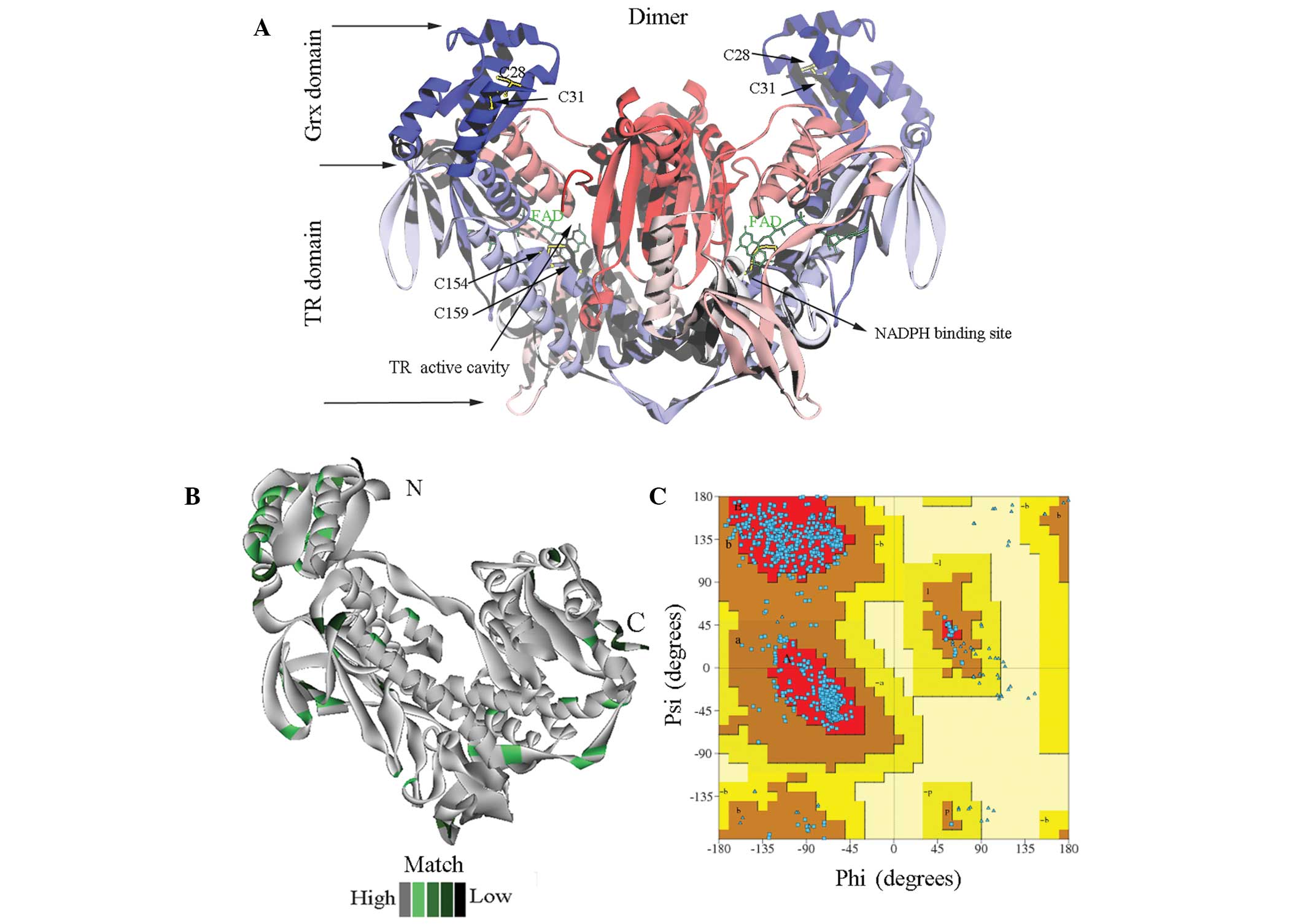
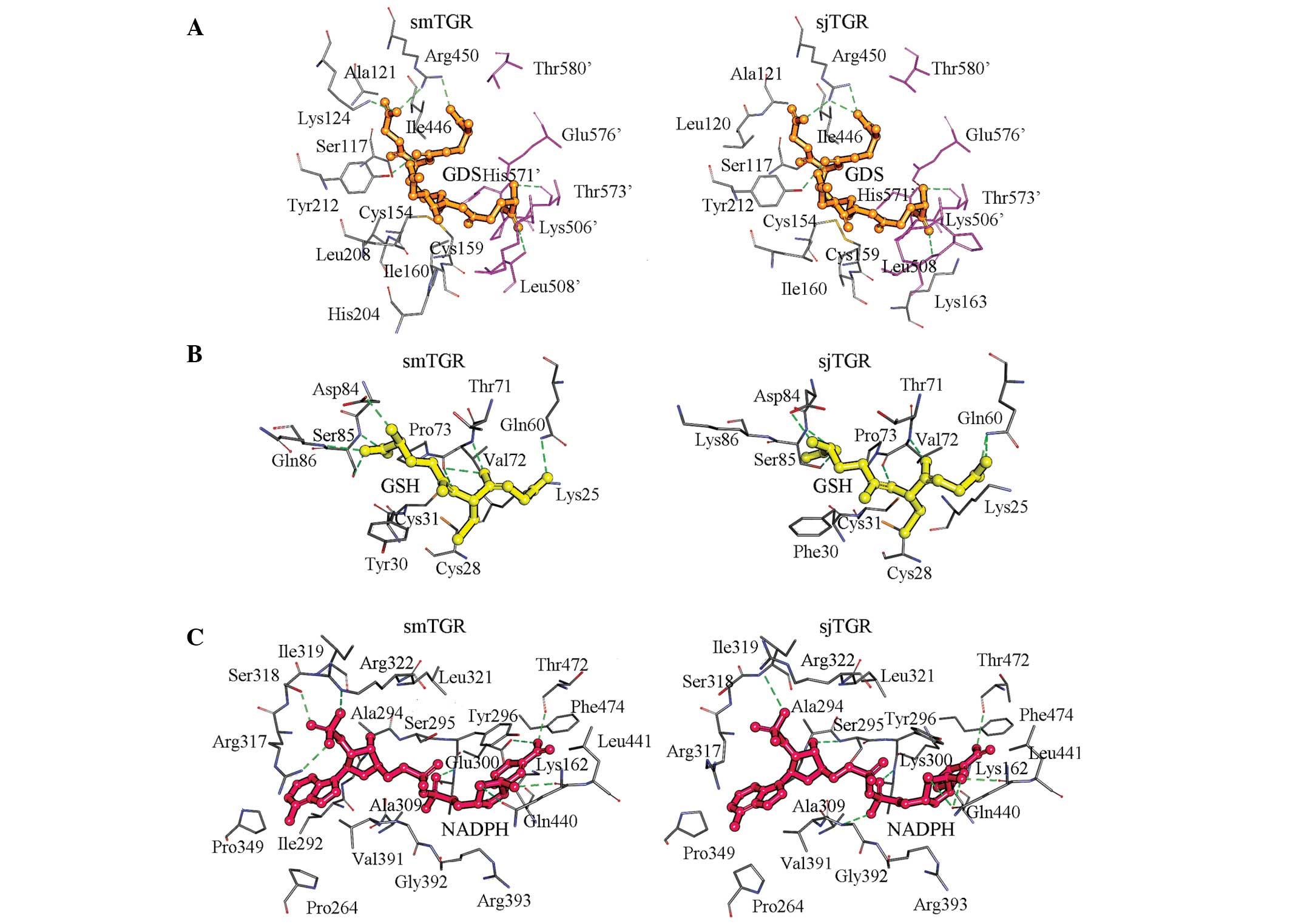
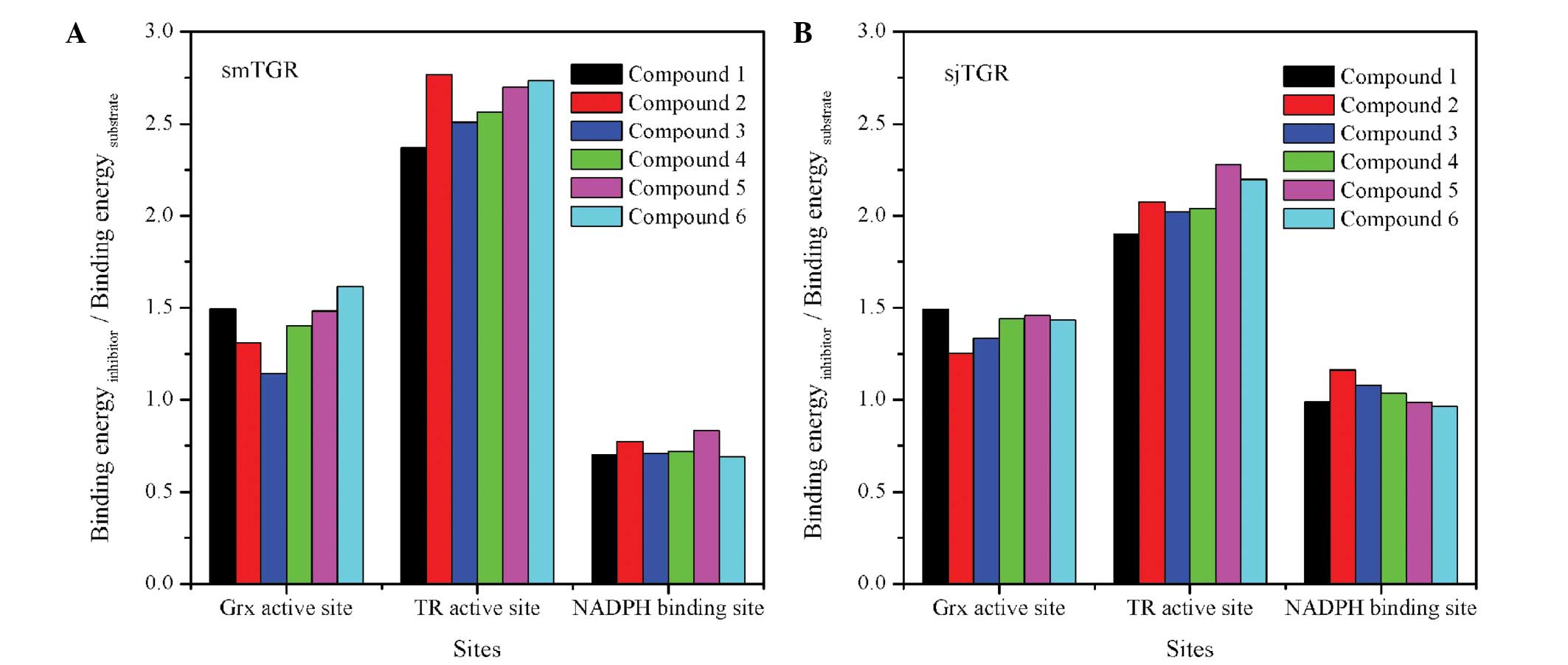
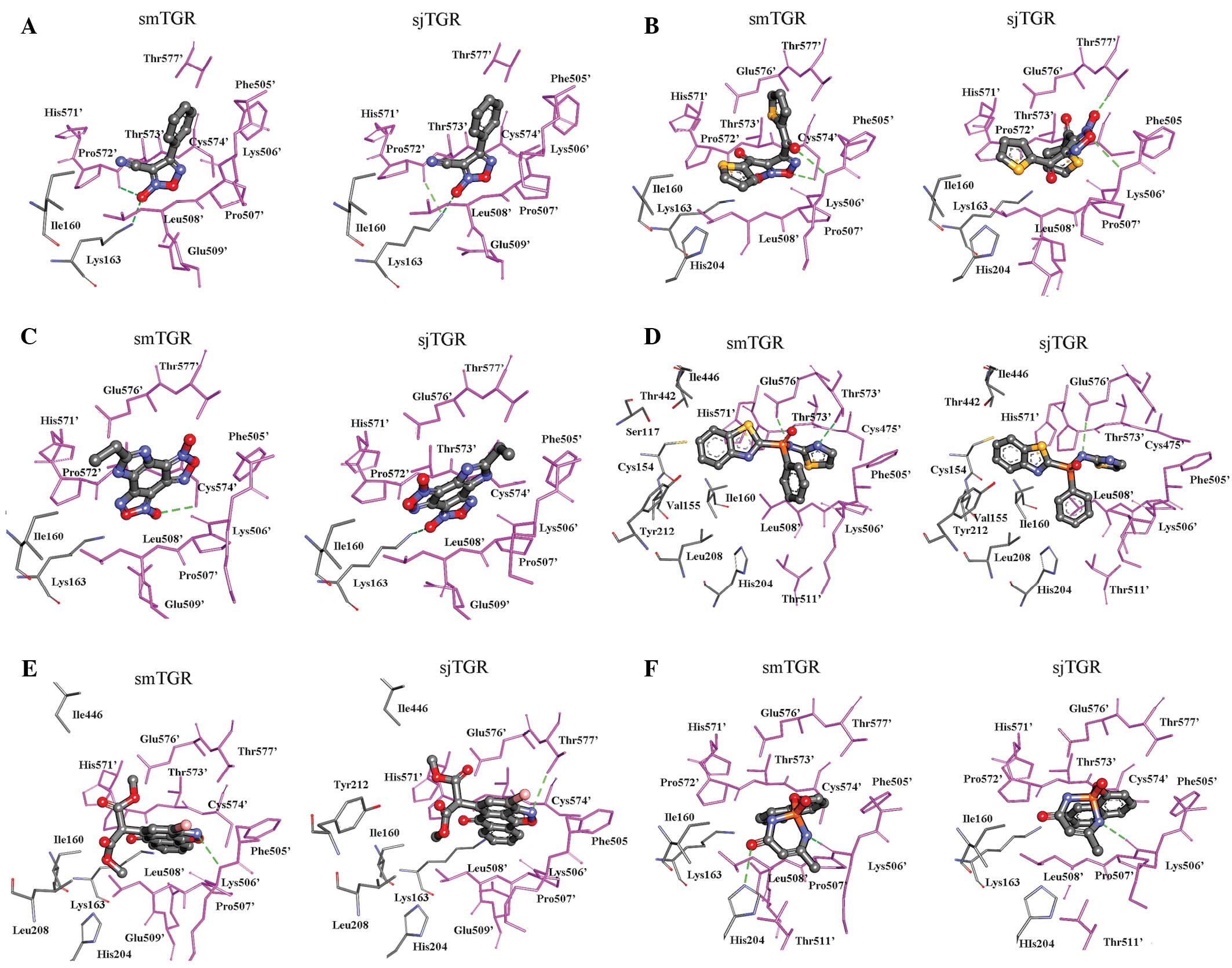
|
1
|
Engels D, Chitsulo L, Montresor A and
Savioli L: The global epidemiological situation of schistosomiasis
and new approaches to control and research. Acta Trop. 82:139–146.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mbanefo EC, Huy NT, Wadagni AA, Eneanya
CI, Nwaorgu O and Hirayama K: Host determinants of reinfection with
schistosomes in humans: A systematic review and meta-analysis. PLoS
Negl Trop Dis. 8:e31642014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung YW and Remais J: Quantitative
