenchymal stem cell
differentiation and modulation of the degenerate human nucleus
pulposus cell phenotype. Regen Med. 5:701–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang SH, Wu CC, Shih TT, Sun YH and Lin
FH: In vitro study on interaction between human nucleus pulposus
cells and mesenchymal stem cells through paracrine stimulation.
Spine (Phila Pa 1976). 33:1951–1957. 2008. View Article : Google Scholar
|
|
21
|
Li X, Zhang Y, Yeung SC, Liang Y, Liang X,
Ding Y, Ip MS, Tse HF, Mak JC and Lian Q: Mitochondrial transfer of
induced pluripotent stem cells-derived mesenchymal stem cells to
airway epithelial cells attenuates cigarette Smoke-induced damage.
Am J Respir Cell Mol Biol. 51:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Risbud MV, Guttapalli A, Stokes DG,
Hawkins D, Danielson KG, Schaer TP, Albert TJ and Shapiro IM:
Nucleus pulposus cells express HIF-1 alpha under normoxic culture
conditions: A metabolic adaptation to the intervertebral disc
microenvironment. J Cell Biochem. 98:152–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karaliotas GI, Mavridis K, Scorilas A and
Babis GC: Quantitative analysis of the mRNA expression levels of
BCL2 and BAX genes in human osteoarthritis and normal articular
cartilage: An investigation into their differential expression. Mol
Med Rep. 12:4514–4521. 2015.PubMed/NCBI
|
|
24
|
Smith LJ, Chiaro JA, Nerurkar NL, Cortes
DH, Horava SD, Hebela NM, Mauck RL, Dodge GR and Elliott DM:
Nucleus pulposus cells synthesize a functional extracellular matrix
and respond to inflammatory cytokine challenge following long-term
agarose culture. Eur Cell Mater. 22:291–301. 2011.PubMed/NCBI
|
|
25
|
Wang Z, Wang Y, Gutkind JS, Wang Z, Wang
F, Lu J, Niu G, Teng G and Chen X: Engineered mesenchymal stem
cells with enhanced tropism and paracrine secretion of cytokines
and growth factors to treat traumatic brain injury. Stem Cells.
32:456–467. 2015. View Article : Google Scholar
|
|
26
|
Kawai T, Katagiri W, Osugi M, Sugimura Y,
Hibi H and Ueda M: Secretomes from bone marrow-derived mesencyhmal
stromal cells enhance periodontal tissue regeneration. Cytotherapy.
17:369–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Figeac F, Lesault PF, Le Coz O, Damy T,
Souktani R, Trébeau C, Schmitt A, Ribot J, Mounier R, Guguin A, et
al: Nanotubular crosstalk with distressed cardiomyocytes stimulates
the paracrine repair function of mesenchymal stem cells. Stem
Cells. 32:216–230. 2014. View Article : Google Scholar
|
|
28
|
Vallabhaneni KC, Haller H and Dumler I:
Vascular smooth muscle cells initiate proliferation of mesenchymal
stem cells by mitochondrial transfer via tunneling nanotubes. Stem
Cells Dev. 21:3104–3113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gerdes HH, Bukoreshtliev NV and Barroso
JF: Tunneling nanotubes: A new route for the exchange of components
between animal cells. FEBS Lett. 581:2194–2201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yagi H, Soto-Gutierrez A, Navarro-Alvarez
N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG,
Parekkadan B and Yarmush Ml: Reactive bone marrow stromal cells
attenuate systemic inflammation via sTNFR1. Mol Ther. 18:1857–1864.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar
|
|
32
|
Groh ME, Maitra B, Szekely E and Koç ON:
Human mesenchymal stem cells require monocyte-mediated activation
to suppress alloreactive T cells. Exp Hematol. 33:928–934. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulivi V, Tasso R, Cancedda R and Descalzi
F: Mesenchymal stem cell paracrine activity is modulated by
platelet lysate: Induction of an inflammatory response and
secretion of factors maintaining macrophages in a proinflammatory
phenotype. Stem Cells Dev. 23:1858–1869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García-Fernández M, Delgado G, Puche JE,
González-Barón S and Castilla Cortázar I: Low doses of insulin-like
growth factor I improve insulin resistance, lipid metabolism and
oxidative damage in aging rats. Endocrinology. 149:2433–2442. 2008.
View Article : Google Scholar
|
|
35
|
Eliopoulos N, Zhao J, Bouchentouf M,
Forner K, Birman E, Yuan S, Boivin MN and Martineau D: Human
marrow-derived mesenchymal stromal cells decrease cisplatin
renotoxicity in vitro and in vivo and enhance survival of mice
post-intraperitoneal injection. Am J Physiol Renal Physiol.
299:F1288–F1298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuwana H, Terada Y, Kobayashi T, Okado T,
Penninger JM, Irie-Sasaki J, Sasaki T and Sasaki S: The
phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular
injury in cisplatin nephrotoxicity. Kidney Int. 73:430–445. 2008.
View Article : Google Scholar
|