Introduction
Nasopharyngeal carcinoma (NPC) is a type of
epithelial squamous cell carcinoma, which is highly malignant with
local invasion and early distant metastasis (1,2).
Pathophysiological studies on the development of NPC have shown
that genetic susceptibility, Epstein-Barr virus infection and
environmental factors are the major etiological factors of NPC
(3). However, the exact molecular
mechanisms involved in NPC pathogenesis and progression have
remained to be fully elucidated (4). In spite of the development and
application of intensity-modulated radiotherapy, the treatment
outcome of NPC has remained poor (5). Therefore, it is required to
investigate the underlying molecular mechanisms of the progression
of NPC and to identify novel molecular targets for the development
of novel therapeutic strategies for NPC patients.
MicroRNAs (miRNAs), small 15–22 nucleotide
non-coding RNA molecules, act as critical regulators of gene
expression by binding to the 3′-untranslated region (3′-UTR) of
target genes (6–8). miRNAs have important roles in
cellular processes of development, differentiation, metastasis and
apoptosis (9). Differential
expression of miRNAs has been demonstrated in various human cancer
types (10–17), including NPC (18–22).
miR-205 is a highly evolutionarily conserved miRNA and was shown to
be involved in tumor proliferation, apoptosis, metastasis and
invasion. Of note, the expression levels of miR-205 in breast
cancer, prostate cancer and laryngeal squamous cell carcinoma are
consistently downregulated. However, overexpressed miR-205 promotes
cancer cell proliferation, migration and invasion in various cancer
types (23–25), including bladder cancer,
endometrial carcinoma and hepatocellular carcinoma (26–28).
To date, the function of miR-205 in NPC has not been fully
elucidated.
The present study detected the expression of miR-205
in NPC tissues and explored the roles of miR-205 in three
NPC-derived cell lines by examining its effects on the
proliferation, colony formation, migration, invasion and apoptosis
of these cells. Furthermore, a luciferase reporter assay was
performed in order to identify a potential target of miR-205.
Materials and methods
Patients and tissue samples
All tissue samples were obtained by surgery and were
quickly frozen in liquid nitrogen and stored at -80°C. A total of
20 fresh nasopharyngeal carcinoma specimens from NPC patients with
non-keratinizing squamous cell nasopharyngeal carcinoma and no
previous chemotherapy or radiotherapy prior to the surgery as well
as 20 fresh normal nasopharyngeal tissues from healthy individuals
who underwent nasopharyngeal endoscopy and were diagnosed as having
inflammation were obtained from the Peking University Shenzhen
Hospital (Shenzhen, China). The study was approved by the ethical
committee of Peking University Shenzhen Hospital, and all patients
provided written informed consent. The clinical tumor staging was
based on the 2003 Union for International Cancer Control staging
system (29). The
clinicopathological characteristics of the patients are shown in
Table I.
 | Table IClinical and pathological features of
patients with nasopharyngeal carcinoma (mean age, 44 years; range,
28–61 years). |
Table I
Clinical and pathological features of
patients with nasopharyngeal carcinoma (mean age, 44 years; range,
28–61 years).
| Characteristic | Number of
cases |
|---|
| Total | 20 |
| Gender | |
| Male | 16 |
| Female | 4 |
| Degree of
differentiation | |
|
Differentiated | 6 |
|
Undifferentiated | 14 |
| Primary tumor
stage | |
| T1 | 3 |
| T2 | 10 |
| T3 | 5 |
| T4 | 2 |
| Union for
International Cancer Control clinical stage | |
| I | 0 |
| II | 3 |
| III | 15 |
| IV | 2 |
Cell culture and transfection
The human nasopharyngeal carcinoma-derived cell
lines CNE2, 6-10B and 9-4E as well as the healthy human
nasopharyngeal epithelial cell line NP69 were used in the present
study. The cell lines used were obtained from Conservation Genetics
Chinese Academy of Sciences Kunming Cell Bank (Kunming Institute of
Zoology, Chinese Academy of Sciences, Kunming, Yunnan, China). The
9-4E and NP69 cells were provided by Peking University Shenzhen
Hospital (Shenzhen, China), and CNE2 and 6-10B cells were a
generous gift from Southern Medical University (Guangzhou, China).