detection of Schistosoma japonicum cercariae in water by real-time
PCR. PLoS Negl Trop Dis. 2:e3372008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doenhoff MJ, Cioli D and Utzinger J:
Praziquantel: Mechanisms of action, resistance and new derivatives
for schistosomiasis. Curr Opin Infect Dis. 21:659–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Dai JR, Li HJ, Shen XH and Liang
YS: Is there reduced susceptibility to praziquantel in Schistosoma
japonicum? Evidence from China. Parasitology. 137:1905–1912. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melman SD, Steinauer ML, Cunningham C,
Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DM, Colley DG,
Black CL, et al: Reduced susceptibility to praziquantel among
naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS
Negl Trop Dis. 3:e5042009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alger HM and Williams DL: The disulfide
redox system of Schistosoma mansoni and the importance of a
multifunctional enzyme, thioredoxin glutathione reductase. Mol
Biochem Parasitol. 121:129–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuntz AN, Davioud-Charvet E, Sayed AA,
Califf LL, Dessolin J, Arnér ES and Williams DL: Thioredoxin
glutathione reductase from Schistosoma mansoni: An essential
parasite enzyme and a key drug target. PLoS Med. 4:e2062007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Angelucci F, Dimastrogiovanni D, Boumis G,
Brunori M, Miele AE, Saccoccia F and Bellelli A: Mapping the
catalytic cycle of Schistosoma mansoni thioredoxin glutathione
reductase by X-ray crystallography. J Biol Chem. 285:32557–32567.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams DL, Bonilla M, Gladyshev VN and
Salinas G: Thioredoxin glutathione reductase-dependent redox
networks in platyhelminth parasites. Antioxid Redox Signal.
