Introduction
Chronic obstructive pulmonary disease (COPD) is a
term used for diseases, which cause lung impairment and
breathlessness. The World Health Organization indicated that COPD
was the fifth leading cause of mortality in 2002 and was predicted
to become the third leading cause of mortality in 2030 (1). A previous large population,
spirometry-based epidemiological investigation reported that, in
China, the prevalence of COPD in adults >40 years was 8.2%
(12.4% in men; 5.1% in women) (2).
Therefore, COPD is likely to become an important health care
problem worldwide.
COPD is associated with characteristic pathological
changes in the small airways, including obstructive bronchiolitis,
and the destruction of lung parenchyma, including emphysema.
Current therapies for COPD, including inhaled corticosteroids and
long-acting agonists, improve the pulmonary function and quality of
life in patients with COPD, however, they cannot reverse the
pathological process of COPD. Therefore, the identification of
novel therapies for COPD is an area of intense investigation
(3–5).
Liebow (6)
demonstrated that the alveolar septa in patients with COPD were
thin and almost avascular, suggesting that a reduction of the small
capillary blood vessels may lead to subsequent alveolar septal
loss. Vascular endothelial growth factor (VEGF) signaling is
important for maintaining lung structure. Kasahara et al
(7) demonstrated that the
expression of VEGF and its receptor in the lung tissues of patients
with emphysema were significantly lower than normal. Therefore, a
decrease in VEGF or disruption to the VEGF signaling pathway may
affect the pathogenesis of emphysema. However, the effect of levels
of VEGF on airway epithelial cells, which are in direct contact
with the environment, remains to be fully elucidated (8). It has been suggested that statins may
modulate VEGF synthesis, and the pleiotropic effects of statins
with regard to their use in COPD treatment has received more
attention (9,10).
Statins are potent inhibitors of
3-hydroxy-3-methylglutaryl coenzyme A reductase, and exhibit
pleiotropic pharmacological effects (11). In addition to a
cholesterol-lowering effect, they exhibit anti-inflammatory,
antioxidant and immunomodulatory properties, and improve
endothelial function in chronic inflammatory lung disease (10). A number of these statin pleiotropic
effects are mediated by the inhibition of isoprenylation of small
guanosine-5′-triphosphate-binding signaling molecules, including
the Rho, Ras and Rac proteins (12). The anti-inflammatory effects
include the suppression of the release of proinflammatory
cytokines, chemokines, adhesion molecules and matrix
metalloproteinases by inflammatory cells. Statins increase the
secretion of VEGF and the expression and activity of endothelial
nitric oxide synthase, which improves endothelial cell function and
promotes angiogenesis (13). There
is evidence that statins exert cell type-dependent effects on
endothelial cell angiogenic activity and on VEGF synthesis
(14). It has been reported that
simvastatin may induce apoptosis in hyper-proliferative pulmonary
vascular lesions (15) and may
inhibit the development of colon cancer via the induction of
apoptosis and suppression of angiogenesis (16).
Cigarette smoking is the most important and common
risk factor for COPD, however, the underlying pathological
mechanisms remain to be elucidated (17,18).
Therefore, the present study performed assays in order to
investigate the effects of statins on the expression of VEGF, based
on the morphometric parameters in cigarette smoke-induced lung
emphysema, using a rat model. A number of cell functions, including
differentiation, proliferation, and apoptosis can be affected by
simvastatin (19). The effect of
statins on the expression of proliferating cell nuclear antigen
(PCNA) was also investigated.
Materials and methods
Reagents and materials
Male (n=12) and female (n=12) Wistar rats
(12-week-old; 190–220 g; Chinese Academy of Medical Sciences,
Beijing, China) were included in the present study, which was
approved by the Division of Laboratory Animal Medicine at the China
Medical University (Shenyang, China; certificate no. SVXK, Liao,
2003–0009). The animals were housed in Plexiglas cages (Guxiu,
Suzhou, China), with males and females housed separately, under a
12:12 h light-darkness cycle in temperature and humidity controlled
rooms. Standard laboratory food and water were provided ad
libitum. The present study was performed in accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (20). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
China Medical University.
Treatment groups
The rats were numbered in ascending order of weight
and divided into four groups. The four groups of rats (n=6 in each
group) were randomly assigned to the following groups: Control
(CtL); smoke-exposed only (Sm); simvastatin-only (St); and smoke +
simvastatin (SmSt). The Sm and SmSt groups were exposed to the
smoke from 16 commercial Da-Sheng-Chan cigarettes (Hongta Liaoning
Tobacco Co., Ltd., Shenyang Cigarette Factory, Liaoning, China) for
1 h each day for 16 weeks. Simvastatin (Merck Sharp & Dohme,
Ltd., Hoddeston, UK) was administered orally to the SmSt and St
groups at a dose of 5 mg/kg (21,22),
once daily for the 16 week period.
