Introduction
Silicosis, which is among the longest known
occupational diseases, is caused by the inhalation of silica
particles, usually at low levels but over long time periods, which
eventually causes irreversible lung fibrosis. Regardless of its
source, purity and age, crystalline silica induces various
pathological reactions in the lung and causes irreversible fibrosis
and damage (1,2). Fibrocytes are a cell type arising
from monocyte precursors expressing surface markers for leukocytes
and mesenchymal cells, which are capable of differentiating into
myofibroblasts (3–5). They were described in 1994 as a
circulating, bone marrow-derived cells with the ability to adopt a
mesenchymal phenotype (4).
Fibrocytes are initially present in injured organs and have the
inflammatory features of macrophages; they have been shown to
participate in granuloma formation, antigen presentation and
various fibrotic disorders (6).
Fibrocytes represent a minor component of the circulating pool of
leukocytes and express a characteristic pattern of markers,
including collagen I and collagen III (4). α smooth muscle actin (α-SMA)
expressed on the surface of fibrocytes is a surface marker of
myofibroblasts (7,8). A previous study demonstrated that
peripheral blood fibrocytes migrate to skin wound chambers in
humans as well as in mice (9).
Fibrocytes have been identified at sites of active fibrosis and in
fibrotic pathologies, including hypertrophic scars, asthma and
idiopathic pulmonary fibrosis (IPF) (10–12).
Fibrocytes contribute to fibrogenesis by directly producing
collagen, hematopoietic growth factors, inflammatory cytokines and
chemokines (13).
It is well known that fibroblasts are responsible
for repair and remodeling within the lung. Circulating fibrocytes
(cFbs) in the blood are involved in tissue damage repair and were
shown to be able to transform into fibrocytes in vitro
(5). cFbs have been described as a
potential source of increased extracellular matrix and
myofibroblasts, markers of fibrotic pathologies, and to contribute
to the pathogenesis of pulmonary fibrosis (14). Without treatment, continued
fibrosis leads to loss of lung function and ultimately, sufferers
succumb to the disease. cFbs locate to the damaged area of lung
tissue via the circulatory system through the action of chemotactic
factors and differentiate into active fibrocytes prior to repairing
and rebuilding the lung tissue (15,16).
cFbs may function as precursors of myofibroblasts in proliferative
vitreoretinopathy membranes (8).
cFbs have an important role in fibrosis damage, including
pneumoconiosis (17).
The effects of crystalline silica (SiO2)
on cFbs and on the development of silicosis have yet to be
determined. cFbs may be recruited to injured lungs as an integral
component of the pathogenesis of pulmonary fibrosis. The aim of the
present study was to evaluate the effects of free SiO2
on the differentiation of cFbs in vitro, specifically with
regard to the effects of free SiO2 on the expression
levels of collagen I, collagen III and α-SMA. Sprague Dawley (SD)
rat cFbs and alveolar macrophages (AMs) were separated and cultured
in order to establish a primary rat culture model of AMs and cFbs
in vitro. AMs were treated with free SiO2 at 0,
20, 40, 60, 80, 100 and 120 µg/ml, the cell culture
supernatant was collected and then used to stimulate cFbs. cFbs
stimulated by the supernatant were then identified by
immunohistochemical analysis. The mRNA expression levels of
collagen I, collagen III and α-SMA in the cFbs were analyzed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The extracellular protein expression levels of collagen
I, collagen III and α-SMA were detected by ELISA. The results of
the present study further clarified the role of cFbs in
silicosis.
Materials and methods
Animals
Specific pathogen-free male SD rats were purchased
from the animal center of Henan province (Henan, China). The rats
(age, 8–13 weeks; weight, 120–150 g) were housed in polycarbonate
isolator cages with autoclaved bedding, and provided with ad
libitum access to autoclaved reverse-osmosis water and standard
rat food. Animals were maintained in accordance with the guidelines
of the Chinese Association of Laboratory Animal Care, all
experiments and surgical procedures complied with the relevant
provisions of the Experimental Animal Ethics Committee of Zhengzhou
University (Zhengzhou, China).
Reagents
The SiO2 dust (purity, >99%; particle
size, <5 µm) was obtained from The Center for Disease
Control and Prevention (Beijing, China) and sterilized by hot air
sterilization at 190°C for 1.5 h. The stock solutions (100 mg/ml)
of SiO2 were suspended in Dulbecco's modified Eagle's
medium (DMEM; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and diluted to the desired working concentrations
for further experimentation.
Cell isolation and culture
The cFbs were prepared as previously described
(4). Total peripheral blood
lymphocytes were first isolated from 45 ml rat blood by
centrifugation in Ficoll® Paque Plus (GE Healthcare Life
Sciences, Little Chalfont, UK) according to the manufacturer's
instructions. Following overnight culture in six-well plates in
high-glucose DMEM supplemented with 20% heat-inactivated fetal
bovine serum (FBS; Hyclone, Logan, UT, USA), non-adherent cells
were removed by gentle aspiration. The experiments were conducted
at 37°C in a 5% CO2 humidified atmosphere after six
weeks of culture.
Rat AMs were collected from the SD rats by
bronchoalveolar lavage. Briefly, the rats were anesthetized with 2%
pentobarbital (Sigma-Aldrich, Shanghai, China) by intraperitoneal
injection, and following tracheal exposure and cannulation, the
airways of the rats were gently treated with 5 ml cold sterile
D-Hank's solution. This procedure was repeated two additional
times. The bronchoalveolar lavage fluid was collected and
centrifuged at 800 × g, for 5 min at 4°C to form pellets of AMs,
which were re-suspended in DMEM supplemented with 10% FBS. The AMs
were counted using a hemocytometer, and the cells were incubated at
37°C for 2 h in an atmosphere containing 5% CO2. The
medium was then discarded and any non-adherent cells were removed
by washing.
Fluorescence-activated cell sorting
analysis
The cells were harvested after six weeks of culture
with 0.25% trypsin (Beijing Solarbio Science & Technology Co.,
Ltd.) and pelleted by centrifugation for 7 min at 250 × g. The
cells were then re-suspended in 0.5 ml phosphate-buffered saline
(PBS), and phycoerythrin-conjugated anti-collagen I and fluorescein
isothiocyanate-conjugated anti-CD45 antibodies (BD Biosciences,
Franklin Lakes, NJ, USA) were added according to the manufacturer's
instructions. Following incubation in a dark environment for 30 min
at 4°C, cell fluorescence was evaluated by flow cytometry (Accuri
C6; BD Biosciences).
Cytotoxicity assay and cellular
activity
The AMs were seeded into 96-well plates at a density
of 3×105 cells/well (250 µl media per well).
After 24 h of incubation, the media was replaced with fresh media
supplemented with SiO2 at 0, 20, 40, 60, 80, 100 or 120
µg/ml. Each concentration was assayed in six replicates, and
the control wells received no SiO2 (0 µg/ml). An
MTT assay was performed after 24 h of culture. cFbs from six wells
in each group were seeded in 96-well plates at a density of
1.5×104 cells/well (200 µl media per well). A
total of 24 h after seeding, the cells were collected and counted
under a light microscope (Eclipse TS100-F; Nikon, Tokyo, Japan)
with trypan blue exclusion (Sigma-Aldrich). The MTT assay was
performed once daily from the first day of culture, the cells from
six wells of each day were assessed for ten consecutive days, and
the cell numbers were used to produce a growth curve. The optical
density (OD) was measured at 490 nm using a plate microreader
(Tecan Infinite M200; Tecan, Wetzlar, Germany). The proliferation
rate was determined using the following formula: Cell proliferation
(%) = ODexperimental samples/ODcontrol × 100% [n=6, mean ± standard
deviation (SD)]. The cell growth curve was used to determine the
activity levels of the cFbs, and to evaluate the inhibitory effects
of SiO2 on the growth of the macrophages in order to
ascertain the maximum non-lethal concentration of
SiO2.