19:735–745. 2013. View Article : Google Scholar :
|
|
11
|
Prast-Nielsen S, Huang HH and Williams DL:
Thioredoxin glutathione reductase: Its role in redox biology and
potential as a target for drugs against neglected diseases. Biochim
Biophys Acta. 1810:1262–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simeonov A, Jadhav A, Sayed AA, Wang Y,
Nelson ME, Thomas CJ, Inglese J, Williams DL and Austin CP:
Quantitative high-throughput screen identifies inhibitors of the
Schistosoma mansoni redox cascade. PLoS Negl Trop Dis. 2. pp.
e1272008, View Article : Google Scholar
|
|
13
|
Sayed AA, Simeonov A, Thomas CJ, Inglese
J, Austin CP and Williams DL: Identification of oxadiazoles as new
drug leads for the control of schistosomiasis. Nat Med. 14:407–412.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krissinel E and Henrick K: Inference of
macromolecular assemblies from crystalline state. J Mol Biol.
372:774–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Beer TA, Berka K, Thornton JM and
Laskowski RA: PDBsum additions. Nucleic Acids Res. 42:D292–D296.
2014. View Article : Google Scholar :
|
|
17
|
Laskowski RA, Hutchinson EG, Michie AD,
Wallace AC, Jones ML and Thornton JM; PDBsum: A Web-based database
of summaries and analyses of all PDB structures. Trends Biochem
Sci. 22:488–490. 1997. View Article : Google Scholar
|
|
18
|
Stoll VS, Simpson SJ, Krauth-Siegel RL,
Walsh CT and Pai EF: Glutathione reductase turned into
trypanothione reductase: Structural analysis of an engineered
change in substrate specificity. Biochemistry. 36:6437–6447. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Angelucci F, Miele AE, Boumis G,
Dimastrogiovanni D, Brunori M and Bellelli A: Glutathione reductase
and thioredoxin reductase at the crossroad: The structure of
Schistosoma mansoni thioredoxin glutathione reductase. Proteins.
72:936–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma M, Khanna S, Bulusu G and Mitra A:
Comparative modeling of thioredoxin glutathione reductase from
Schistosoma mansoni: A multifunctional target for antischistosomal
therapy. J Mol Graph Model. 27:665–675. 2009. View Article : Google Scholar
|
|
21
|
Song L, Li J, Xie S, Qian C, Wang J, Zhang
W, Yin X, Hua Z and Yu C: Thioredoxin glutathione reductase as a
novel drug target: Evidence from Schistosoma japonicum. PLoS One.
7:e314562012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rai G, Thomas CJ, Leister W and Maloney
DJ: Synthesis of oxadiazole-2-oxide analogues as potential
antischistosomal agents. Tetrahedron Lett. 50:1710–1713. 2009.
View Article : Google Scholar : PubMed/NCBI
|