Morphometric evaluation of the lungs
Following 16 weeks of treatment, the rats were
anesthetized using pentobarbital sodium (50 mg/kg,
intraperitoneally; Sigma-Aldrich, St. Louis, MO, USA) and
sacrificed by cervical vertebra dislocation. The bilateral lung
were excised from the chest. The right lung tissues were fixed in
10% neutral buffered formalin (Sigma-Aldrich), embedded in paraffin
blocks (Leica Microsytems GmbH, Wetzlar, Germany), cut into
4-µm serial sections. The right lung was used for
histological analyses. The inflammation score of the small airways
(300–1,100-µm) consisted of the following: Epithelial loss,
erosion and ulcer formation; goblet cell hyperplasia and
hypertrophy; ciliated epithelium lodging; inflammatory cell
infiltration; lymph follicle formation; bronchial stenosis; airway
smooth-muscle cell proliferation disorder; connective tissue
proliferation; squamous metaplasia; wall congestion, edema; and
wall pigmentation. Hematoxylin-and-eosin (HE; Leica Microsystems
GmbH) was used to stain hilar areas of the lung tissue, in order to
assess the small-airway pathology score, using two independent
investigators, in a blinded-manner [Three small-airway sections,
composite score 100/3, total score 100/33 (3 × 11)] (23). The tissues were viewed under a
light microscope (BX51, Olympus Corporation, Tokyo, Japan).
Standard morphometric measurements were used to
determine emphysematous changes. A total of 10 randomly selected
HE-stained hilar areas from each sample (magnification, low power
field) were used to analyze changes in air space size, which was
determined by the average interalveolar septal wall distance (mean
linear intercept; MLI). The alveolar density was determined by the
mean alveolar number (MAN) (24).
Measurements of VEGF and PCNA
Measurements of VEGF in the bronchoalveolar lavage
fluid (BALF) were performed in order to investigate the effect of
simvastatin. For preparation of BALF and measurement of VEGF in the
BALF, each rats was anesthetized using 10% neutral buffered
formalin (Sigma-Aldrich), the trachea were exposed and intubated
and the left lung was washed three times using 2 ml sterile saline,
at 0°C. BALF was collected using a 5 ml syringe and placed on ice.
Following centrifugation at 400 × g for 5 min at 4°C, the VEGF
concentration was measured in the supernatants using an
enzyme-linked immunosorbent assay (ELISA; Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA), according to the manufacturer's
instructions.
To determine the levels of VEGF in the lungs in each
group, the lung tissues were homogenized in phosphate-buffered
saline (PBS; Wake Pure Chemical Industries, Osaka, Japan), and the
supernatant was obtained by ultracentrifugation at 11,100 × g for
10 min at 4°C. The level of VEGF in the supernatant was determined
using a commercially available ELISA kit (Quantikine Mouse VEGF
kit; R&D Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer's instructions.
Immunohistochemical staining and
quantification
In order to perform immunohistochemical scoring
analysis for VEGF and PCNA, hilar sections (5-µm) were
deparaffinized in xylene (Shenyang Chemical Reagent Plant,
Shenyang, China) and rehydrated. Antigen retrieval was performed by
heating the lung tissue in a microwave (MYE-1870MEG; Haier,
Qingdao, China) in 10 mmol/l citric acid monohydrate for 5 min at
900 W and three times for 5 min at 600 W. Endogenous peroxidase
activity was inhibited by incubating the samples with 0.5%
H2O2 for 10 min. The slides were incubated
overnight at 4°C in the appropriate dilutions of the primary
antibodies (mouse anti-PCNA monoclonal antibody (1:100; cat. no.
sc-25280; Santa Cruz Biotechnology, Inc.) and VEGF monoclonal
antibody (1:100; cat. no. sc-7269; Santa Cruz Biotechnology, Inc.).
The slides were incubated for 60 min at room temperature (20–22°C),
rinsed for 2 min with PBS three times, and incubated in 3,
3′-diaminobenzidine. The slides were then evaluated using light
microscopy (BX51; Olypmus Corporation).
The percentages of VEGF- and PCNA-positive
small-airway and alveolar epithelial cells (AECs) were calculated.
The staining was scored as follows: Expression of VEGF was
localized in the cytoplasm, and the expression of PCNA was
localized in the cell nucleus. The 500 cells were randomly selected
in the bronchiolar epithelium, alveolar epithelium and vascular
endothelial cells in each slide at 400× magnification. The number
of cells expressing VEGF or PCNA were counted, respectively
The entire tissue samples were scored using the same
magnification factor. The staining intensities of VEGF and PCNA in
the SAECs, AECs and vascular endothelial cells (VECs were scored by
independent investigators in a blinded-manner, in order to avoid
observer bias.