These cell lines were cultured in RPMI 1640 (Gibco-BRL, Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen Life Technologies), 1% antibiotics
(100 µ/ml penicillin and 100 mg/ml streptomycin sulfates;
Invitrogen Life Technologies) and 1% glutamate (Invitrogen Life
Technologies) at 37°C in a humidified 5% CO2 atmosphere.
Transfection of miR-205 (5′-UCCUUCAUUCCACCGGAGUCUG-3′) and negative
control (5′-UUCUCCGAACGUGUCACGUTT) (GenePharma, Shanghai, China)
were performed with Lipofectamine 2000 (Invitrogen Life
Technologies), which were mixed in the Opti-MEM® I
Reduced Serum medium (Invitrogen Life Technologies) following the
manufacturer's instructions. Fluorescence microscopy (BX51TF;
Olympus, Tokyo, Japan) was used to verify the transfection
efficiency.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the clinical tissue
samples by using the TRIzol reagent (Invitrogen Life Technologies)
according to the manufacturer's instructions. Reverse transcription
of the extracted RNA was performed using the miScript II RT kit
(Qiagen GmbH, Hilden, Germany) according to the manufacturer's
instructions. PCR reactions were performed by using the miScript
SYBR® green PCR kit (Qiagen GmbH) according to the
manufacturer's instructions using the Lightcycler 480 Real-Time PCR
System (Roche Diagnostics, Basel, Switzerland). The reaction
mixture contained: 2 µl cDNA template, 2µl specific
microRNA primer, 2µl 10X miScript Universal Primer,
10µl 2X QuantiTect SYBR Green PCR Master Mix and RNase-free
water was added to 20 µl in total. The amplification
conditions were as follows: 95°C for 15 mins, followed by 94°C for
15 sec, 55°C for 30 sec and 70°C for 30 sec, for 45 cycles. The
2−ΔΔCT method was used to quantify the relative miRNA
expression (30). U6 was used as
an internal control for the normalization and the forward primer
was 5′-ACGCAAATTCGTGAAGCGTT-3′ (Invitrogen Life Technologies). The
forward primer for miR-205 amplification was
5′-TCCTTCATTCCACCGGAGTCTG-3′ (Invitrogen Life Technologies), and
the reverse primer of U6 and miR-205 was the universal primer
provided in the miScript SYBR® green PCR kit (Qiagen
GmbH).
Cell proliferation assay
CNE2, 6-10B or 9-4E cells were seeded in 96-well
plates (7×103 cells/well). Following transfection for
various time periods (0, 24, 48 and 72 h), 20 µl MTT
solution was added and the mixture was incubated for 4 h at 37°C.
Subsequently 150 µl dimethylsulfoxide (Sigma-Aldrich, St.
Louis, MO, USA) was added to each well followed by thorough mixing
for 10 min. The absorbance was measured using an ELISA microplate
reader (680; Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm
(with 630 nm as the reference wavelength).
Colony formation assay
Following transfection for 24 h, the cells were
trypsinized and cells were seeded into six-well plates
(1,000/well), cultured for 10 days, then fixed with methanol and
stained with 0.1% crystal violet. Images of colonies were captured
and the colonies were counted. All steps were performed in
triplicate.
Analysis of apoptosis
The percentage of apoptotic cells was determined by
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
staining followed by flow cytometric analysis (Gallios Flow
Cytometer and Kaluza for Gallios software; Beckman Coulter, Inc.,
Brea, California, USA). An Annexin V/FITC Detection kit
(Invitrogen, Life Technologies) was used to stain the cells
following the manufacturer's instructions. A total of
2×105 CNE-2 or 6-10B cells were seeded on six-well
plates, followed by transfection and incubation for 48 h. The cells
were then washed twice with ice-cold phosphate-buffered saline,
re-suspended in 1X binding buffer and stained with Annexin V-FITC
(5 µl) and PI (3 µl). The experiments were repeated
at least three times.