Cell treatment
A primary culture model of rat AMs and cFbs was
established in vitro. After 24 h of culture, the AMs were
treated with various concentrations of SiO2 (0, 20, 40,
60, 80, 100 and 120 µg/ml). After 24 h of SiO2
treatment, the cell culture supernatant and cells were collected
for the subsequent experiments. After three days of cFbs culture,
the cells were treated with supernatant for 24 h. The supernatant
was collected from the AMs, which were stimulated with
SiO2 at various concentrations (0, 20, 40, 60 and 80
µg/ml), and each concentration was assayed in six
replicates. The cell culture supernatant was collected from AMs and
was then transferred to cFbs each day for 28 days consecutively,
the cFbs were cultured with DMEM supplemented with 10% FBS
(control), 0 µg/ml SiO2-treated AM supernatant (0
µg/ml, 2 ml for 1×105 cells) and 80 µg/ml
SiO2-treated AM supernatant (80 µg/ml, 2 ml for
1×105 cells) respectively. After cultivation for 3, 6,
9, 12, 15 and 18 days for collagen I and Collagen III detection,
and 13, 16, 19, 22, 25 and 28 days for α-SMA detection, the cell
culture supernatant and cells were collected for the subsequent
experiments.
Immunohistochemistry
Immunofluorescence staining was used to determine
the levels of collagen I, collagen III and α-SMA proteins in the
cFbs. The cFbs were fixed in 4% (v/v) paraformaldehyde
(Sigma-Aldrich) for 20 min at 4°C. To suppress endogenous
peroxidase activity, the samples were treated with 3% hydrogen
peroxide solution (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 15 min and rinsed with PBS. To prevent non-specific
immune reactions, the samples were treated with 3% normal horse
serum (Wuhan Boster Biological Technology, Ltd.) for 20 min.
Non-specific staining was prevented by omitting primary antibodies
and rat non-immune serum. The slides were gently agitated and then
incubated with mouse anti-rat α-SMA monoclonal antibody (dilution
1:50; cat. no. BM0002; Wuhan Boster Biological Technology, Ltd.),
mouse anti-rat collagen I polyclonal antibody (dilution, 1:50; cat.
no. BA0325; Wuhan Boster Biological Technology, Ltd.) and mouse
anti-rat collagen III polyclonal antibody (dilution, 1:50; cat. no.
BA0326; Wuhan Boster Biological Technology, Ltd.) at 37°C for 90
min. Following washing with PBS, horseradish peroxidase-conjugated
goat-anti-mouse monoclonal antibody (dilution, 1:200; OriGene
Technologies, Inc., Beijing, China) was added and the solution was
incubated at 37°C for 30 min, followed by rinsing with PBS.
Subsequently, avidin-biotin complex solution was added to the
solution and incubated for 30 min. The slides were rinsed with PBS,
stained with 3–3′-diaminobenzidene (Origene Technologies, Inc.) for
5 min and rinsed with the buffer solution, as previously described
(18,19).
Extraction and RT-qPCR analysis
RNA extraction and RT-qPCR analysis of the mRNA
expression levels of collagen I, collagen III and α-SMA were
performed as previously described (20). The primers were designed and
synthesized by Takara Biotechnology Co., Ltd. (Dalian, China) and
the annealing temperatures used for the PCR are shown in Table I. A PrimerScript RT reagent kit
(Tiangen Biotech Co., Ltd., Beijing, China) was used to
reverse-transcribe the total RNA according to the manufacturer's
instructions. The resulting cDNA was sequentially amplified with 2X
PCR Master Mix (Tiangen Biotech Co., Ltd.) and 10 pmol of each
primer in a Biometra TPersonal Thermocycler (Biometra GmbH,
Goettingen, Germany). The thermocycling conditions were as follows:
Initial denaturation at 94°C for 3 min, and three-step PCR for 30
cycles (denaturation at 94°C for 30 sec, annealing for 30 sec at
the appropriate annealing temperature depending on the primer (51°C
for collagen I, 52°C for collagen III, 51°C for α-SMA and 54°C for
β-actin), and extension at 72°C for 60 sec), with a final extension
at 72°C for 5 min. The PCR products were combined and then
separated by 1.0% agarose gels containing ethidium bromide
(Sigma-Aldrich). Autoradiographic films of the RT-qPCR assays were
subjected to densitometric analyses using a Kodak Gel Logic 100
(Kodak, Rochester, NY, USA). The PCR bands were analyzed using
Quantity One 4.3.0 software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The amount of gene-specific PCR products was expressed as
the ratio of the intensity of each band to that of the
corresponding β-actin internal reference.
 | Table IPrimers for β-actin, collagen I,
collagen III and α-SMA mRNA and their annealing temperatures. |
Table I
Primers for β-actin, collagen I,
collagen III and α-SMA mRNA and their annealing temperatures.
| Gene | Primers | Predicted size
(bp) | Annealing
temperature (°C) |
|---|
| β-actin | F,
5′-CCCATCTATGAGGGTTACGCT-3′ | 519 | 54 |
| R,
5′-TTTAATGTCACGCACGATTTC-3′ | | |
| Collagen I | F,
5′-CCCACCCCAGCCGCAAAGAT-3′ | 352 | 51 |
| R,
5′-TTGGGTCCCTCGACTCCTACA-3′ | | |
| Collagen III | F,
5′-TGCCCACAGCCTTCTACACCT-3′ | 240 | 52 |
| R,
5′-CAGCCATTCCTCCCACTCCAG-3′ | | |
| α-SMA | F,
5′-CCGAGATCTCACCGACTACC-3′ | 121 | 51 |
| R,
5′-TCCAGAGCGACATAGCACAG-3′ | | |
Detection of the protein expression
levels of collagen I, collagen III and α-SMA in the cell culture
supernatant by ELISA
After cultivation for 3, 6, 9, 12, 15 and 18 days,
the levels of collagen I and collagen III in the cell culture
supernatant were detected by ELISA, whereas the levels of α-SMA
were detected after culture for 13, 16, 19, 22, 25 and 28 days.
Collagen I, collagen III and α-SMA in the cFbs culture supernatant
were detected using a commercially available ELISA kit (Yixing
Qianchen Bioengineering Company, Shanghai, China) according to the
manufacturer's instructions.
Statistical analysis
All values are expressed as the mean ± SD. SPSS 12.0
(SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Statistical analysis of the differences between treated and
untreated groups was performed by one-way analysis of variance, and
by Dunnett's test for multigroup comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Graphic representation was performed using GraphPad Prism 5.0 for
Windows (GraphPad Software, Inc., La Jolla, CA, USA).
Results
SiO2 inhibits the growth of
rat AMs and cFbs
The primary culture model of rat AMs and cFbs in
vitro is shown in Fig. 1A.
