Introduction
Myocardial fibrosis refers to excessive accumulation
of collagen fibers in the heart that results from upregulated
collagen fibers or altered components of collagen fibers in
myocardial tissues, which is implicated in numerous physiological
and pathological conditions (1–6).
Although the precise mechanism remains elusive, one hypothesis is
that the imbalance between the synthesis and degradation of
myocardial collagen fibers leads to fibrosis in the heart (5,6).
Numerous studies have shown that cytokines,
including transforming growth factor (TGF), insulin-like growth
factor (IGF) and fibroblast growth factor (FGF) have important
roles in proliferation, extracellular matrix (ECM) deposition and
organ fibrosis by interacting with the corresponding receptors
(7–9). IGF-1 was found to protect against
cardiac fibrosis in vivo, although it may promote fibrosis
in other organs (10, 11).
The mouse model of isoproterenol (ISO)-induced
myocar-dial fibrosis offers a tool for studying the functions of
various genes. ISO is a synthetic non-selective β-adrenoceptor
agonist whose subcutaneous injection induces heart failure and
suppressed cardiac function due to myocardial hypertrophy and
fibrosis (11,12). The amplified infammatory response
after ISO injection is likely to be the cause of myocardial
injury.
Heat shock proteins (HSPs) are protective proteins,
which are present under normal conditions and are upregulated
during stress (heat stress in particular). Being molecular
chaperones, HSPs take part in protein folding, disaggregation,
renaturing and transportation (13–15).
HSPs are a superfamily of proteins, which are regulated by heat
shock factor 1 (HSF1) (16,17).
They are classified into numerous sub-families according to their
molecular weight, including HSP90, −70, −60 and small-molecular
HSPs. HSP47 belongs to the small molecular family. As a molecular
chaperone, HSP47 is involved in processing, folding, aggregation
and secretion processes of collagen proteins in the endoplasmic
reticulum, which has an important role in quality control of mature
collagen, preventing generation and secretion of misfolded
pro-collagen during stress (18).
Thus, HSP47 may have an important role in the process of fibrosis
in various organs. HSF1 is the main regulator of the HSP genes,
suggesting it may also have an important role in the fibrotic
process.
Although numerous studies have focused on the roles
of HSF1 in liver (19–24), kidney or pulmonary fibrosis, there
have been few studies on the role of HSF1 in experimentally induced
myocardial fibrosis. In the present study, it was hypothesized that
HSF1 has a promoting role in experimentally induced cardiac
fibrosis, and the effect of HSF1 on ISO-induced myocardial fibrosis
was investigated via histomorphological observation and
determination of the expression of collagens and HSP47 as well as
the activation of HSF1 itself in mice by using Kunm ing and HSF1 k
nockout mice (HSF1−/− mice).
Materials and methods
Materials
IGF-1 was purchased from PeproTech (Rocky Hill, NJ,
USA). Anti-HSF1 (cat. no. SPA901) and anti-HSP47 (cat. no. SPA470)
antibodies were from Stressgen (Enzo Life Sciences, Farmingdale, N
Y, USA). Anti-phospho HSF1 (ser307) (cat. no. ab47369), collagen
(COL)-I (cat. no. ab6308) and COL-III (cat. no. ab82354) monoclonal
antibodies were purchased from Abcam (Cambridge, UK). β-actin
polyclonal antibody (cat. no. AF4000) was from R&D Systems
(Minneapolis, MN, USA), horseradish-peroxidase-labeled goat
anti-rabbit, rabbit anti-goat immunoglobulin G antibodies and a
diamminobenzidine (DAB) test kit were from Boster Biological
Technology (Wuhan, China). Dulbecco's modifed Eagle's medium (DMEM)
and RPMI 1640 were from Gibco-BRL (Invitrogen Life Technologies,
Carlsbad, CA, USA) and SDS, agarose, Hoechst 33258 and proteinase K
were from Invitrogen Life Technologies. Acrylamide and
N,N'-methylene-bis-acrylamide were from Fluka
(Sigma-Aldrich, St Louis, MO, USA). Dithiothreitol (DTT), ammonium
persulfate, toluidine blue, tetramethylethylenediamine (TEMED) and
an MTT kit were from Promega Corp. (Madison, WI, USA).
Polyvinylidene difluoride (PVDF) membranes were purchased from
Millipore (Billerica, MA, USA).
Adult Kunming mice (age, 7–8 weeks) were provided by
the Animal Center of Central South University (Changsha, China).
HSF1+/− and HSF−/− mice were kindly provided by Professor Ivor
Benjamin (Division of Cardiology, University of Utah, Salt Lake
City, UT, USA). The present study was app roved by the ethics
committee of Centra l South University.
Methods
Experimental groups and preparation of
animal model
The Kunming mice were maintained at 25°C, in an
atmosphere containing ~60% humidity, with free access to food and
water. Kunming mice were randomly divided into three groups
(n=4/group): i) Control: Kunming mice were injected subcutane-ously
with normal saline; ii) ISO group: ISO (Shanghai Harvest
Pharmaceutical Co., Ltd., Shanghai, China) was subcutaneously
injected beside the thoracic vertebrae of the mice at a dose of 40
mg/kg on the first day, 20 mg/kg on the second day, 10 mg/kg on the
third day and 5 mg/kg on the fourth day. The dose (5 mg/kg) was
then kept for 10 more days. iii) ISO + IGF-1 intervention group:
After the mice were injected with ISO, IGF-1 (2 µg/kg) was
immediately injected into the same location.
HSF-1 knockout mice were randomly divided into six
groups: i) HSF−/− control; ii) ISO HSF−/− group; iii) ISO + IGF-1
HSF−/− group: iv) HSF−/+ control; v) ISO HSF−/+ group; and vi) ISO
+ IGF-1 HSF−/+ group. The animal model was prepared as described in
the corresponding groups of Kunming mice.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Cardiac tissue was ground in liquid nitrogen. Total
RNA was extracted with TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer's instructions. 1
µg total RNA was reverse-transcribed by using a reverse
transcription kit (Reverse Transcription system; Promega Corp.).