Wound-healing assay
For the wound-healing experiments, 6×105
cells were plated on six-well plates, transfected for 24 h and
cultured for 48 h to confluency. A line-shaped wound was generated
with a 200-µl pipette tip, and images were captured at the
0-, 12- and 24-h time-points to measure the wound-healing distance.
A DM4000B microscope (Leica, Wetzlar, Germany) was used. The
migration distance (µm) was assessed using MIAS-2000
software (Sichuan University, Chengdu, China). Each experiment was
performed at least three times.
Cell invasion assay
Matrigel (1:5; 50 µl/well; BD Biosciences,
Franklin Lakes, NJ, USA) was added to Transwell chambers in a
24-well plate. Following 24 h of transfection, a total of
2×104 transfected CNE2, 6-10B or 9-4E cells in 100
µl serum-free RPMI 1640 medium were seeded into the upper
chambers (24-well insert; pore size, 8 µm; Corning-Costar,
Corning, NY, USA), and the lower chambers were filled with 500
µl RPMI 1640 medium containing 10% FBS. After 24 h of
incubation, the non-invaded cells on the upper side of the membrane
were removed and the lower sides of the membranes were fixed with
methanol, stained with 0.1% crystal violet (Sigma-Aldrich) and
washed three times. Finally, the stained cells were visualized
under the DM4000B microscope and manually counted. All experiments
were performed in triplicate.
Luciferase reporter assay
For generating a reporter construct, the miRNA
target sequences were inserted between the XhoI-NotI
restriction sites in the 3′-untranslated region (UTR) of the hRluc
gene of the psiCHECK™-2 luciferase vector (Promega,
Madison, WI, USA). The primer sequences used for the amplification
of the 3′-UTR of TP53INP1 were as follows: Forward, 5′-CGC TCG AGA
TTT GTT GTC TAA TTA GGG GCC AAG-3′ and reverse, 5-AAG GAA AAA AGC
GGC CGC CAA CTT CTG TAG CTT GTG AGG TAC TA -3′ (Invitrogen Life
Technologies). Takara EmeraldAmp PCR Master Mix (Takara Bio, Inc.,
Otsu, Japan) was used for this assay. The reaction mixture
contained: cDNA template (2 µl), forward primer (2
µl), reverse primer (2 µl), Master Mix (25
µl), and RNase-free water (19 µl). The amplification
conditions were as follows: 98°C for 2 mins, followed by 35 cycles
of 98°C for 10 sec, 55°C for 39 sec and 72°C for 1 min, followed by
a final extension at 72°C for 5 min, then the mixture was
maintained at 12°C. The mutant construct of the 3′UTR was generated
in the same way after substituting G with T and A with C to
deactivate the potential binding sites. Co-transfection of the
reporter vectors and miR-205 mimic or negative control were
performed. After 48 h, dual-luciferase activity was measured using
the dual-luciferase assay system (Promega, Madison, WI, USA)
according to the manufacturer's instructions. The experiments were
repeated at least three times.
Bioinformatics analysis
miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
was used to verify the targets of miR-205. In addition to its own
algorithm, miRWalk used eight additional computational algorithms -
miRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/miRDB/), RNA22 (https://cm.jefferson.edu/rna22/), RNAhybrid
(http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html),
PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html),
PICTAR (http://pictar.mdc-berlin.de/),
Diana-microT (http://diana.cslab.ece.ntua.gr/microT/) and TargetScan
(http://www.targetscan.org/) to predict
the targets of the miRNAs.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(International Business Machines, Armonk, NY, USA). All values are
expressed as the mean ± standard deviation of results from three
independent experiments. The differences were assessed using a
two-tailed Student's t-test, while the MTT data were
examined by analysis of variance. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Expression of miR-205 is up-regulated in
NPC tissues and enforced expression of miR-205 promotes NPC-cell
growth and colony formation
As shown in Fig. 1,
the expression of miR-205 was significantly upregulated in NPC
tissues compared with that in the normal nasopharyngeal epithelial
tissues, suggesting that overexpression of miR-205 may be important
in NPC development and metastasis.