Following treatment of macrophages with various concentrations of
SiO2 (0, 20, 40, 60, 80, 100 and 120 µg/ml) for
24 h, the cell viability was detected using an MTT assay. As shown
in Fig. 1B, compared with the
control group (0 µg/ml SiO2), treatment with
SiO2 at concentrations of 100 and 120 µg/ml
significantly inhibited the growth of AMs, with a significant
decrease in cellular activity (P<0.05). To further confirm the
effects of SiO2 on the proliferative rate of cFbs, the
cFbs were cultured with AM supernatant, treated with 80
µg/ml SiO2 and subjected to an MTT assay at 1 day
intervals. The cFbs were seeded and cultured for 1–10 days, and
harvested and counted each day. As shown in the growth curve
(Fig. 1C), the doubling time of
the cells was 96 h, and the proliferative activity of the cFbs
reached a peak between the fourth and eighth day.
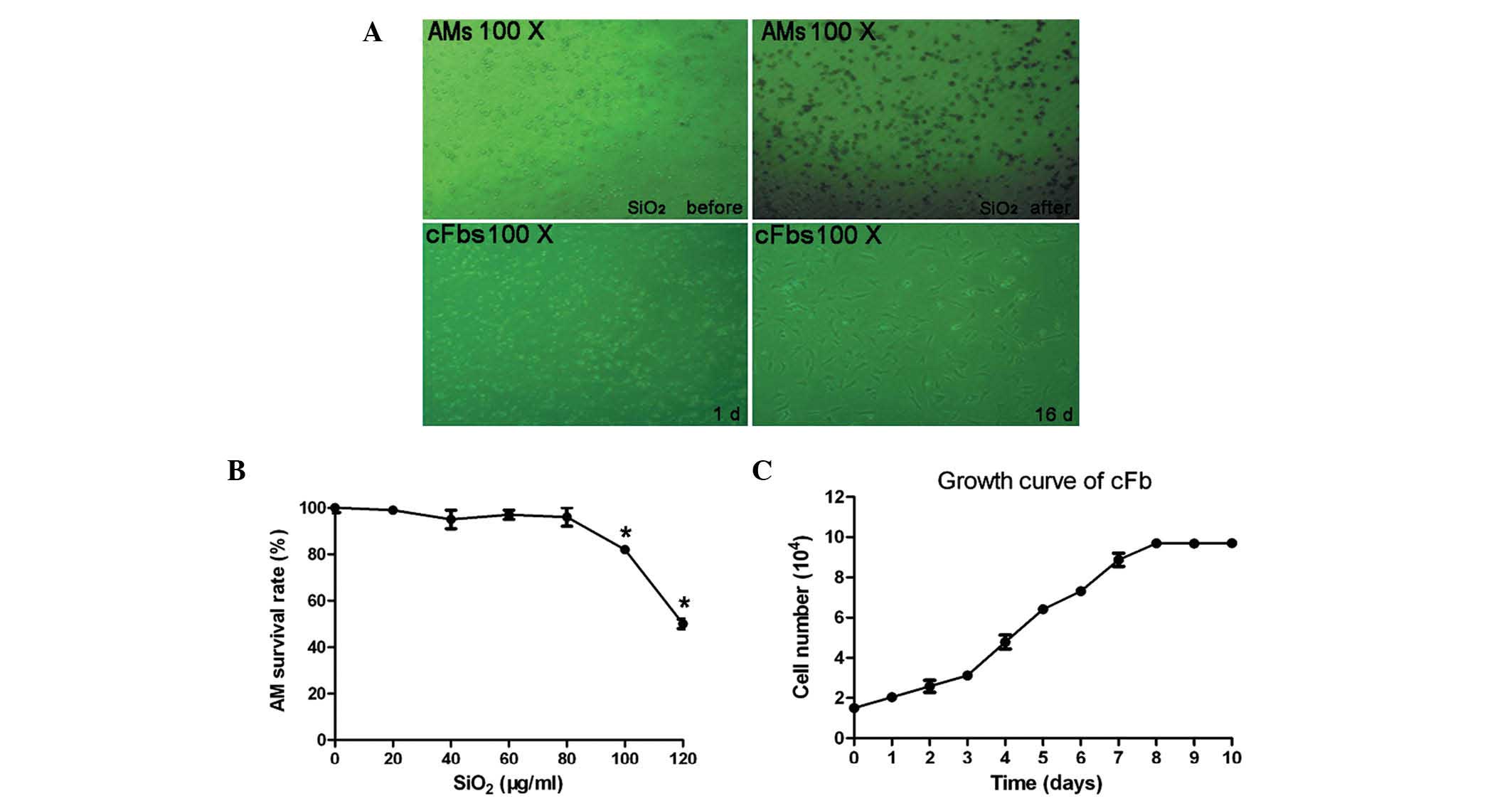 | Figure 1(A) Microscopic images of macrophages
treated with SiO2 for 24 h and of cFbs treated with
macrophage supernatant. (B) Growth curve of cFbs. The survival rate
of macrophages stimulated with SiO2 at 0, 20, 40, 60,
80, 100 or 120 µg/ml (n=6 per treatment group) as determined
by MTT assay. (C) Assessment the effects of SiO2 on the
proliferative rate of the cFbs. The cFbs were seeded and cultured
with 80 µg/ml SiO2 treated AM supernatant for 1,
2, 3, 4, 5, 6, 7, 8, 9 or 10 days. Viable cells were detected by
MTT assay and the cells were counted. Values are expressed as the
mean ± standard deviation. *P<0.05, vs. the control group (0
µg/ml SiO2-treated group). cFbs, circulating
fibrocytes; AMs, alveolar macrophages; SiO2, crystalline
silica. |
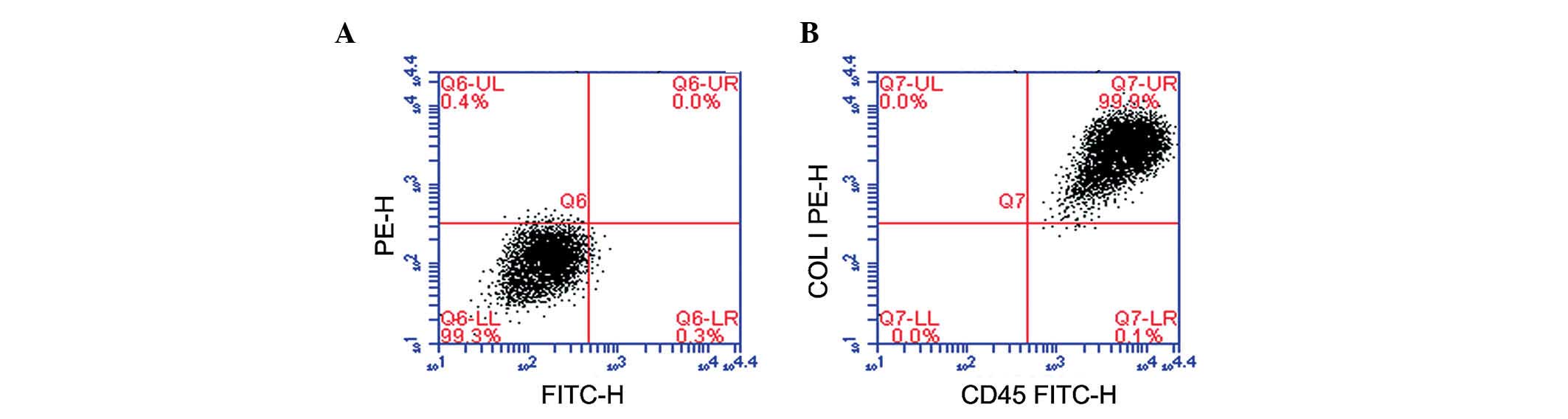
Characterization of cFbs
cFbs were identified by flow cytometry, and the
purity of the cFbs was assayed by staining with collagen I and CD45
antibodies after six weeks of culture. The results demonstrated
that 99% of the cultured cells co-expressed collagen I and CD45
(Fig. 2).