For quantitative real-time PCR, a total of 100 ng cDNA was used to
assess HSP47 expression, whereas 10 ng cDNA was used for PCR of
GAPDH as a control. The primer sequences were as follows: HSP47,
forward 5′-TCACCACAGGATGGTGGACA-3′ and reverse
5′-TGGTCAGCAGCTTCTCCAAG-3′; GAPDH, forward 5′-ACCA
CAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTG CTGTA-3′ (SYBR
Green I fuorescence dye was used to bind specifically to the minor
groove of double-stranded DNA). The primers were chemically
synthesized by Invitrogen Life Technologies (Shanghai, China). PCR
was conducted using an Applied Biosystems 7500 Real-Time PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA). The
PCR mixture contained: 2 µl cDNA, 0.2 µl forward
primer, 0.2 µl reverse primer, 0.5 µl 20X Quantifast
SYBR® Green PCR kit (Qiagen, Hilden, Germany), 0.4
µl dNTP (2.5 mM), 2.4 µl Mg2+ (25 mM), 2
µl 10X buffer, 0.2 µl enzyme (5 units/µl),
diluted to 20 µl with ddH2O. PCR cycling
conditions were as follows: HSP47, 95°C for 2 min, 95°C for 10 sec,
61°C for 10 sec and 72°C for 45 sec, 40 cycles; GAPDH, 95°C for 2
min, 95°C for 10 sec, 61°C for 10 sec, 72°C for 45 sec, 40 cycles.
All reactions followed the typical sigmoidal reaction profile, and
the cycle threshold was used to measure amplicon abundance.
Protein extraction
Myocardial tissue was washed with Buffer A [20 mM
4-(2-hydroxyethyl)-1-piperazineethanesul-fonic acid-KOH, pH 7.5; 10
mM KCl; 1.5 mM MgCl2; 1.0 mM EDTA-Na; 1.0 mM ethylene
glycol tetraacetic acid-Na; 250 mM sucrose] for 10 min,
subsequently homogenized on ice using a glass homogenizer and
centrifuged at 100,000 × g for 30 min at 4°C. The supernatant was
pooled and protein concentrations were determined by the Bradford