The transfection efficiency was >90% when the
cells were transfected with fluorescence-conjugated miRNAs
(Fig. 2).
The MTT assay showed that the cell proliferation was
significantly increased by overexpression of miR-205 in CNE2, 6-10B
and 9-4E cells compared with that of the negative control cells.
(Fig. 3A).
Furthermore, the effect of miR-205 on the colony
formation ability of NPC cells was assessed. Overexpression of
miR-205 significantly increased the colony numbers of CNE2, 6-10B
and 9-4E cells (Fig. 3B). These
results proved that miR-205 may act as an oncogenic miRNA in NPC
and that the upregulation of miR-205 enhanced the proliferation of
NPC cells.
miR-205 decreases apoptosis of NPC cells
and increases NPC-cell migration and invasion
Flow cytometric analysis showed that overexpression
of miR-205 significantly decreased the percentage of apoptotic
cells in CNE2 and 6-10B cells (Fig.
4).
The wound-healing and invasion assays indicated that
overexpression of miR-205 increased the rates of migration and
invasion of CNE2, 6-10B and 9-4E cells compared with those of the
negative control cells (Fig. 5A and
B).
Tumor protein p53-inducible nuclear
protein 1 (TP53INP1) is a direct target of miR-205
Bioinformatics analysis with miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
was used to identify potential targets of miR-205, and from these
results, TP53INP1 was selected as a potential target for further
study. To verify whether miR-205 directly targets TP53INP1,
dual-luciferase reporter assays were performed. The sequences of
the short fragments are shown in Fig.
6A. As shown in Fig. 6B,
co-transfection of CNE2, 6-10B and 9-4E cells with TP53INP1-3′UTR
and miR-205 mimic caused a significant decrease in the luciferase