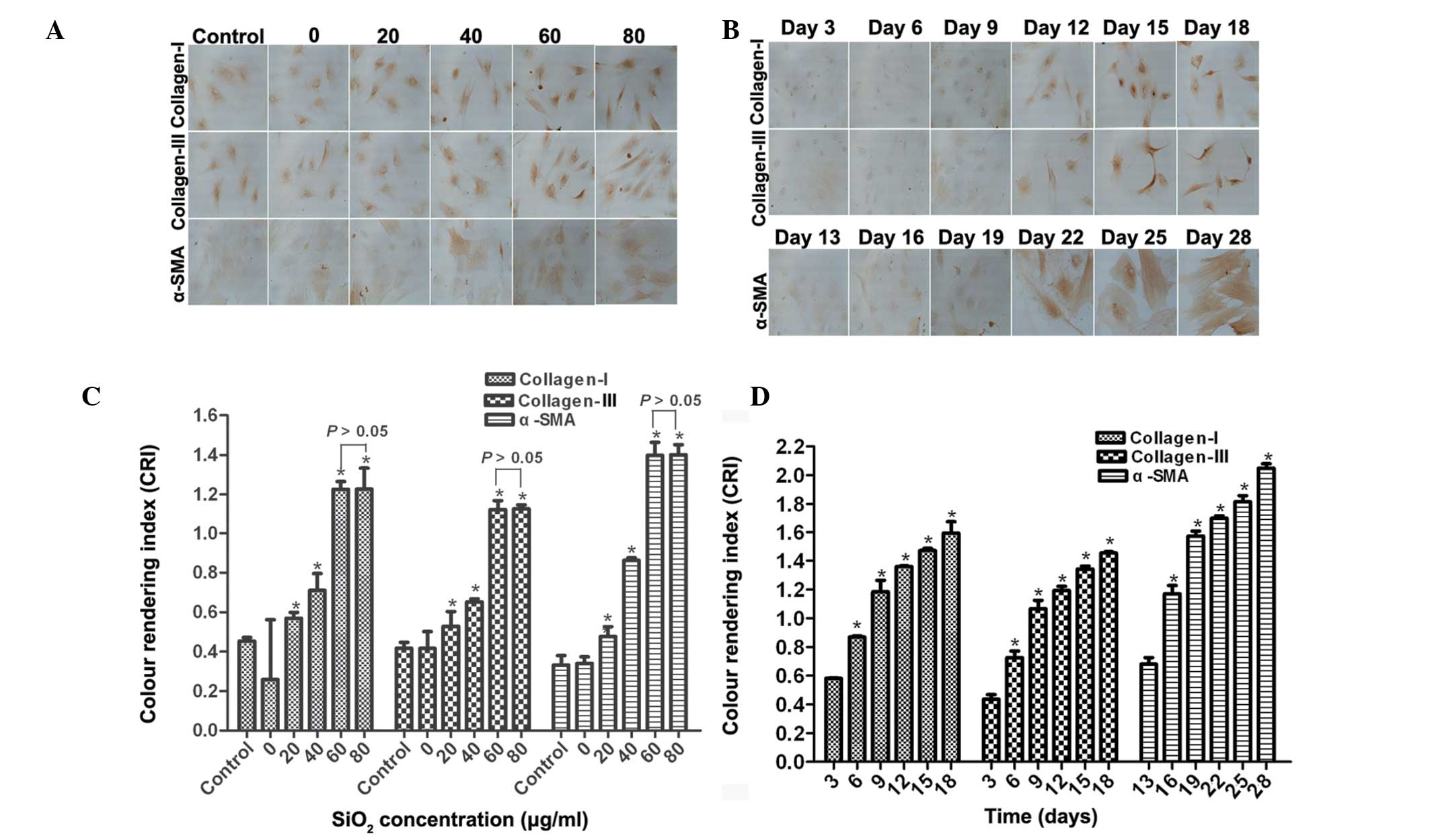
SiO2 stimulates the protein
expression of collagen I, collagen III and α-SMA in cFbs
The present study investigated the effects of
SiO2 on collagen I, collagen III and α-SMA protein
expression at various concentrations and incubation times by
immunohistochemistry. As shown in Fig.
3A, collagen I, collagen III and α-SMA was significantly
increased in the cFbs following treatment with the supernatant of
AM treated with SiO2 (0, 20, 40, 60 and 80 µg/ml)
for 24 h. A dose-dependent increase in the expression levels of
collagen I, collagen III and α-SMA was observed in the cFbs treated
with the supernatant of AM treated with 20, 40 and 60 µg/ml
SiO2, as compared with that in the control group (n=6;
P<0.05), whereas in the 80 µg/ml SiO2 group,
the expression of the respective proteins was similar to that in
the 60 µg/ml SiO2 group (P>0.05; Fig. 3C). Fig. 3B shows representative staining for
collagen I, collagen III and α-SMA in cFbs following treatment for
various durations with the supernatant of AM treated with 80
µg/ml SiO2. The protein expression levels of
collagen I, collagen III began to increase on day 3, and those of
α-SMA on day 13. As compared with the control group, the expression
levels of collagen I and collagen III were significantly
upregulated on days 6, 9, 12, 15 and 18 (P<0.05), whereas the
expression levels of α-SMA were significantly upregulated on days
16, 19, 22, 25 and 28 (P<0.05; Fig.
3D). These results suggested that SiO2 stimulated
the expression levels of collagen I, collagen III and α-SMA in a
dose- and time-dependent manner.
In a further experiment, an ELISA was used to
investigate the effects of SiO2 on the protein
expression levels of collagen I, collagen III and α-SMA in the cFbs
cultured with the supernatant of AM treated with various
concentrations of SiO2 for various durations. The cFbs
were treated with AM supernatant treated with SiO2 at
concentrations of 0, 20, 40, 60 and 80 µg/ml for 24 h, and
untreated cFbs served as a control group. The protein expression
levels of collagen I, collagen III and α-SMA were quantified and
normalized relative to the control group. As shown in Fig. 4A, a marked increase was observed in
collagen I, collagen III and α-SMA expression in the cFbs treated
with SiO2 at concentrations of 20, 40, 60 and 80
µg/ml for 24 h, as compared with that in the control group.
No statistically significant difference was observed between the 60
µg/ml and 80 µg/ml treatment groups (P>0.05; n=6).
Following cFbs treatment with 80 µg/ml SiO2 for
various durations, the protein expression levels of collagen I and
collagen III began to increase on day 3, and those of α-SMA on day
13, whereas the expression levels of collagen I and collagen III in
the 0 µg/ml SiO2 and the control groups began to
increase on day 6, and those of α-SMA on day 19. In the treatment
group, the expression levels of collagen I and collagen III were
significantly upregulated on days 3, 6, 9, 12, 15 and 18, as
compared with those in the control group (P<0.05; Fig. 4B and C), whereas the expression of
α-SMA was significantly upregulated on days 13, 16, 19, 22, 25 and
28 (P<0.05; Fig. 4D). These
results suggested that SiO2 stimulated collagen I,
collagen III and α-SMA expression at the protein level and
accelerated the processes of transdifferentiation of cFbs into
myofibroblasts in a dose-dependent manner.