Analysis of the expression of VEGF
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was used to analyze the expression levels of
VEGF by amplifying the coding parts of the VEGF gene. Total lung
RNA was isolated using TRIzol reagent (Invitrogen Life
Technologies), according to the manufacturer's instructions. RNA
concentration was measured by absorption at 260 nm and the purity
of the RNA was guaranteed based on a absorption ratio of 260 nm/280
nm. Following quantification of the RNA samples, cDNA was
synthesized from 50–100 mg of total lung RNA using a Takara RNA PCR
kit (AMV) Ver.3.0 (Takara, Bio, Inc.) and subjected to a
semi-quantitative PCR using rTaq (Takara Bio, Inc.), according to
the manufacturer's instructions. Briefly, the first-strand cDNA was
synthesized in a 10 µl reaction mixture containing total
RNA, AMV Reverse Transcriptase, RNase Inhibitor, dNTP Mixture,
MgCl2, 10X RT buffer, and Random 9-mer as the reverse
primer. The reverse-transcription reactions were carried out at
30°C for 10 min, 50°C for 30 min, and 95°C for 2 min, prior to
being chilled to 5°C for 5 min. The PCR cycle conditions were as
follows: 94°C for 5 min, followed by 28 cycles of 94°C for 30 sec,
56°C for 30 sec and 72°C for 60 sec, with a final extension step of
7 min at 72°C in a thermal cycler (Applied Biosystems Life
Technologies, Foster City, CA, USA). The PCR products were
electrophoresed on agarose gel (Takara, Bio, Inc.), stained with
ethidium bromide (Sigma-Aldrich) and then visualized in a UV
transilluminator (GAS7001B; UVItec Limited, Cambridge, UK), with
images captured. GAPDH was used as an internal control. The primer
sequences used in the present study were as follows: Forward:
5′-ATCTTCAAGCCGTCCTGTGT-3′ and reverse:
5′-TGTTCTATCTTTCTTTGGTCTGC-3′, for VEGF. Primers were obtained from
Takara Bio, Inc., Dalian, China). The total lung RNA (50–100 mg)
was reverse-transcribed and amplified using RT-qPCR as