method (25), using Coomassie
Brilliant Blue (Fluka). Protein samples were kept at −80°C for
future use.
Western blot analysis
Western blot analysis was performed as previously
described (26). Briefly, 20 µg
protein was loaded in each lane. After SDS-PAGE, proteins were
electrically transferred to a PVDF membrane. After blocking,
primary antibodies (1:1,000) were added and incubated at room
temperature for 1 h. The primary antibodies used were as follows:
Anti-COL-I, anti-COL-III, anti-phospho-HSF1, anti-HSP7, and
anti-β-actin. After washing, secondary antibodies were added and
incubated for about 45 min. After thorough washing, the membrane
was visualized with a DAB kit according to manufacturer's
instructions. The blots were analyzed using Bandleader 3.0
(Magnitec Ltd., Tel Aviv, Israel).
Histochemistry
For histological analysis, hearts were fixed by
perfusion with 10% formalin. Fixed hearts were embedded in paraffin
and sectioned (4 µm). The myocyte cross-sectional diameter
was measured in the sections stained with hematoxylin and eosin,
and suitable cross-sections were defined as having nearly circular
capillary profiles and nuclei (n=100 in each group).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Multiple group comparison was performed by one-way
analysis of variance (ANOVA), followed by the Bonferroni procedure
for comparison of means. Comparison between two groups were
analyzed by the tow-tailed Student's t-test or two-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
HSF1 is required for the establishment of
an ISO-induced mouse model of myocardial fibrosis
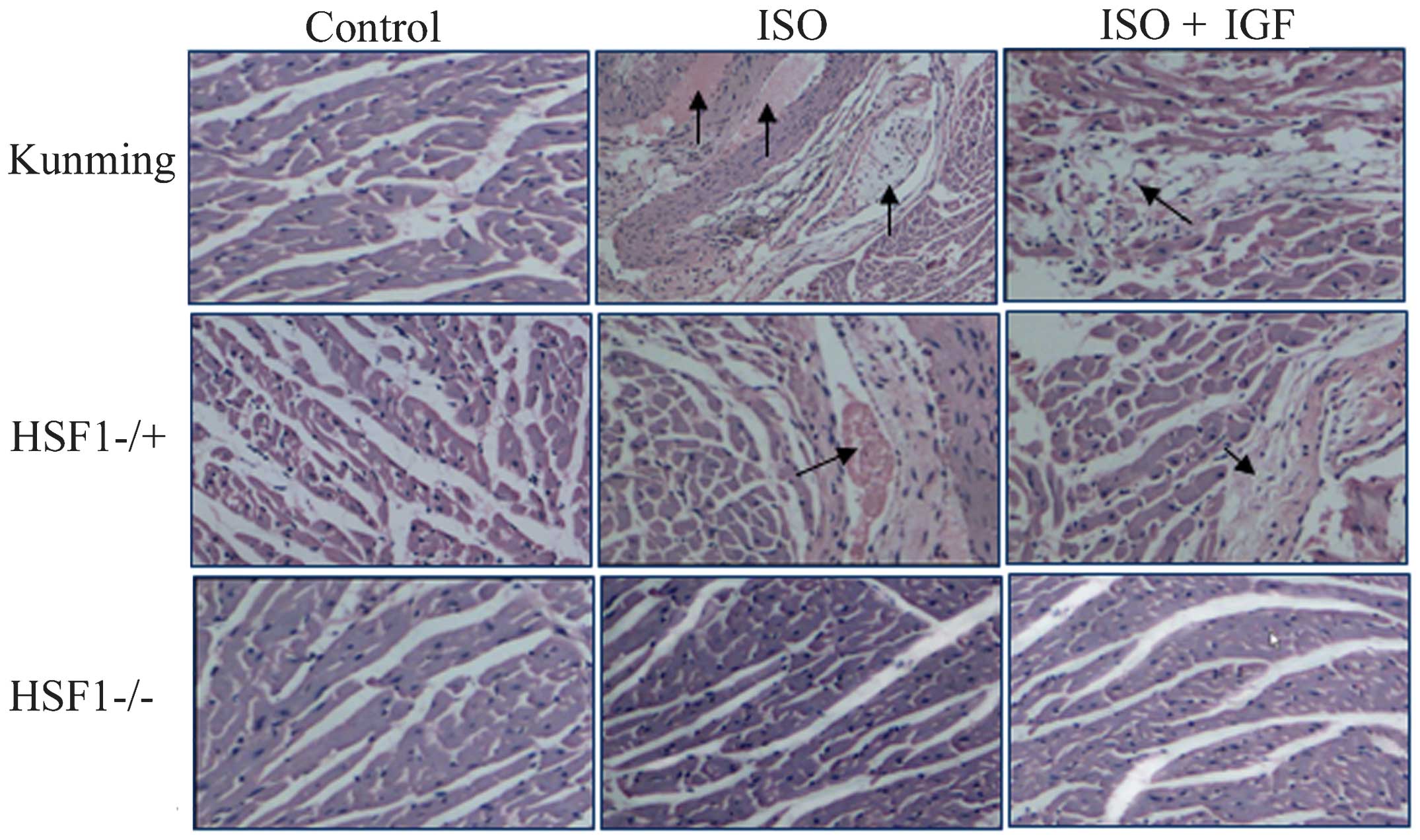
To observe the effects of HSF1 on cardiac fibrosis,
a myocardial fibrosis model was prepared using HSF−/+ mice. After