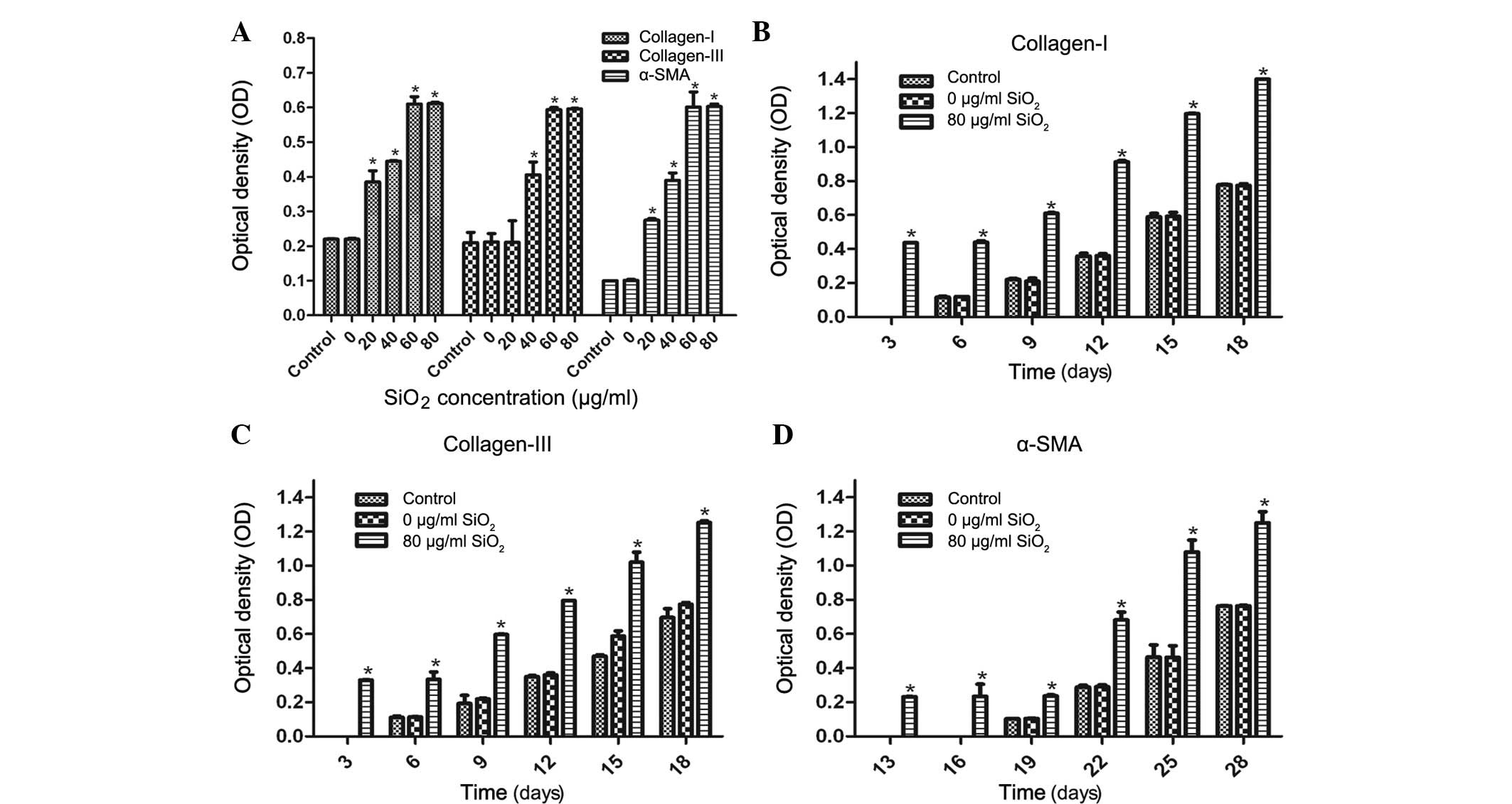 | Figure 4Protein expression levels of collagen
I, collagen III and α-SMA in cFbs, as determined by ELISA. (A) The
cFbs were treated with the supernatant of AM treated with
SiO2 (0, 20, 40, 60 and 80 µg/ml) for 24 h, and
untreated cells served as the control group. The protein expression
levels of collagen I, collagen III and α-SMA were quantified and
normalized relative to the control group. The bands were quantified
by densitometric analysis. (B-D) Expression levels of collagen I,
collagen III and α-SMA following treatment with AM supernatant (0
and 80 µg/ml SiO2) for 3, 6, 9, 12, 15 and 18
days for collagen I and collagen III, and for 13, 16, 19, 22, 25
and 28 days for α-SMA. The cFb supernatant was collected and
protein expression was determined by ELISA. The expression levels
of collagen I and collagen III were quantified and normalized
relative to the control group. The bands were quantified by
densitometric analysis. Values are expressed as the mean ± standard
deviation from three independent experiments (n=6).
*P<0.01, vs. the control. cFbs, circulating
fibrocytes; α-SMA, α smooth muscle actin; SiO2,
crystalline silica; AM, alveolar macrophages. |
SiO2 stimulates the mRNA
expression of collagen I, collagen III and α-SMA in cFbs
To evaluate the effects of SiO2 on
collagen I, collagen III and α-SMA mRNA expression in cFbs, the
cFbs were treated with the supernatant of SiO2-treated
AM (20, 40, 60 and 80 µg/ml for 24 h). Treatment with the
supernatant of AM treated with various concentrations of
SiO2 increased the mRNA expression levels of collagen I,
collagen III and α-SMA in the cFbs. This increase in expression
levels became apparent at 20 µg/ml SiO2 and
markedly apparent at 80 µg/ml SiO2. The
expression of collagen I, collagen III and α-SMA was significantly
and dose-dependently upregulated following treatment with 20, 40
and 60 µg/ml SiO2, as compared with that in the
control group (P<0.05), while no statistically significant
difference was observed between the 60 µg/ml and 80
µg/ml groups (P>0.05; Fig.
5A and B). Following treatment of the cFbs with the supernatant
of AM treated with 80 µg/ml SiO2 for 28 days, the
mRNA expression levels of collagen I, collagen III and α-SMA were
determined by RT-qPCR. As shown in Fig. 6, increases in collagen I and
collagen III expression were detected on day three, and increases
in α-SMA expression was observed on day 13 of incubation, whereas
in the control and 0 µg/ml groups, elevated collagen I and
collagen III expression was detected on day 6, and elevated α-SMA
expression on day 19. A significant upregulation of the expression
levels of collagen I and collagen III in the treated groups was
observed on days 3, 6, 9, 12, 15 and 18 (P<0.05). The expression
levels of α-SMA were significantly upregulated on days 13, 16, 19,
22, 25, 28, as compared with those in the control group
(P<0.05). These results suggested that SiO2 was able
to stimulate collagen I, collagen III and α-SMA activity at the
transcriptional level, and increase the mRNA synthesis of collagen
I, collagen III and α-SMA.