activity compared with that of the negative control. By contrast,
the activity of the reporter containing the mutated seed sequence
showed no obvious change in fluorescence. This result indicated
that miR-205 exerts an inhibitory effect on TP53INP1 expression via
binding to the 3′-UTR of TP53INP1, and that TP53INP1 is therefore a
direct target of miR-205.
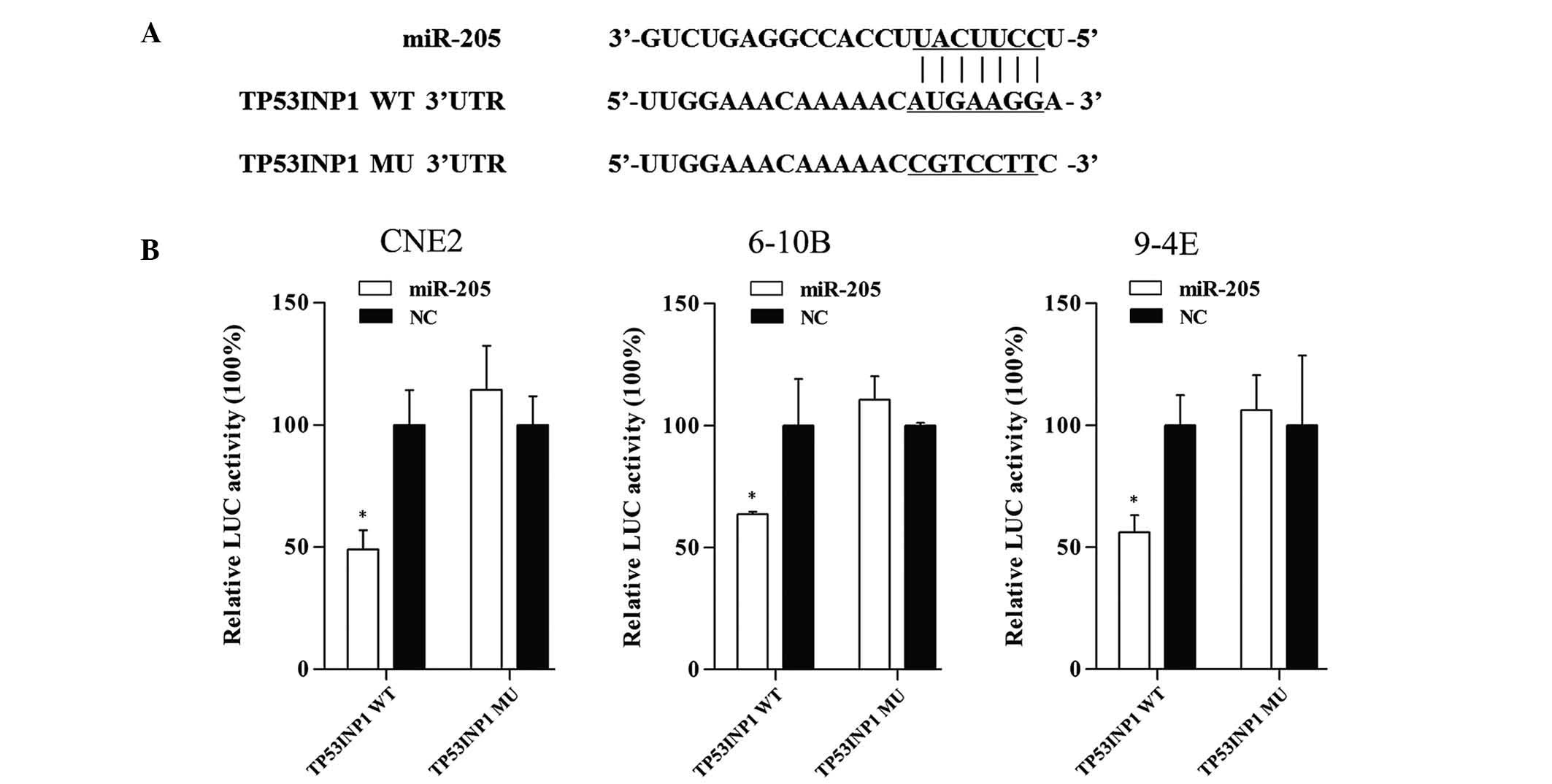 | Figure 6TP53IPN1 is a target gene of miR-205.
(A) Fragments of TP53INP1 3′UTR, which contained the WT potential
binding sites was synthesized and the MU fragments were generated
by replacing G with T and A with C on the putative binding sites.
(B) The target gene was verified by luciferase reporter assay.
Co-transfection with miR-205 and TP53INP1-3′UTR significantly
decreased the luciferase activity by 50.9% (P=0.006), 36.4%
(P=0.03) and 43.9% (P=0.006) in CNE2, 6-10B and 9-4E cells,
respectively. However, the activity of the reporter containing the
mutated seed sequence was not obviously changed. Values are
expressed as the mean ± standard deviation. *P<0.05
vs. NC. LUC, luciferase; WT, wild-type; Mut; mutant; UTR,
untranslated region; miR, microRNA; NC, negative control; TP53INP1,
tumor protein p53-inducible nuclear protein 1. |
Discussion
Tumorigenesis is a complex process, which is
controlled by oncogenes and anti-oncogenes. Increasing evidence has
indicate that microRNAs have significant roles in regulating the
expression of their targets genes, and influence cell biological
behavior, including proliferation, motility and apoptosis (31–33).
MiR-205 has opposite roles in various cancer types by serving as an
oncogene or an anti-oncogene. Luo et al (34) found that miR-205 was upregulated in
NPC biopsy specimens and correlated with the stage of NPC.