described.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD). Following a test of homogeneity of variance, a
one-way analysis of variance was used for multiple group
comparisons. Pairwise multiple comparisons were performed using
Dunnett's or the least significant difference test. A
Kruskal-Wallis rank test was used for multiple comparisons of
groups with unequal variances, and Dunn's method was used for
pairwise multiple comparisons. All analyses were performed using
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Condition of the rats
All 24 rats in the four groups survived the 16 week
treatment period. The mean ± SD body weights in the CtL, Sm, St and
SmSt groups following 16 weeks of treatment were 390.81±76.75,
371.50±62.89, 366.67±75.69 and 322.83±66.03 g, respectively.
Although the body weights in the Sm, St and SmSt groups were lower
than those in the CtL group, the differences were not
significant.
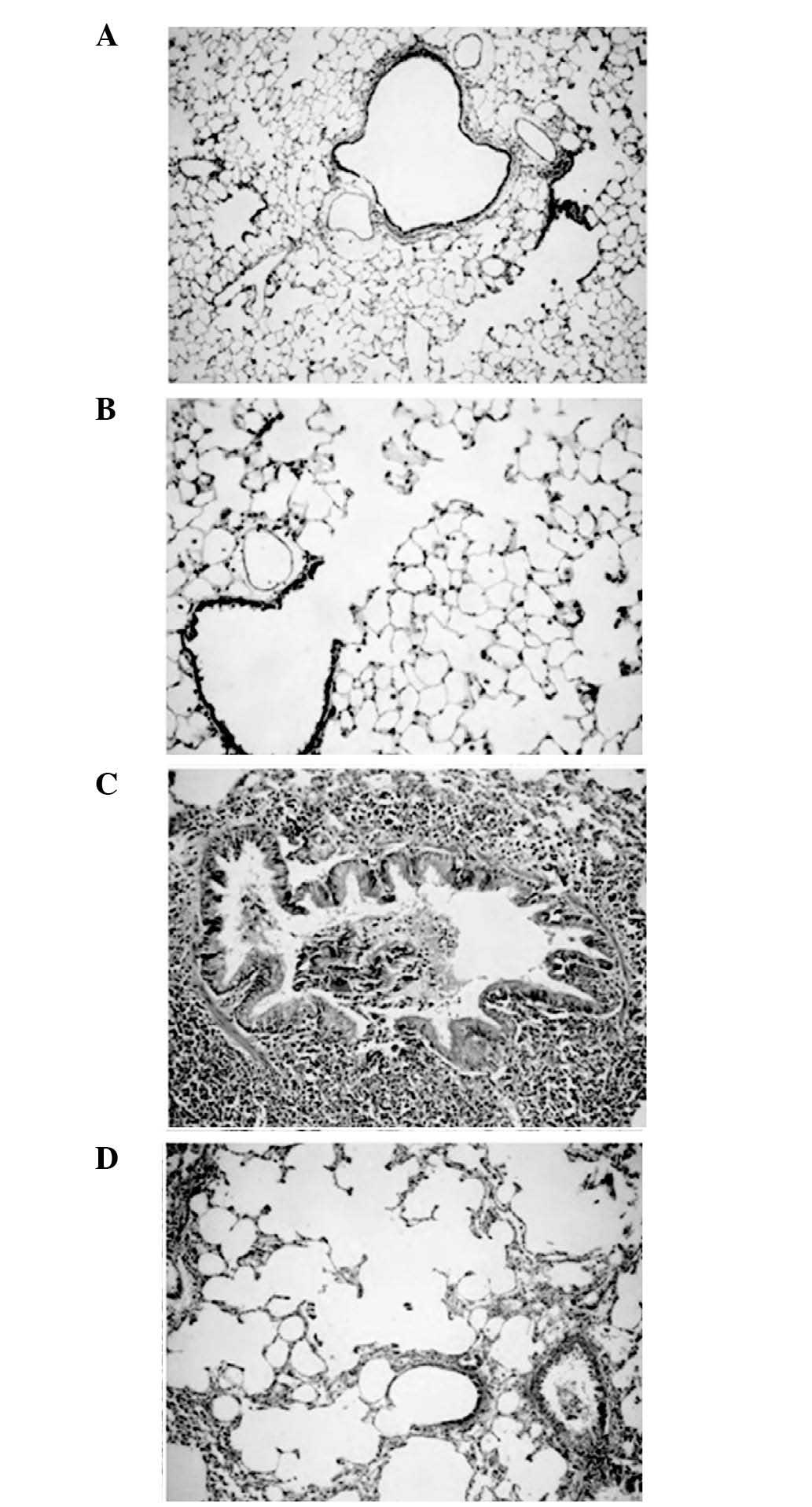
Reduction of small-airway inflammation
and inhibition of cigarette smoke-induced emphysema by
simvastatin
The histological changes in inflammatory cell
infiltration, goblet cell hyperplasia/hypertrophy and airway
smooth-muscle cell proliferation were significantly reduced in the
SmSt group compared with those in the Sm group (Table I). The histological scoring
revealed that simvastatin treatment led to lower TIS in the SmSt
group compared with the Sm group (P<0.05). No significant
differences were observed between the TIS values in the SmSt and
CtL groups (Table I; Fig. 1A–D)
 | Table ITotal inflammation scores and results
of morphometric evaluation in the four study groups 16 weeks after
treatment. |
Table I
Total inflammation scores and results
of morphometric evaluation in the four study groups 16 weeks after
treatment.
| Group | TIS | MLI (µm) | MAN
(n/nm2) |
|---|
| CtL | 43.43±24.97 | 58.72±5.98 | 138.07±6.74 |
| Sm | 84.85±48.96a | 79.31±25.31a | 84.56±31.83b |
| St | 47.78±31.24 | 50.64±6.02 |
128.42±33.55c |