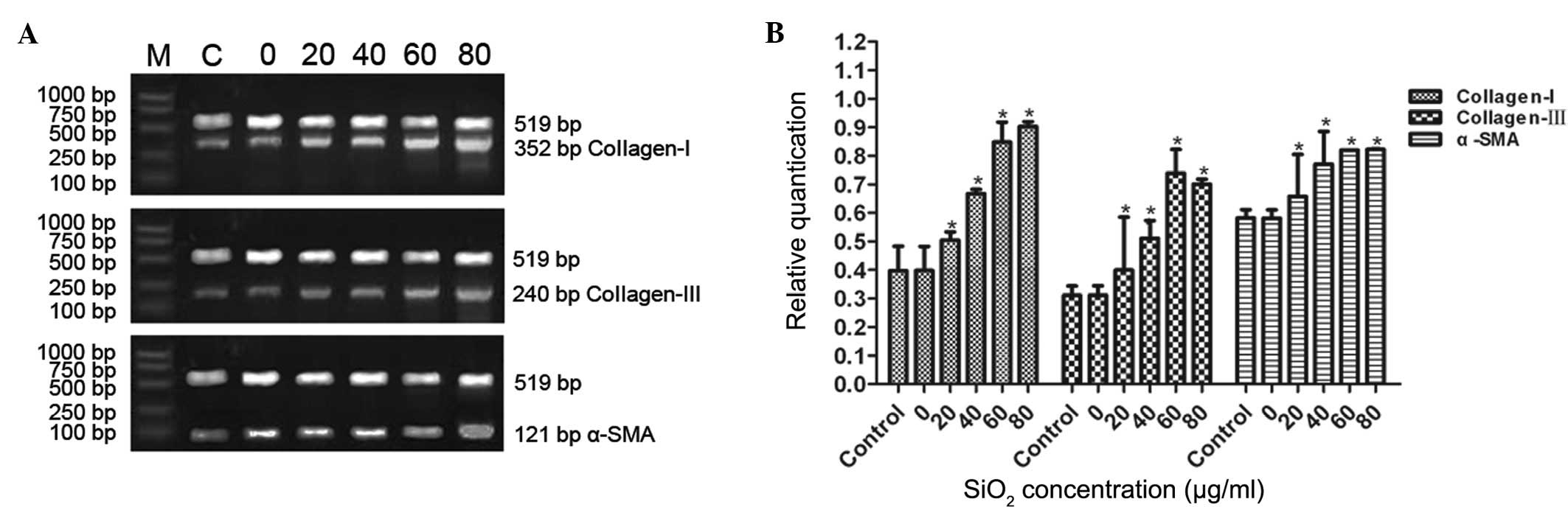 | Figure 5mRNA expression levels of collagen I,
collagen III and α-SMA in cFbs treated with the supernatant of AM
treated with various concentrations of SiO2. The cFbs
were treated with the supernatant of AM treated with
SiO2 at concentrations of 0, 20, 40, 60 or 80
µg/ml for 24 h, and the control group was not treated. (A)
The cFbs were harvested, total RNA was extracted and the mRNA
expression levels were determined by reverse
transcription-quantitative polymerase chain reaction. (B) The mRNA
expression levels of collagen I, collagen III and α-SMA were
quantified and normalized relative to internal β-actin mRNA. The
bands were quantified by densitometric analysis. Values are
expressed as the mean ± standard deviation from three independent
experiments (n=6). *P<0.01, vs. the control group.
cFbs, circulating fibrocytes; α-SMA, α smooth muscle actin;
SiO2, crystalline silica; AM, alveolar macrophages. |
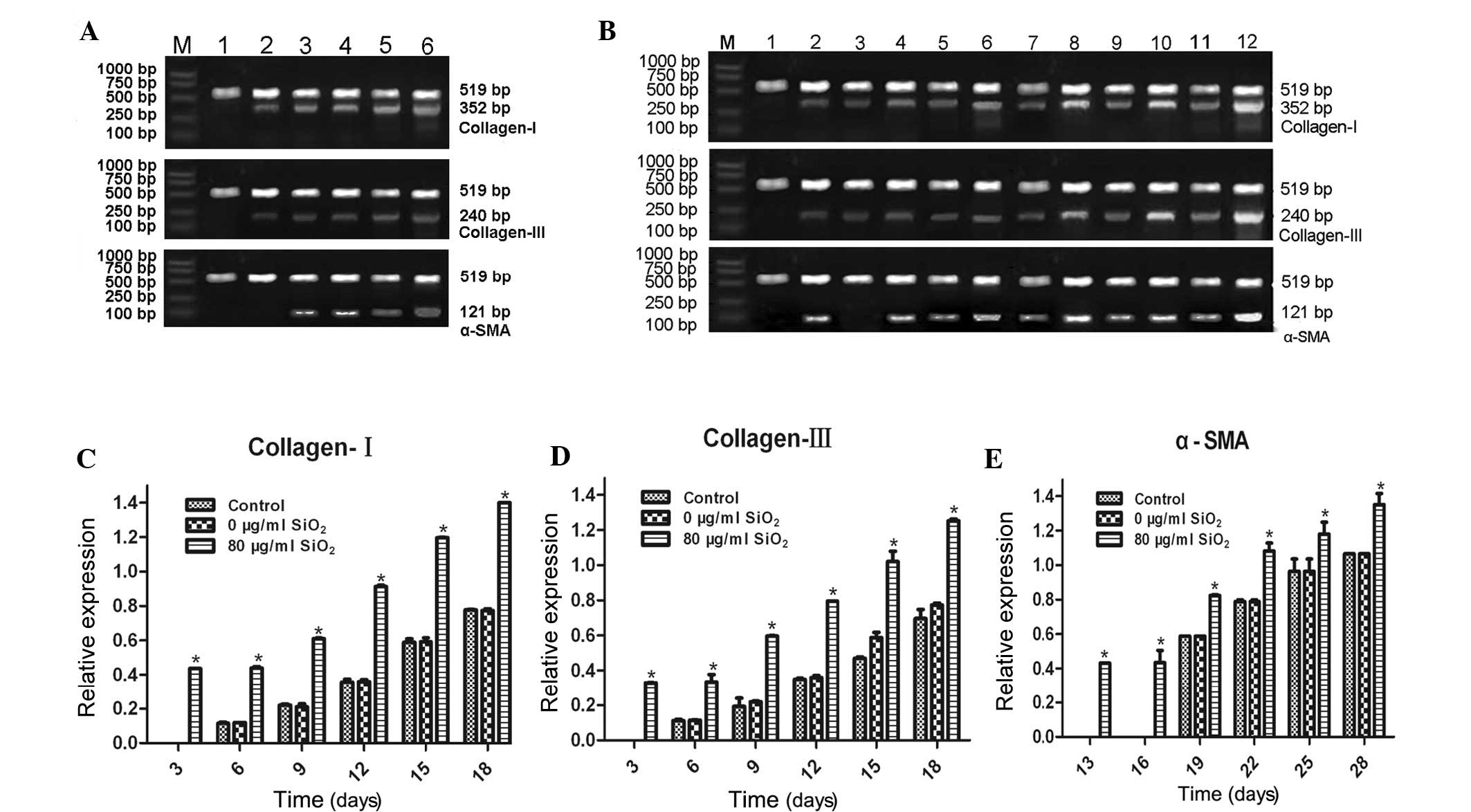 | Figure 6mRNA expression levels of collagen I,
collagen III and α-SMA in cFbs. The cFbs were treated with alveolar
macrophage supernatant treated with SiO2 at
concentrations of 0 and 80 µg/ml for 24 h, and untreated
cells served as the control group. (A) Collagen I and collagen III
detection was performed following cultivation with AM supernatant
(0 µg/ml SiO2) for (1) 3, (2)
6, (3) 9, (4) 12, (5) 15 and (6) 18 days, and α-SMA detection was
performed following cultivation for (1) 13, (2) 16, (3) 19, (4) 22, (5) 25 and (6) 28 days. (B) Collagen I and collagen
III detection was performed following treatment with AM supernatant
(80 µg/ml SiO2) for (1) 3, (2)
6, (3) 9, (4) 12, (5) 15 and (6) 18 days, and α-SMA detection was
performed following cultivation for (1) 13, (2) 16, (3) 19, (4) 22, (5) 25 and (6) 28 days. Total RNA was extracted and
quantified by reverse transcription-quantitative polymerase chain
reaction. The mRNA expression levels of collagen I, collagen III
and α-SMA were quantified and normalized relative to the mRNA
expression levels of β-actin. (C-E) The bands were quantified by
densitometric analysis and are shown in histograms. Values are
expressed as the mean ± standard deviation from three independent
experiments (n=6). *P<0.01, vs. the control group.