Furthermore, Qu et al (35)
demonstrated that overexpression of miR-205 reduced NPC-cell
apoptosis. However, the function of miR-205 in NPC has remained
elusive. The expression of miR-205 in NPC tissues was found to be
upregulated in the present study. Furthermore, the present study
investigated the role of miR-205 in NPC-derived cells and revealed
that overexpression of miR-205 promoted NPC-cell proliferation,
migration and invasion, and suppressed apoptosis, suggesting that
miR-205 may act as an oncogene in NPC.
To shed further light on the tumor-promoting effect
of miR-205 in NPC, the present study identified a target gene of
miR-205, TP53INP1, via a bioinformatics target analysis. TP53INP1
is known to function depending on p53 and to promote apoptosis by
phosphorylating Ser46 of p53 (36,37).
TP53INP1 encodes two protein isoforms which are able to modulate
p53 activity to induce G1-arrest and enhance the p53-mediated
apoptosis by coordinating with homeodo-main-interacting protein
kinase 2, which binds to p53 and induces its phosphorylation in
promyelocytic leukemia protein nuclear bodies (38,39).
In gastric cancer, it has been demonstrated that TP53INP1
accelerated cancer cell apoptosis and was closely associated with
the progression and prognosis of gastric cancer (40). Furthermore, TP53INP1 was confirmed
to be a target of several other miRNAs, which enhance the growth of
several cancer types, including esophageal squamous cell carcinoma
and breast cancer, cervical cancer (41–43).
At the same time, TP53INP1 has been gradually considered to be a
therapeutic target of certain cancer types (44,45).
In the present study, TP53INP1 was found to be a direct target of
miR-205, suggesting that the effect of miR-205 on NPC may be
partially mediated through this protein. However, the explicit
function of TP53INP1 in NPC requires further verification.
In conclusion, the present study provided evidence
that miR-205 has a pivotal role in NPC tumorigenesis, at least in
part via TP53INP1, and may be utilized for the early detection and
molecular-targeted therapy of NPC.
Acknowledgments
The present study was supported by the Science and
Technology Development Fund Project of Shenzhen (grant nos.
JCYJ20120827150357364 and JCYJ20130402114702127) and the Medical
Research Project of the Health and Family Planning Commission of
Shenzhen (grant no. 201302005).
References
|
1
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang GL, Lu Y, Pu XX, He YX, Chen ML, Li
YZ, Tang SY, Che H and He Z: Association study between miR-149 gene
polymorphism and nasopharyngeal carcinoma. Biomed Rep. 1:599–603.
2013.
|
|
3
|
McDermott AL, Dutt SN and Watkinson JC:
The aetiology of nasopharyngeal carcinoma. Clin Otolaryngol Allied
Sci. 26:82–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu N, Jiang N, Guo R, Jiang W, He QM, Xu
YF, Li YQ, Tang LL, Mao YP, Sun Y and Ma J: MiR-451 inhibits cell
growth and invasion by targeting MIF and is associated with
survival in nasopharyngeal carcinoma. Mol Cancer. 12:1232013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu
LZ, Sun Y, Lin AH, Liu MZ and Ma J: How does intensity-modulated
radiotherapy versus conventional two-dimensional radiotherapy
influence the treatment results in nasopharyngeal carcinoma
patients? Int J Radiat Oncol Biol Phys. 80:661–668. 2011.
View Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho HJ, Liu G, Jin SM, Parisiadou L, Xie
C, Yu J, Sun L, Ma B, Ding J, Vancraenenbroeck R, et al:
MicroRNA-205 regulates the expression of Parkinson's
disease-related leucine-rich repeat kinase 2 protein. Hum Mol
Genet. 22:608–620. 2013. View Article : Google Scholar :
|
|
8
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Huang Z and Liu Y: Reduced
miR-125a-5p expression is associated with gastric carcinogenesis
through the targeting of E2F3. Mol Med Rep. 10:2601–2608.