| SmSt | 45.96±23.27b | 60.46±4.28b |
132.11±11.22c |
| F-statistic | 3.321 | 4.789 | 5.700 |
| P-value | <0.05 | <0.05 | <0.01 |
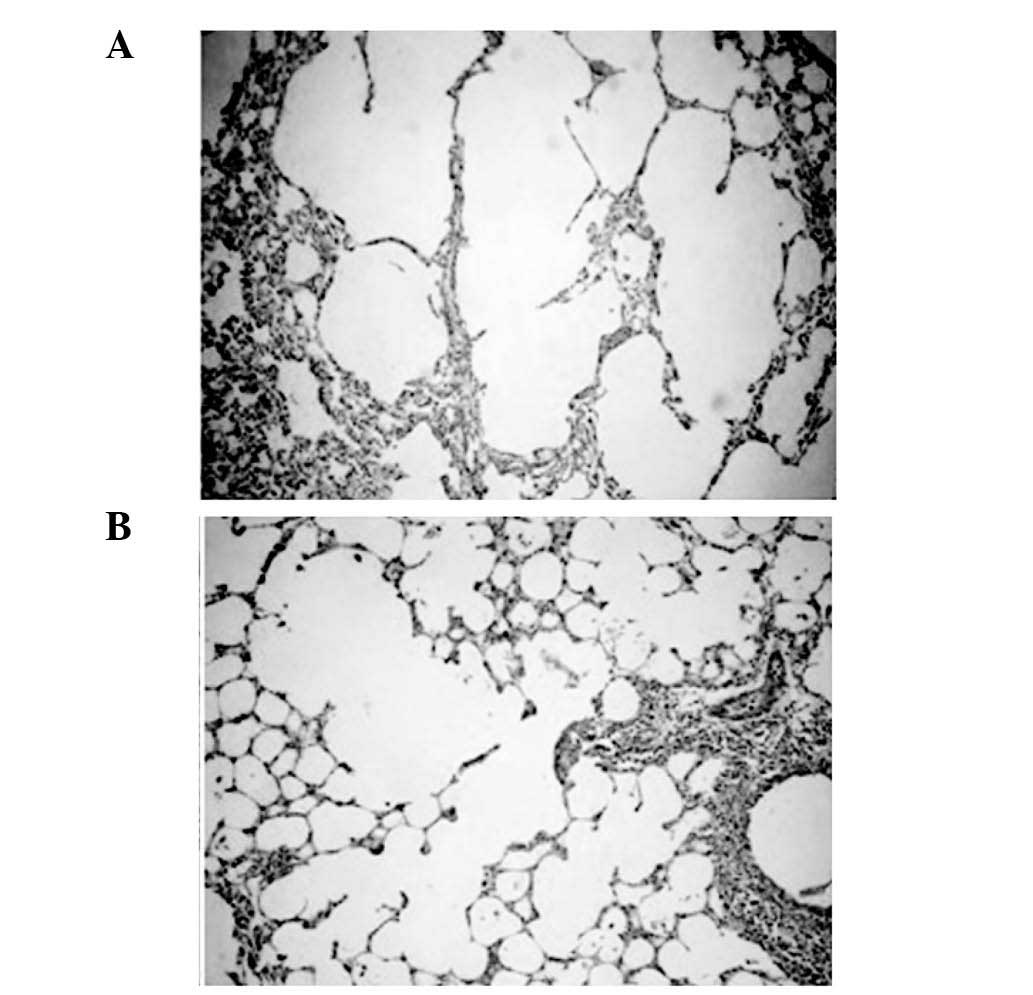
Evaluation of the destruction of the alveolar
architecture demonstrated that chronic exposure to cigarette smoke
for 16 weeks caused higher lung parenchymal destruction in the Sm
group compared with the CtL group, leading to the enlargement of
air space. By contrast, simvastatin significantly inhibited lung
destruction in the SmSt group. Following 16 weeks of treatment, the
MLI levels in the Sm group were 35% higher than in the CtL group,
and the MAN levels in the Sm group were 60% higher than in the CtL
group. No significant differences were observed in MLI and MAN
between the St and the control groups, however, in the SmSt group,
MLI was significantly lower and MAN was significantly higher
compared with the Sm group, the levels of MLI and MAN in the SmSt
group were similar to those in the CtL group (Table I; Fig.
2A and B).
Simvastatin increases the smoke-induced
reduction in VEGF
The mean concentrations of VEGF in the BALF and in
the lung tissue samples were significantly lower in the Sm group,
compared with those in the CtL group (P<0.01). In the SmSt
group, simvastatin treatment led to significantly higher
concentrations of VEGF in the BALF samples (P<0.01) and
homogenate samples (P<0.01), compared with those in the Sm group
(Table II).
 | Table IILevels of VEGF in lung homogenates
and BALF. |
Table II
Levels of VEGF in lung homogenates
and BALF.
| Group | BALF (pg/ml) | Homogenate
(ng/ml) |
|---|
| CtL | 199.85±20.46 | 7.56±0.33 |
| Sm | 71.49±16.22a | 4.15±0.11a |
| St |
201.30±17.61b | 6.86±0.16b |
| SmSt |
187.14±15.18b | 6.78±0.05b |
| F-statistic | 81.253 | 368.44 |
| P-value | <0.01 | <0.01 |
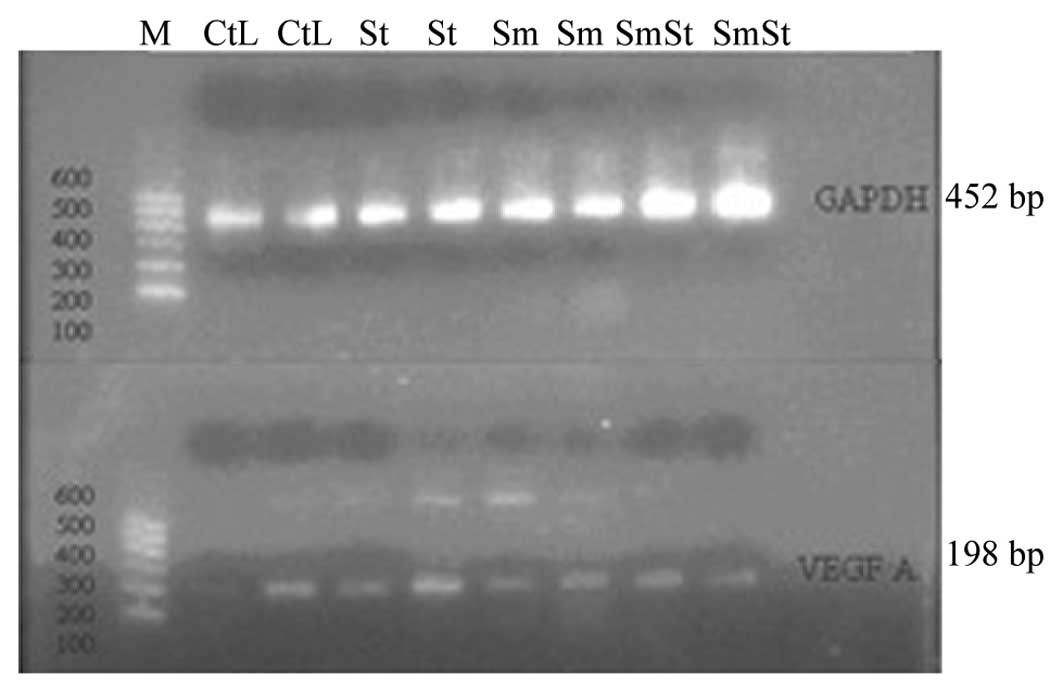
Immunohistochemical VEGF staining and
RT-PCR
The mRNA expression of VEGF198 was lower
in the lung tissue samples from the Sm group than in the CtL group.