injection of ISO for 8 or 14 days, a large number of fibres were
found to accumulate between cardiomyocytes or around aortic
ventricles of the heart in Kunming mice, and this accumulation was
significantly inhibited by intervention with IGF-1, and similar
results were observed in HSF−/+ mice treated with ISO (Fig. 1), indicating that myocardial
fibrosis was induced by ISO but reversed by IGF-1 treatment, in
accordance to other studies (27,28).
By contrast, in HSF1−/− mice, cardiac fibrosis was not found to be
induced by ISO (Fig. 1). These
results suggested that HSF1 is required for myocardial fibrosis in
mice, at least that induced by ISO.
HSF1 is required for the expression of
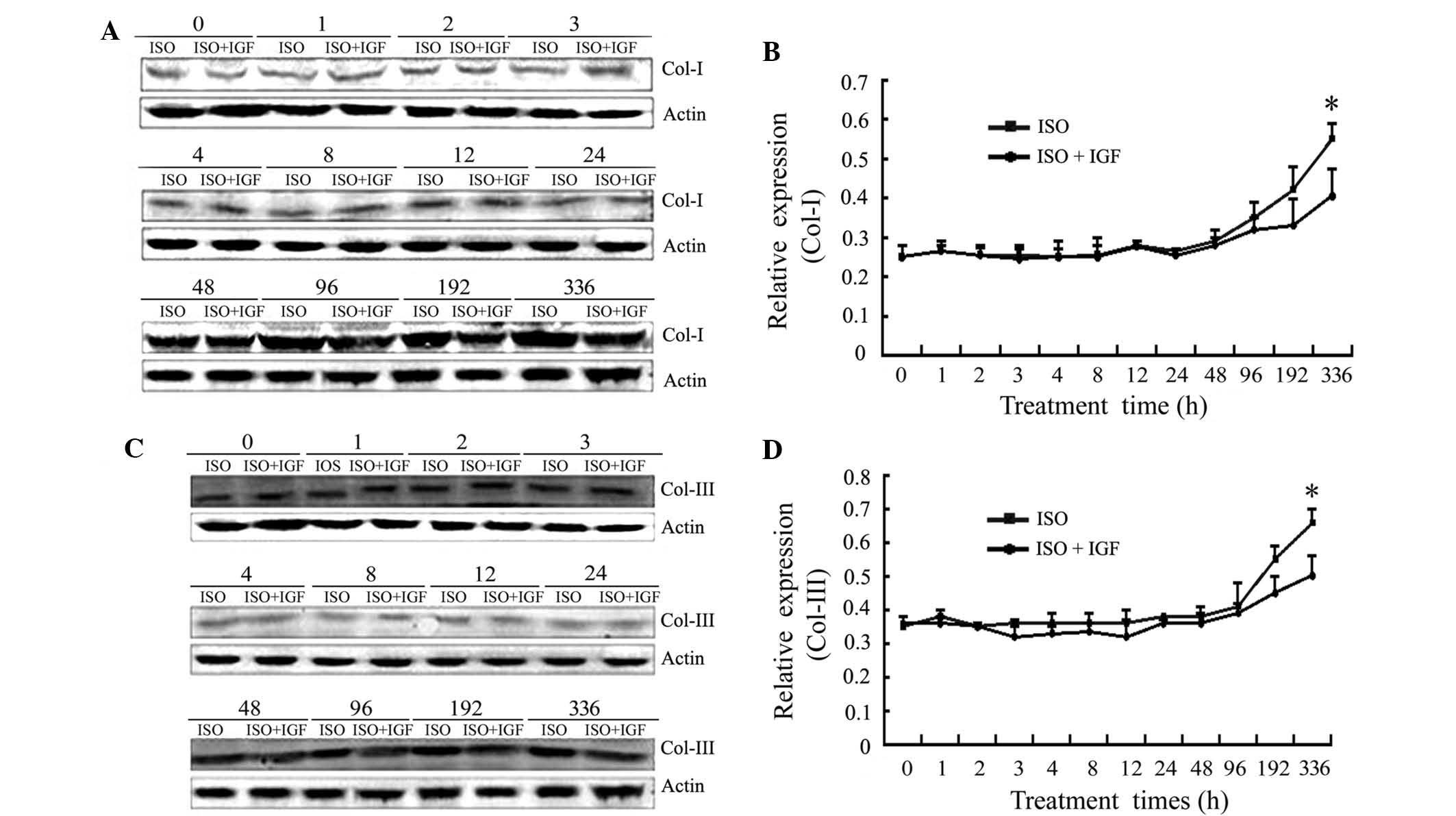
myocardial collagen proteins induced by ISO
Though disease processes and origins may differ,
excess deposition of fibrillar collagens type I and III
characterizes fibrosis in the heart (29). To investigate the effect of HSF1 on
the protein expression of western blot analysis was performed. As
shown in Fig. 2, the protein
expression of collagen type I and III only began to increase after
8 days, and was significantly upregulated after 14 days of ISO
injection. However, IGF-1 treatment inhibited the upregulation of
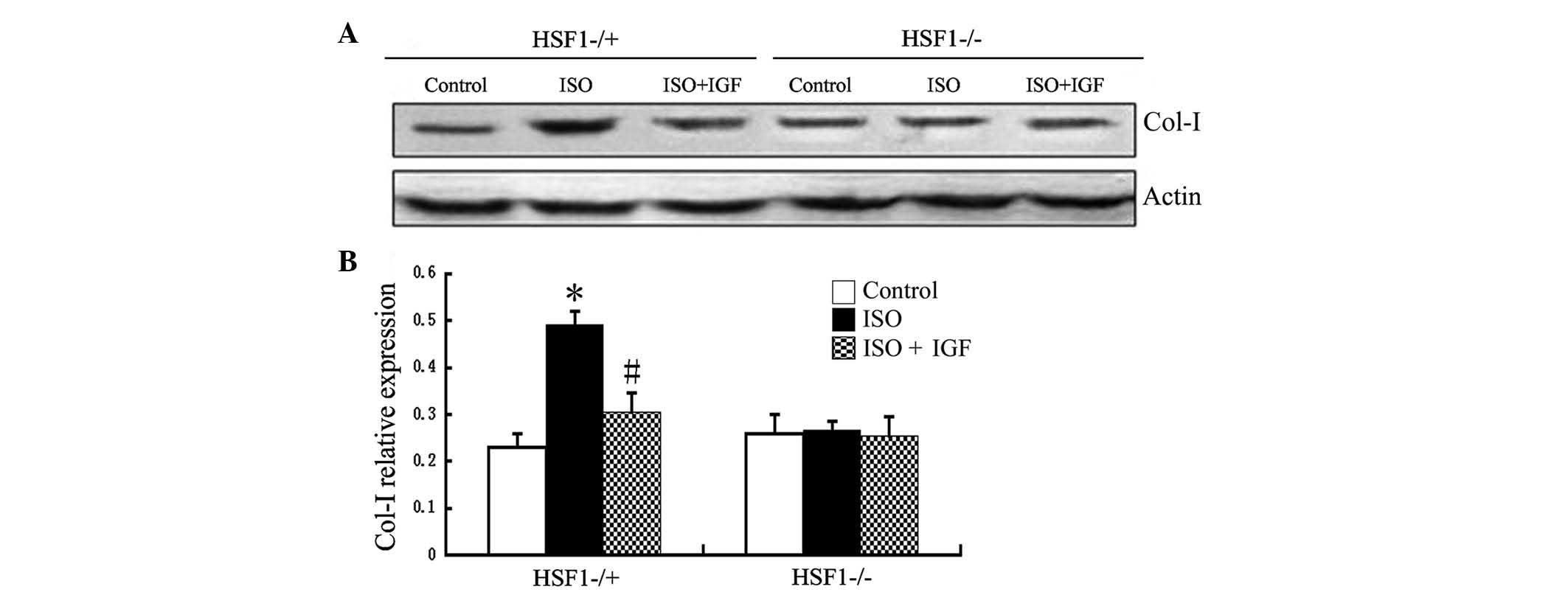
the protein expression of collagen type I and III. In HSF−/+ mice,
the effect of ISO was similar to that in Kunming mice (Fig. 3). By contrast, in HSF−/− mice, the
expression of collagen proteins (types I and III) was not found to
significantly alter, even after 14 days of ISO injection (Fig. 3). These results suggested that HSF1
is required for expression of myocardial collagen proteins (type I
and III) induced by ISO in mice.