2014.PubMed/NCBI
|
|
11
|
Zhou JJ, Zheng S, Sun LF and Zheng L:
MicroRNA regulation network in colorectal cancer metastasis. World
J Biol Chem. 5:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Li X, Kong X, Luo Q, Zhang J and
Fang L: MiRNA-26b inhibits cellular proliferation by targeting CDK8
in breast cancer. Int J Clin Exp Med. 7:558–565. 2014.PubMed/NCBI
|
|
15
|
Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z,
Zhang S, Nie L and Yu Z: MiR-145 functions as tumor suppressor and
targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J
Cancer Res Clin Oncol. 140:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li J, Tong L, Zhang J, Zhai A, Xu
K, Wei L and Chu M: The prognostic value of miR-21 and miR-155 in
non-small-cell lung cancer: A meta-analysis. Jpn J Clin Oncol.
43:813–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ and
Li GX: MicroRNA-338-3p inhibits colorectal carcinoma cell invasion
and migration by targeting smoothened. Jpn J Clin Oncol. 44:13–21.
2014. View Article : Google Scholar
|
|
18
|
Huang GL, Lu Y, Pu XX, He YX, Chen ML, Li
YZ, Tang SY, Che H and He Z: Association study between miR-149 gene
polymorphism and nasopharyngeal carcinoma. Biomed Rep. 1:599–603.
2013.
|
|
19
|
Huang GL, Chen ML, Li YZ, Lu Y, Pu XX, He
YX, Tang SY, Che H, Zou Y, Ding C and He Z: Association of miR-146a
gene polymorphism with risk of nasopharyngeal carcinoma in the
central-southern Chinese population. J Hum Genet. 59:141–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Wang Y, Sun Y, Zheng J and Zhu D:
MiR-155 up-regulation by LMP1 DNA contributes to increased
nasopharyngeal carcinoma cell proliferation and migration. Eur Arch
Otorhinolaryngol. 271:1939–1945. 2014. View Article : Google Scholar
|
|
21
|
Lyu X, Fang W, Cai L, Zheng H, Ye Y, Zhang
L, Li J, Peng H, Cho WC, Wang E, et al: TGFβR2 is a major target of
miR-93 in nasopharyngeal carcinoma aggressiveness. Mol Cancer.
13:512014. View Article : Google Scholar
|
|
22
|
Liu Y, Li Z, Wu L, Wang Z, Wang X, Yu Y,
Zhao Q and Luo F: MiRNA-125a-5p: A regulator and predictor of
gefitinib's effect on nasopharyngeal carcinoma. Cancer Cell Int.
14:242014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Liao H, Deng Z, Yang P, Du N,
Zhanng Y and Ren H: MiRNA-205 affects infiltration and metastasis
of breast cancer. Biochem Biophys Res Commun. 441:139–143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalogirou C, Spahn M, Krebs M, Joniau S,
Lerut E, Burger M, Scholz CJ, Kneitz S, Riedmiller H and Kneitz B:
MiR-205 is progressively down-regulated in lymph node metastasis
but fails as a prognostic biomarker in high-risk prostate cancer.
Int J Mol Sci. 14:21414–21434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Zhang J, Ge J, Xiao H, Lu J, Fu S,
Liu M and Sun Y: MicroRNA-205 suppresses proliferation and promotes
apoptosis in laryngeal squamous cell carcinoma. Med Oncol.
31:7852014. View Article : Google Scholar
|
|
26
|
Su N, Qiu H, Chen Y, Yang T, Yan Q and Wan
X: MiR-205 promotes tumor proliferation and invasion through
targeting ESRRG in endometrial carcinoma. Oncol Rep. 29:2297–2302.
2013.PubMed/NCBI
|
|
27
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui M, Xiao Z, Sun B, Wang Y, Zheng M, Ye
L and Zhang X: Involvement of cholesterol in hepatitis B virus X
protein-induced abnormal lipid metabolism of hepatoma cells via
up-regulating miR-205-targeted ACSL4. Biochem Biophys Res Commun.
445:651–655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Mao YP, Ma J, Huang Y, Tang LL,
Wang Y, Liu LZ and Lu TX: Influences of magnetic resonance imaging
on the staging system of nasopharyngeal carcinoma. Ai Zheng.