However, no significant differences were observed between the SmSt
and CtL groups (Fig. 3). The
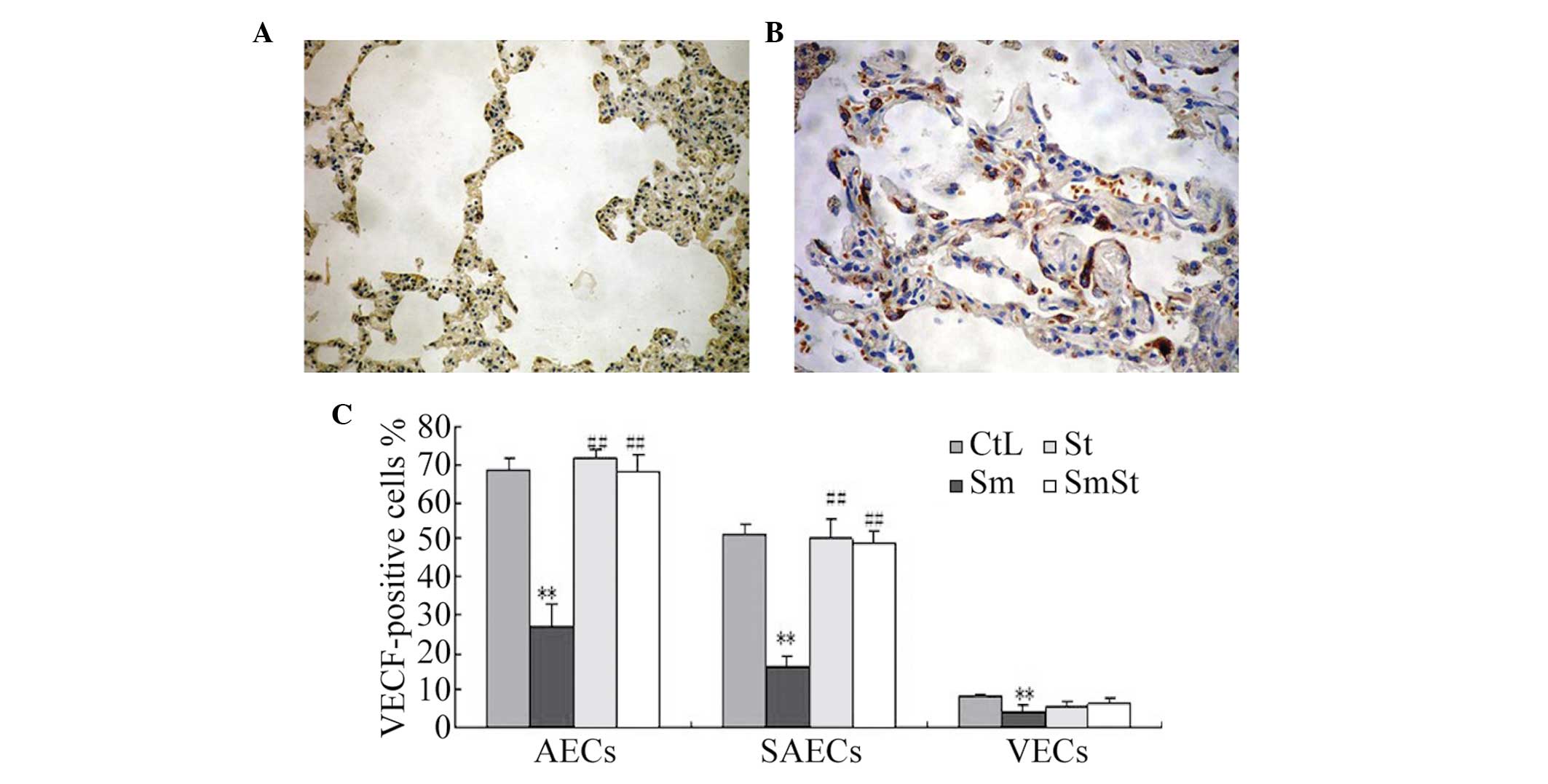
expression of VEGF occurred predominantly in the SAECs (51.3±2.9%)
and AECs (68.3±3.3%) of the CtL group, but was also present to a
lesser extent, in the VECs (8.5±0.8%) of the CtL group. Following
16 weeks of smoke exposure, the expression of VEGF was
significantly lower in the Sm group compared with the CtL group in
the SAECs (16.3±2.7 vs. 51.3±2.9%, respectively; P<0.01) and in
the AECs (27.0±5.9 vs. 68.3±3.2%, respectively; P<0.01),
however, simvastatin alone did not affect the expression of VEGF.