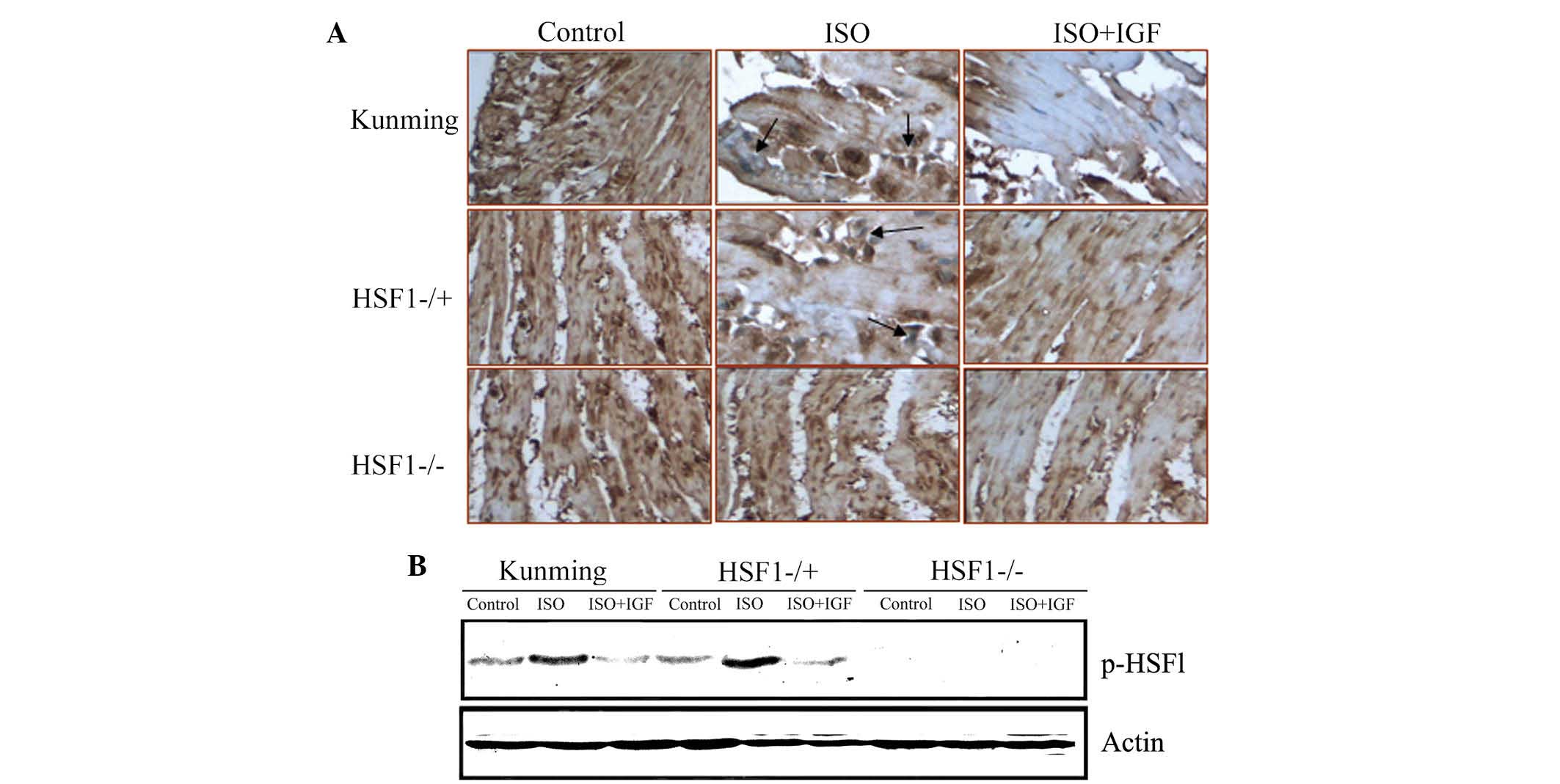
ISO mediates HSF1 protein activation
The above results demonstrated that HSF1 is required
for myocardial fibrosis and collagen protein expression induced by
ISO in mice. As HSF1 is a transcription factor, the present study
hypothesized that ISO-induced expression of collagen proteins is
directly or indirectly dependent on the transcriptional functions
of HSF1. To test this hypothesis, HSF1 activation was first
examined by immunohistochemistry. As shown in Fig. 4A, HSF1 protein expression in the
nuclei of cardiac cells from Kunming mice and HSF1−/ + mice after
treatment with ISO for 14 days was significantly increased, but
this induced increase in nuclear HSF1 protein was inhibited by
IGF-1 (Fig. 4A).
The activation of HSF1 protein involves its
phosphorylation, which leads to its nuclear tanslocation. To
further investigate whether HSF1 activation is induced by ISO,
phosphorylated HSF1 (p-HSF1) was detected using western blot
analysis. As shown in Fig. 4B, ISO
treatment induced an increase in p-HSF1 in Kunming as well as in
HSF1−/+ mice. The results suggested that HSF1 activation is
required for ISO-induced myocardial fibrosis in mice.