cFbs, circulating fibrocytes; α-SMA, α smooth muscle actin;
SiO2, crystalline silica. |
Discussion
The present study demonstrated that SiO2
treatment accelerated cFb differentiation in a concentration- and
time-dependent manner. In patients with ongoing lung fibrosis,
blood monocytes may penetrate into the alveolar space, where they
differentiate into fibrocytes (21). cFbs have previously been reported
to be increased in patients with idiopathic pulmonary fibrosis
(7). Silicosis is characterized by
persistent inflammation, leading to fibroblast formation and
excessive collagen deposition, which causes interstitial fibrosis
and silicotic nodule formation (22). The presence of cFbs may serve as a
prognostic biomarker for silicosis.
In healthy individuals, fibrocytes comprise 0.1–1.0%
of the peripheral blood nucleated cells, and are present in a
variety of healthy as well as diseased tissue types (5). Fibrocytes from human peripheral blood
are circulating progenitor cells with the ability to differentiate
into osteoblasts and chondrocyte-like cells (23). These cells remain active during
tissue remodeling through the elaboration of extracellular matrix
(ECM) proteins (collagen I and collagen III) and secrete various
inflammatory mediators (cytokines, chemokines and growth factors)
(5). Of note, fibrocytes deposit
ECM components during various fibroproliferative disorders of the
lung (14). The present study
aimed to investigate differentiated fibrocytic cell lines
expressing the myofibroblast marker α-SMA. The present study
observed changes in collagen I, collagen III and α-SMA expression
associated with the development of cFbs treated with
SiO2. The proliferative activity levels of AMs treated
with SiO2 were assessed using an MTT assay and the
effects of their supernatants on cFbs were investigated by
observing the mRNA and protein expression levels of collagen I,
collagen III and α-SMA over the course of 28 days. A previous study
reported that there are three stages in the process of cFbs
cultivation in vitro: An early developmental stage over the
course of the first three days, a differentiation stage from the
fourth to the sixth day, and a ripening stage from the seventh day
to the 18th day (17). The results
of the present study demonstrated that the expression levels of
collagen I, collagen III and α-SMA in cFbs increased at different
time-points: Collagen I and collagen III expression increased on
day 3, while α-SMA expression increased on day 13, and these
expression levels continued to increase on days 3, 5, 7, 9 and 12
for collagen I and collagen III, and on days 13, 15, 17, 19 and 23
for α-SMA.
The mRNA expression levels of collagen I, collagen
III and α-SMA in the experimental groups were significantly higher
as compared with those in the control group, and the expression
levels were increased by SiO2 treatment in a
dose-dependent manner. No statistically significant difference was
observed between the 0 µg/ml SiO2 treatment group
and the control group (P>0.05). Therefore, the mRNA expression
levels of collagen I, collagen III and α-SMA were increased by
SiO2 in a dose-dependent manner. As compared with the
control group, marked increases were observed in the mRNA
expression levels of collagen I, collagen III and α-SMA in the
treated groups. These results indicated that SiO2
promoted the transdifferentiation of cFbs into myofibroblasts, as
deduced from the marked increment of α-SMA expression. Increases in
the mRNA expression levels of collagen I and collagen III were
detected on day 6, those of α-SMA were detected on day 19, and mRNA
levels continued to increase in a time-dependent manner.
The majority of liver injuries feature collagen
accumulation and the destruction of normal liver architecture by
fibrosis, a process that involves activated myofibroblasts that
arose from local fibroblast precursors (24). α-SMA is expressed on the surface of
fibrocytes and is a surface marker of myofibroblasts (7,8). As
compared with the control group, following treatment with AM
supernatant treated with SiO2 at concentrations of 0,
20, 40, 60 and 80 µg/ml for 24 h, a marked increase in the
expression levels of collagen I, collagen III and α-SMA was
observed in the cFbs, as determined by immu-nohistochemistry and
ELISA. No statistically significant difference was observed between
the 60 µg/ml and 80 µg/ml treatment groups. These
results suggested that 80 µg/ml SiO2 may exhibit
toxicity to cFbs, inhibiting the growth of cFbs. As determined by
immunohistochemistry, following cFb treatment with 80 µg/ml
SiO2 for various durations, the expression levels in the
80 µg/ml treatment group were markedly elevated as compared
with those in the control group, and these expression levels
increased in a dose-dependent manner with increasing
SiO2 concentration (P<0.05). In the 0 µg/ml
treatment and the control group, the protein expression levels of
collagen I and collagen III were markedly increased on day 3,
whereas those of α-SMA were markedly increased on day 16, as
determined by ELISA. The protein expression levels of collagen I
and collagen III in the 0 mg/kg treatment and the control groups
markedly increased on day 6, whereas those of α-SMA increased on
day 19. The variation in the results obtained by
immunohistochemical analysis and ELISA indicated that collagen I,
collagen III and α-SMA were expressed earlier in the cells, as
compared with their presence in the culture supernatant. These
results suggested that SiO2 was able to stimulate
translational expression of collagen I, collagen III and α-SMA and
stimulate cFb transformation in a dose-dependent manner and the
protein expressions of α-SMA, collagen I and collagen III in cells
were similar to that of 3 days earlier in the culture supernatant
at the same concentration of SiO2.
The expression levels of collagen I and collagen III
observed in the cFbs treated with 80 µg/ml SiO2
were significantly higher, as compared with those in the 0
µg/ml treatment or control groups. Similar cellular
distribution and appearance was observed in cells expressing α-SMA,
as demonstrated by immunohistochemical analysis of cFbs. These
results indicated upon treatment with the supernatant of AM treated
with SiO2, cFbs produced more collagen I, collagen III
and α-SMA. It has been proposed that fibrocytes may be a
transitional stage between a monocyte and a fibroblast (25). Previous studies have demonstrated
the role of myofibroblasts in pulmonary fibrotic processes
(26), and cFbs are capable of
differentiating into myofibroblasts in vitro and in
vivo (27). Concordant with
these previous observations, in the present study, the presence of
significantly higher levels of α-SMA expression by fibrocytes
treated with the supernatant of AM treated with SiO2, as
compared with those in the control group (untreated), suggested and
important role for α-SMA in the transformation of cFbs. The
observations of the present study suggested that the time required
for leukocytes to differentiate into cFbs was accelerated by
SiO2 treatment in a concentration-dependent manner.