26:158–163. 2007.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Liu Y, Yang K, Sun X, Fang P, Shi H, Xu J,
Xie M and Li M: MiR-138 suppresses airway smooth muscle cell
proliferation through the PI3K/AKT signaling pathway by targeting
PDK1. Exp Lung Res. Jul 7–2015.Epub ahead of print. View Article : Google Scholar
|
|
32
|
Li R, Shi X, Ling F, Wang C, Liu J, Wang W
and Li M: MiR-34a suppresses ovarian cancer proliferation and
motility by targeting AXL. Tumor Biol. Apr 21–2015.Epub ahead of
print.
|
|
33
|
Chen JJ, Liu SX, Chen MZ and Zhao ZY:
Has-miR-125a and 125b are induced by treatment with cisplatin in
nasopharyngeal carcinoma and inhibit apoptosis in a p53-dependent
manner by targeting p53 mRNA. Mol Med Rep. 12:3569–3594.
2015.PubMed/NCBI
|
|
34
|
Luo Z, Zhang L, Li Z, Li X and Li G, Yu H,
Jiang C, Dai Y, Guo X, Xiang J and Li G: An in silico analysis of
dynamic changes in microRNA expression profiles in stepwise
development of nasopharyngeal carcinoma. BMC Med Genomics. 5:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomasini R, Samir AA, Pebusque MJ, Calvo
EL, Totaro S, Dagorn JC, Dusetti NJ and Iovanna JL: P53-dependent
expression of the stress-induced protein (SIP). Eur J Cell Biol.
81:294–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okamura S, Arakawa H, Tanaka T, Nakanishi
H, Ng CC, Taya Y, Monden M and Nakamura Y: p53DINP1, a
p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell.
8:85–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tomasini R, Samir AA, Carrier A, Isnardon
D, Cecchinelli B, Soddu S, Malissen B, Dagorn JC, Iovanna JL and
Dusetti NJ: TP53INP1s and homeodomain-interacting protein kinase-2
(HIPK2) are partners in regulating p53 activity. J Biol Chem.
278:37722–37729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Orazi G, Cecchinelli B, Bruno T, Manni
I, Higashimoto Y, Saito S, Gostissa M, Coen S, Marchetti A, Del Sal
G, et al: Homeodomain-interacting protein kinase-2 phosphorylates
p53 at Ser 46 and mediates apoptosis. Nat Cell Biol. 4:11–19. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang PH, Motoo Y, Garcia S, Iovanna JL,
Pébusque MJ and Sawabu N: Down-expression of tumor protein
p53-induced nuclear protein 1 in human gastric cancer. World J
Gastroenterol. 12:691–696. 2006.PubMed/NCBI
|
|
41
|
Zhang J, Cheng C, Yuan X, He JT, Pan QH
and Sun FY: microRNA-155 acts as an oncogene by targeting the tumor
protein 53-induced nuclear protein 1 in esophageal squamous cell
carcinoma. Int J Clin Exp Pathol. 7:602–610. 2014.PubMed/NCBI
|
|
42
|
Zhang CM, Zhao J and Deng HY: MiR-155
promotes proliferation of human breast cancer MCF-7 cells through
targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci.
20:792013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei Q, Li YX, Liu M, Li X and Tang H:
MiR-17-5p targets TP53INP1 and regulates cell proliferation and
apoptosis of cervical cancer cells. IUBMB Life. 64:697–704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shahbazi J, Lock R and Liu T: Tumor
protein 53-induced nuclear protein 1 enhances p53 function and
represses tumorigenesis. Front Genet. 4:802013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giusiano S, Baylot V, Andrieu C, Fazli L,
Gleave M, Iovanna JL, Taranger-Charpin C, Garcia S and Rocchi P:
TP53INP1 as new therapeutic target in castration-resistant prostate
cancer. Prostate. 72:1286–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|




