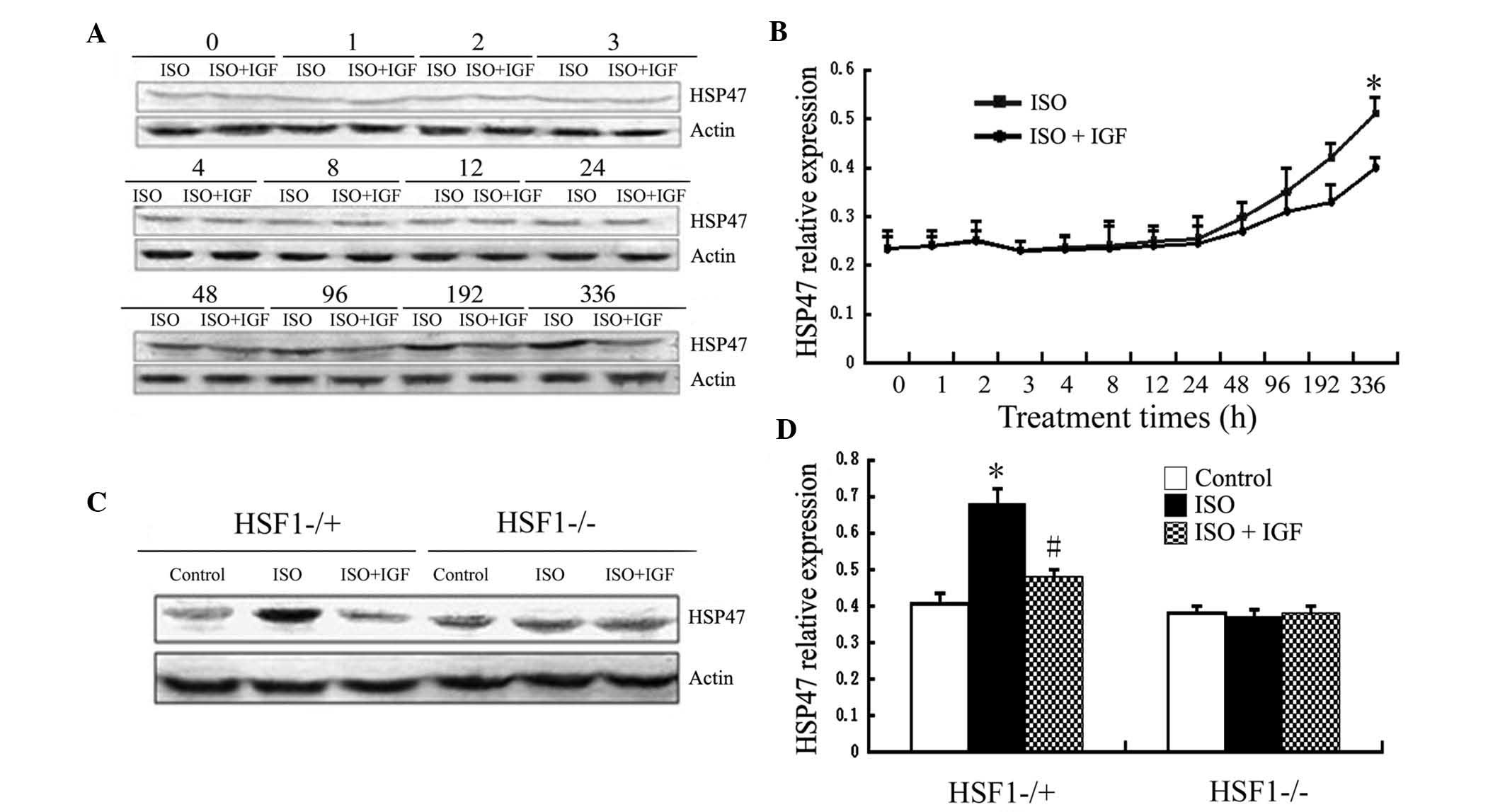
HSP47 upregulation induced by ISO is
dependent on HSF1 and correlates with that of collagen
proteins
HSF1 regulates expression of HSPs. Among HSPs, HSP47
is closely associated with fibrosis (18). Therefore, the present study
investigated the expression of HSP47 in mouse models of myocardial
fibrosis induced by ISO. As shown in Fig. 5, in Kunming mice, HSP47 protein
expression was induced after 96 h of ISO injection and was markedly
increased after injection for 336 h, while IGF-1 treatment
inhibited this induced expression (Fig. 5A and B), which correlates with
upregulation of collagen I and III. Similarly, after treatment with
ISO for 336 h, HSP47 protein expression was markedly increased in
HSF1−/+ mice, while this increase was inhibited by IGF-1
intervention (Fig. 5C and D).
However, HSP47 expression was not i nduced by ISO treatment i n
HSF1−/− mice, even after 336 h (Fig.
5). These results suggested that HSF1 is required for HSP47
expression induced by ISO.
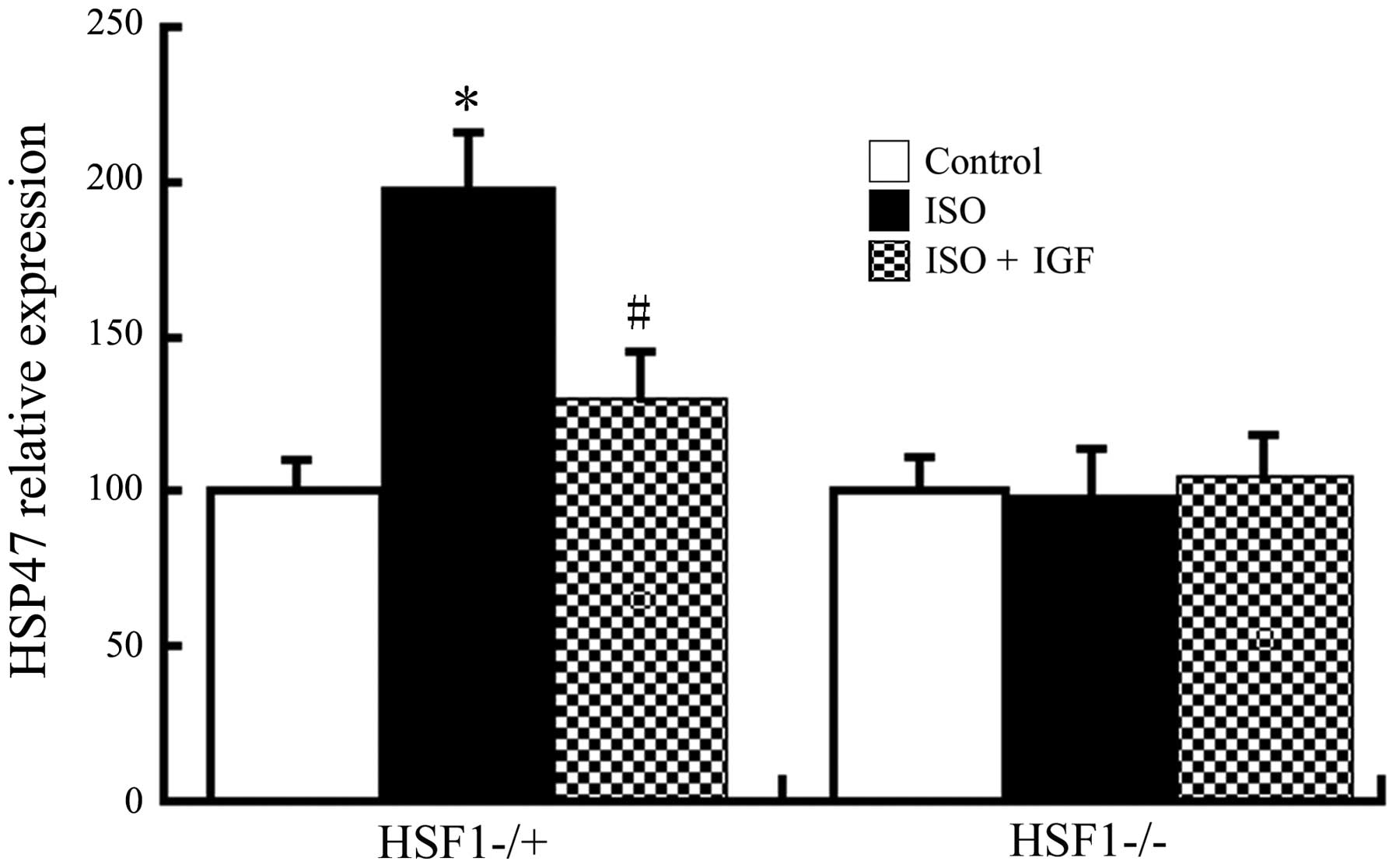
In order to investigate whether the increase in
HSP47 protein is dependent on its transcription, HSP47 RNA was
examined by RT-qPCR. As shown in Fig.