The present study provided numerous important
implications which serve as a basis for future studies. Considering
that the silicosis-induced fibrotic process is similar to that of
other fibrotic pulmonary diseases, the fibroproliferative effects
of SiO2 on cFbs are of paramount importance. In
addition, the effects of cFbs in the development and progression of
silicosis prompt further investigation. Future studies
investigating the mechanisms underlying the role of cFbs in
silicosis in the human body would be of great value.
Despite the significant aforementioned results, the
present study has notable limitations. It remains elusive whether
cFbs differentiate into myofibroblasts during the development and
progression of silicosis in vivo. As the present study only
provided results based on in vitro findings, future studies
are required to further characterize the changes in cFbs that occur
during the development and progression of silicosis. However, the
present study represents an important contribution to scientific
research and indicated that cFbs may represent a novel therapeutic
target in the treatment of silicosis.
In conclusion, the present study provided a novel
therapeutic approach, and determined that the overexpression of
collagen I, collagen III and α-SMA protein as well as mRNA in cFbs
in the peripheral blood may have an important role in the
development of silicosis. In addition, the results of the present
study may provide novel methods of silicosis prevention and
control. The present study investigated the effects of
SiO2 on collagen I, collagen III and α-SMA protein and
mRNA in cFbs in vitro. To the best of our knowledge, the
present study was the first to demonstrate that the
transdifferentiation of cFbs into myofibroblasts was accelerated by
SiO2. cFbs may represent a novel therapeutic target for
developing more effective treatments against silicosis
Acknowledgments
The authors of the present study are grateful to the
staff of the Department of Public Health of the Zhengzhou
University. The present study was supported by a grant from the
National Natural Science Foundation of China (grant. no.
81102109).
References
|
1
|
Athavale A, Iyer A, Sahoo D, Salgia K,
Raut A and Kanodra N: Incidence of silicosis in flourmill workers.
Indian J Occup Environ Med. 15:104–108. 2011. View Article : Google Scholar
|
|
2
|
Sharawy MH, El-Agamy DS, Shalaby AA and
Ammar el-SM: Protective effects of methyl palmitate against
silica-induced pulmonary fibrosis in rats. Int Immunopharmacol.
16:191–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ekert JE, Murray LA, Das AM, Sheng H,
Giles-Komar J and Rycyzyn MA: Chemokine (C-C motif) ligand 2
mediates direct and indirect fibrotic responses in human and murine
cultured fibrocytes. Fibrogenesis Tissue Repair. 4(23)2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bucala R, Spiegel LA, Chesney J, Hogan M
and Cerami A: Circulating fibrocytes define a new leukocyte
subpopulation that mediates tissue repair. Mol Med. 1:71–81.
1994.PubMed/NCBI
|
|
5
|
Quan TE, Cowper S, Wu SP, Bockenstedt LK
and Bucala R: Circulating fibrocytes: Collagen-secreting cells of
the peripheral blood. Int J Biochem Cell Biol. 36:598–606. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia D, Moyana T and Xiang J: Combinational
adenovirus-mediated gene therapy and dendritic cell vaccine in
combating well-established tumors. Cell Res. 16:241–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moeller A, Gilpin SE, Ask K, Cox G, Cook
D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et
al: Circulating fibrocytes are an indicator of poor prognosis in
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
179:588–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu El-Asrar AM, Struyf S, Van Damme J and
Geboes K: Circulating fibrocytes contribute to the myofibroblast
population in proliferative vitreoretinopathy epiretinal membranes.
Br J Ophthalmol. 92:699–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe R, Donnelly SC, Peng T, Bucala R and
Metz CN: Peripheral blood fibrocytes: Differentiation pathway and
migration to wound sites. J Immunol. 166:7556–7562. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore BB, Murray L, Das A, Wilke CA,
Herrygers AB and Toews GB: The role of CCL12 in the recruitment of
fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol.
35:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abe S, Boyer C, Liu X, Wen FQ, Kobayashi
T, Fang Q, Wang X, Hashimoto M, Sharp JG and Rennard SI: Cells
derived from the circulation contribute to the repair of lung
injury. Am J Respir Crit Care Med. 170:1158–1163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore BB, Kolodsick JE, Thannickal VJ,
Cooke K, Moore TA, Hogaboam C, Wilke CA and Toews GB: CCR2-mediated
recruitment of fibrocytes to the alveolar space after fibrotic
injury. Am J Pathol. 166:675–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murray LA, Argentieri RL, Farrell FX,
Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff
HL, et al: Hyper-responsiveness of IPF/UIP fibroblasts: Interplay
between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol.
40:2174–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomer RH: Circulating progenitor cells and
scleroderma. Curr Rheumatol Rep. 10:183–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong KM, Belperio JA, Keane MP, Burdick MD
and Strieter RM: Differentiation of human circulating fibrocytes as
mediated by transforming growth factor-beta and peroxisome
proliferator-activated receptor gamma. J Biol Chem.
282:22910–22920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hrckova G, Velebný S and Solár P: Dynamics
of hepatic stellate cells, collagen types I and III synthesis and
gene expression of selected cytokines during hepatic fibrogenesis
following Mesocestoides vogae (Cestoda) infection in mice. Int J
Parasitol. 40:163–174. 2010. View Article : Google Scholar
|
|
18
|
Joddar B and Ramamurthi A: Fragment size-
and dose-specific effects of hyaluronan on matrix synthesis by
vascular smooth muscle cells. Biomaterials. 27:2994–3004. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Li J, Mao S and Zhu H: Comparison
of immunohistology using pan-CK and EMA in the diagnosis of lymph
node metastasis of gastric cancer, particularly micrometastasis and
isolated tumor cells. Oncol Lett. 5:768–772. 2013.PubMed/NCBI
|
|
20
|
Feldmann G, Fendrich V, McGovern K, Bedja
D, Bisht S, Alvarez H, Koorstra JB, Habbe N, Karikari C, Mullendore
M, et al: An orally bioavailable small-molecule inhibitor of
Hedgehog signaling inhibits tumor initiation and metastasis in
pancreatic cancer. Mol Cancer Ther. 7:2725–2735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reese C, Lee R, Bonner M, Perry B, Heywood
J, Silver RM, Tourkina E, Visconti RP and Hoffman S: Fibrocytes in
the fibrotic lung: Altered phenotype detected by flow cytometry.
Front Pharmacol. 5(141)2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thakur SA, Beamer CA, Migliaccio CT and
Holian A: Critical role of MARCO in crystalline silica-induced
pulmonary inflammation. Toxicol Sci. 108:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi YH, Burdick MD and Strieter RM: Human
circulating fibrocytes have the capacity to differentiate
osteoblasts and chondrocytes. Int J Biochem Cell Biol. 42:662–671.
2010. View Article : Google Scholar :
|
|
24
|
Guyot C, Lepreux S, Combe C, Doudnikoff E,
Bioulac-Sage P, Balabaud C and Desmoulière A: Hepatic fibrosis and
cirrhosis: The (myo)fibroblastic cell subpopulations involved. Int
J Biochem Cell Biol. 38:135–151. 2006.
|
|
25
|
Reilkoff RA, Bucala R and Herzog EL:
Fibrocytes: Emerging effector cells in chronic inflammation. Nat
Rev Immunol. 11:427–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phan SH: The myofibroblast in pulmonary
fibrosis. Chest. 122(Suppl 6): 286S–289S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt M, Sun G, Stacey MA, Mori L and
Mattoli S: Identification of circulating fibrocytes as precursors
of bronchial myofibroblasts in asthma. J Immunol. 171:380–389.
2003. View Article : Google Scholar : PubMed/NCBI
|