The expression of VEGF was significantly higher in the SmSt group
compared with the Sm group in the SAECs (49.0±2.9 vs. 16.3±2.7%,
respectively; P<0.01) and the AECs (67.7±4.9 vs. 27.0±5.9%,
respectively; P<0.01). However, no significant differences were
observed in the expression of VEGF in the VECs between the SmSt and
Sm groups (6.3 ± .6 vs. 4.5±1.5%, respectively; P>0.05). These
findings are shown in Fig.
4A–C.
 | Figure 4Immunohistochemical staining for VEGF.
(A) Weak positive staining for VEGF was observed in the alveolar
epithelial cells of the Sm group. (B) Marked positive staining for
VEGF was observed in the alveolar epithelial cells of the SmSt
group. (C) Immunohistochemical staining for VEGF in the lung cells
from the four groups of rats. The percentages of VEGF-positive
SAEC, AEC and VEC in the Sm group were significantly lower than
those in the CtL group (**P<0.01). The expression
levels of VEGF in the SAEC and AEC in the SmSt group were similar
to those in the W group. There were significant difference sin the
expression of VEGF between the SmSt and Sm groups in the AEC and
SAEC (##P<0.01), but not the VEC. VEGF, vascular
endothelial growth factor; Sm. smoke exposed; St, simvastatin
treated; SmSt, smoke exposed ± simvastatin treated; SAEC, small
airway epithelial cells; VEC, vascular endothelial cells; AEC,
alveolar epithelial cells; CtL, control; PCNA, proliferating cell
nuclear antigen. |
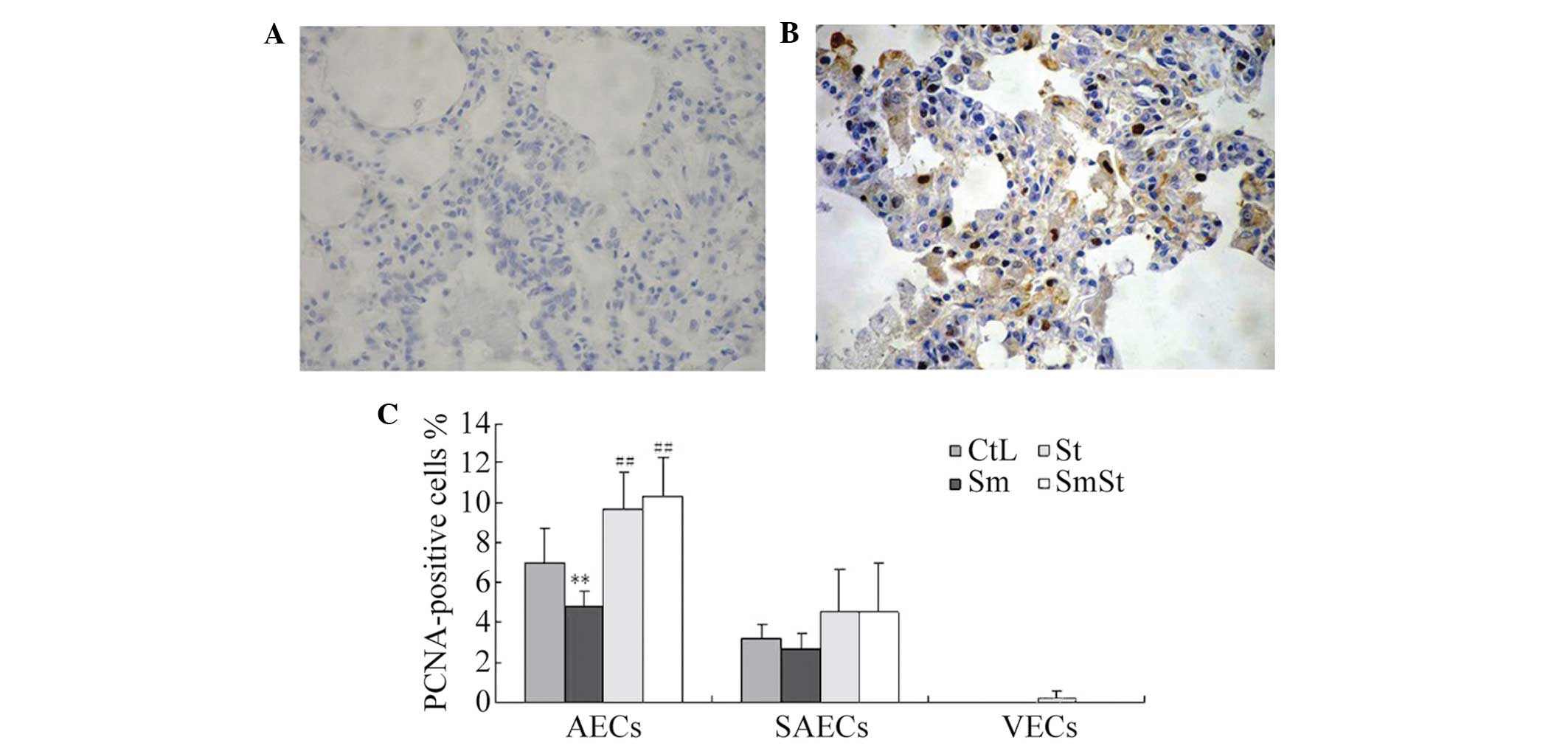
Increase of PCNA-positive AECs by
simvastatin
The percentage of PCNA-positive AECs was
significantly higher in the SmSt group compared with the Sm group
(10.3±1.9 vs. 4.8±0.8%, respectively; P<0.01) and CtL group
(7.0±1.7%, P<0.01). However, the expression of PCNA was lower in
the SAECs and VECs (Fig. 5A–C;
Table III).