6, in Kunming and HSF1−/+ mice, treatment with ISO induced an
increase in HSP47 mRNA levels, which was inhibited by IGF-1, but
ISO did not induce any increases in HSP47 mRNA in HSF1−/− mice,
which was consistent with the effects observed regarding protein
expression. All of these results indicated that ISO induces HSP47
expression, possibly through HSF1-mediated increases in its
transcription.
Discussion
It is well known that continuous use of β-agonist
ISO causes myocardial hypertrophy and myocardial fibrosis in rats,
which may be due to cardiac hypertrophy and the activation of
fibrosis-associated signaling pathways, including
mitogen-acti-vated protein kinase, c-Jun N-terminal kinase/signal
transducer and activator of transcription and nuclear factor κB by
ISO (30,31). In the present study, it was
demonstrated that subcutaneous injection of ISO in Kunming mice and
HSF1−/+ mice caused myocardial hypertrophy, leading to severe
fibrosis, accompanied by a significant increase in the expression
of collagen type I and III, indicating that the animal model of
myocardial fibrosis was successfully established, consistent with
other studies (27,32–34).
Of note, subcutaneous injection of ISO did not cause any myocardial
fibrosis in HSF1−/− mice, indicating that HSF1 is required for
myocardial fibrosis in mice, at least in the mouse model induced by
ISO. As a transcription factor activated HSF1 regulates the
expression of other genes (35,36).
Immunohistochemical analysis showed that, in Kunming and HSF1−/ +
mice, ISO induced large amounts of HSF1 protein in cardiac nuclei,
indicating that it may function as a transcription factor in the
process of cardiac fibrosis.
As a transcription factor, HSF1 mainly regulates the
expression of HSPs. Among the known HSPs, HSP47 is most closely
associated with myocardial fibrosis. Therefore, the present study
assessed the possibility of HSF1 regulating the expression of
HSP47. It was found that in Kunming and HSF1−/+ mice, ISO induced
myocardial fibrosis, accompanied with HSP47 upregulation. However,
the use of ISO did not induce myocardial fibrosis in HSF1−/− mice,
nor did it increase in HSP47 expression, suggesting that HSP47
upregulation is regulated by HSF1. Bioinformatics analysis further
showed that the HSP47 promoter region contains HSF1 binding sites.
Therefore, HSF1 directly regulates the expression of HSP47 in this
model.
In the present study, the role of IGF-1 in cardiac
fibrosis was also examined. The results showed that IGF-1 inhibited
ISO-induced myocardial fibrosis and also inhibited HSP47 expression
and activation of HSF1. Taking into account the regulation of the
expression of HSP47 by HSF1 and the inhibition of fibrosis and
HSP47 expression in HSF1−/− mice, it was speculated that IGF-1
downregulates HSP47 expression by inhibiting the activation of
HSF1, and that HSP47 may affect the synthesis of collagen protein;
however, the detailed underlying molecular mechanisms remain to be
elucidated.
In conclusion, the present study successfully
induced myocardial fibrosis in mice through treatment with ISO. It
was demonstrated that HSF1 is required for myocardial fibrosis in
this animal model. As myocardial fibrosis was accompanied by
elevated HSP47 levels, it was suggested that HSP47 may have an
important role in myocardial fibrosis. The exact underlying
molecular mechanisms remain to be elucidated in future studies.
Acknowledgments
The authors of the present study are grateful to
Professor Xianzhong Xiao (Central South University, Changsha,
China) for providing good conditions for this study.
References
|
1
|
Ghosh AK, Bradham WS, Gleaves LA, De Taeye
B, Murphy SB, Covington JW and Vaughan DE: Genetic deficiency of
plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged
mice: Involvement of constitutive transforming growth factor-beta
signaling and endothelial-to-mesenchymal transition. Circulation.
122:1200–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krieg T, Abraham D and Lafyatis R:
Fibrosis in connective tissue disease: The role of the
myofibroblast and fibroblast-epithelial cell interactions.
Arthritis Res Ther. 9(Suppl 2): S42007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwak HB, Kim JH, Joshi K, Yeh A, Martinez
DA and Lawler JM: Exercise training reduces fibrosis and matrix
metalloproteinase dysregulation in the aging rat heart. FASEB J.
25:1106–1117. 2011. View Article : Google Scholar :
|
|
4
|
Yu XY, Qiao SB, Guan HS, Liu SW and Meng
XM: Effects of visfatin on proliferation and collagen synthesis in
rat cardiac fibroblasts. Horm Metab Res. 42:507–513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezzani R, Angoscini P, Rodella L and
Bianchi R: Alterations induced by cyclosporine A in myocardial
fibers and extracellular matrix in rat. Histol Histopathol.
17:761–766. 2002.PubMed/NCBI
|
|
6
|
Gao HC, Zhao H, Zhang WQ, Li YQ and Ren
LQ: The role of the Rho/Rock signaling pathway in the pathogenesis
of acute ischemic myocardial fibrosis in rat models. Exp Ther Med.
5:1123–1128. 2013.PubMed/NCBI
|
|
7
|
Díez J: Mechanisms of cardiac fibrosis in
hypertension. J Clin Hypertens (Greenwich). 9:546–550. 2007.
View Article : Google Scholar
|
|
8
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato M, Shegogue D, Gore EA, Smith EA,
McDermott PJ and Trojanowska M: Role of p38 MAPK in transforming
growth factor beta stimulation of collagen production by
scleroderma and healt hy der ma l fibroblasts. J Invest Dermatol.
118:704–711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhattacharyya S, Wei J and Varga J:
Understanding fibrosis in systemic sclerosis: Shifting paradigms,
emerging opportunities. Nat Rev Rheumatol. 8:42–54. 2012.
View Article : Google Scholar
|
|
11
|
Huynh K, McMullen JR, Julius TL, Tan JW,
Love JE, Cemerlang N, Kiriazis H, Du XJ and Ritchie RH:
Cardiac-specifc IGF-1 receptor transgenic expression protects
against cardiac fibrosis and diastolic dysfunction in a mouse model
of diabetic cardiomyopathy. Diabetes. 59:1512–1520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haleagrahara N, Chakravarthi S and Mathews
L: Insulin like growth factor-1 (IGF-1) causes overproduction of
IL-8, an angiogenic cytokine and stimulates neovascularization in
isoproterenol-induced myocardial infarction in rats. Int J Mol Sci.
12:8562–8574. 2011. View Article : Google Scholar
|
|
13
|
Yousefi K, Fathiazad F, Soraya H,
Rameshrad M, Maleki-Dizaji N and Garjani A: Marrubium vulgare L.
methanolic extract inhibits infammatory response and prevents
cardiomyocyte fibrosis in isoproterenol-induced acute myocardial
infarction in rats. Bioimpacts. 4:21–27. 2014.
|
|
14
|
Xiao X and Benjamin IJ: Stress-response
proteins in cardiovascular disease. Am J Hum Genet. 64:685–690.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benjamin IJ and McMillan DR: Stress (heat
shock) proteins: Molecular chaperones in cardiovascular biology and
disease. Circ Res. 83:117–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kregel KC: Heat shock proteins: Modifying
factors in physiological stress responses and acquired
thermotolerance. J Appl Physiol 1985. 92:2177–2186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellaye PS, Burgy O, Causse S, Garrido C
and Bonniaud P: Heat shock proteins in fibrosis and wound healing:
Good or evil? Pharmacol Ther. 143:119–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SJ, Sohn HY and Park SI: TRAIL
regulates collagen production through HSF1-dependent Hsp47
expression in activated hepatic stellate cells. Cell Signal.
25:1635–1643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang Y and Zheng J: Roles of HSP47/HSF1 in
pulmonary fibrosis. Shandong Yi Yao. 50:117–118. 2010.In
Chinese.
|
|
21
|
Xiao H, Lv J, Chen Q, Liu R and Ling G:
Role of HSP47 in renal tubulointerstitial fibrosis induced by
transforming growth factor β1. Chin J Nephrol. 27:923–927. 2011.In
Chinese.
|
|
22
|
Deng J, Wang D, Zhang Y and Yuan F: The
expression of HSP47 in renal tissues of chronic renal disease
patients and its significance. Chongqing Yi Xue. 34:1029–1031.
2005.In Chinese.
|
|
23
|
Hai G, Li J, Jia Y, Xia W, Chen Y and Liu
J: The effect of TGF-β1 on the expression of HSP47 and collagen in
HFL cells. Guangdong Yi Xue. 34:2632–2634. 2013.In Chinese.
|
|
24
|
Li Y, Wu W, Jiang Y and Wang K: Effect of
heat shock protein 47 on the expression of collagen I induced by
TGF-beta(1) in hepatic stellate cell-T6 cells. Zhong Nan Da Xue Xue
Bao Yi Xue Ban. 32:650–655. 2007.In Chinese. PubMed/NCBI
|
|
25
|
Bradford MM: A rapid and sensitive method
for the quanititation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagata K: Hsp47: A collagen-specific
molecular chaperone. Trends Biochem Sci. 21:22–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siu PM, Wang Y and Alway SE: Apoptotic
signaling induced by H2O2-mediated oxidative stress in
differentiated C2C12 myotubes. Life Sci. 84:468–481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong RQ, Wang ZF, Zhao C, Gu HR, Hu ZW,
Xie J and Wu YQ: Toll-like receptor 4 knockout protects against
isoproterenol-induced cardiac fibrosis: The role of autophagy. J
Cardiovasc Pharmacol Ther. 20:84–92. 2015. View Article : Google Scholar
|
|
29
|
Hribal ML, Procopio T, Petta S, Sciacqua
A, Grimaudo S, Pipitone RM, Perticone F and Sesti G: Insulin-like
growth factor-I, infam-matory proteins, and fibrosis in subjects
with nonalcoholic fatty liver disease. J Clin Endocrinol Metab.
98:E304–E308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roche P and Czubryt MP: Transcriptional
control of collagen I gene expression. Cardiovasc Hematol Disord
Drug Targets. 14:107–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ocaranza MP, Díaz-Araya G, Chiong M, Muñoz
D, Riveros JP, Ebensp erger R, Sabat S, Irarrázaval P, Jalil JE and
Lavandero S: Isoproterenol and angiotensin I-converting enzyme in
lung, left ventricle, and plasma during myocardial hypertrophy and
fbrosis. J Cardiovasc Pharmacol. 40:246–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takemoto Y, Yoshiyama M, Takeuchi K, Omura
T, Komatsu R, Izumi Y, Kim S and Yoshikawa J: Increased JNK, AP-1
and NF-kappa B DNA binding activities in isoproterenol-induced
cardiac remodeling. J Mol Cell Cardiol. 31:2017–2030. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gallego M, Espiña L, Vegas L, Echevarria
E, Iriarte MM and Casis O: Spironolactone and captopril attenuates
isopro-terenol-induced cardiac remodelling in rats. Pharmacol Res.
44:311–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leenen FH, White R and Yuan B:
Isoproterenol-induced cardiac hypertrophy: Role of circulatory
versus cardiac renin-angiotensin system. Am J Physiol Heart Circ
Physiol. 281:H2410–H2416. 2001.PubMed/NCBI
|
|
35
|
Ciocca DR, Arrigo AP and Calderwood SK:
Heat shock proteins and heat shock factor 1 in carcinogenesis and
tumor development: an update. Arch Toxicol. 87:19–48. 2013.
View Article : Google Scholar
|
|
36
|
Vihervaara A and Sistonen L: HSF1 at a
glance. J Cell Sci. 127:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|