 | Table IIIPositive expression of PCNA in rat
lung tissue cells. |
Table III
Positive expression of PCNA in rat
lung tissue cells.
| Group | AECs | SAECs | VECs |
|---|
| CtL | 7.00±1.67 | 3.17±0.75 | 0.00±0.00 |
| Sm | 4.83±0.75a | 2.67±0.82 | 0.00±0.00 |
| St | 9.67±1.86b | 4.50±2.17 | 0.17±0.41 |
| SmSt | 10.33±1.97b | 4.50±2.51 | 0.00±0.00 |
| F-statistic | 14.387 | 1.721 | 1.000 |
| P-value | <0.01 | <0.195 | <0.413 |
Discussion
The present study examined whether treatment with
simvastatin attenuates cigarette smoke-induced emphysema in rats by
analyzing the expression of VEGF and lung morphology. Observations
and epidemiological studies have demonstrated that statins may be
beneficial for the treatment of COPD (3,5,25).
Different pathological abnormalities, including emphysema and
chronic bronchitis, coexist in a number of patients with COPD, and
emphysema, loss of elastic recoil and intrinsic airway
abnormalities synergistically contribute to disease severity. There
is evidence that significant small-airway pathology not only exists
in patients with a chronically and radiographically documented
emphysematous phenotype of COPD, but it also affects the outcome
(26). Cigarette smoke causes
primary lymphocyte infiltration into the small-airways, whereas
simvastatin inhibits inflammatory infiltration around the bronchial
branches. Animal model systems represent useful methods to assess
the impact of cigarette smoke in patients with COPD (27). Emphysema is an anatomical lesion,
which has been the focus of the majority of animal models (27,28).
Therefore, the present study selected a rat model to investigate
the therapeutic effects of simvastatin on lung emphysema. The
production of alveolar ducts was observed in lung samples from the
model of smoke-induced emphysema; this change is anatomically
similar to a mild form of centrilobular emphysema, commonly
observed in cigarette smokers. The parenchyma between the dilated
alveolar ducts is typically abnormal, with increases in the size
and number of pores of Kohn, which connect adjacent alveoli
(28), and is also observed in the
lungs of human cigarette smokers. The lesions produced in
laboratory animals can be subtle and, therefore, morphometric
analysis, including MLI and MAN measurements is required to assess
the degree of damage.
Small-airway remodeling, including increased matrix
components, inflammatory-cell and goblet-cell metaplasia in the
airways, with luminal narrowing, mucus-associated distortion and
obstruction, is an important cause of airflow obstruction in human
smokers (4). The results of the
present study demonstrated that, following 16 weeks of cigarette
smoke exposure, airway pathological scores were higher in the Sm
group compared with those in the CtL group. These results suggested
that the COPD rat model was a successful, appropriate experimental
approach to for subsequent investigation. The results of the
present study also demonstrated that simvastatin attenuated
cigarette smoke-induced inflammatory infiltration, goblet-cell
metaplasia and smooth-muscle proliferation disorders in the airway
walls. The results of the present study are in accordance with
those of other studies. Murphy et al (29) reported the effects of simvastatin
on primary bronchial epithelial cells (PBECs) derived from stable
lung allografts, which demonstrated the ability of simvastatin to
attenuate airway neutrophilia, remodel mediators and inhibit their
upregulation in response to transforming growth factor and
interleukin-17. This finding and those of other studies have
demonstrated the potential of simvastatin in alleviating
neutrophilic airway inflammation and causing lung remodeling
(30–32).
Previous studies have identified the presence of
VEGF and its receptors in several cell types of a number of organs.
It has been reported that the expression levels of VEGF levels in
the lungs is the highest among normal tissues (33). The observations that increased
expression of VEGF or VEGF signaling causes experimental emphysema
and that the lungs of patients with COPD have decreased expression
levels of VEGF and VEGF receptor-2 (VEGFR) have led to the
suggestion that alveolar maintenance is required for structural
preservation of the lungs (34).
In the present study, the expression levels of VEGF in lung tissues
and BALF samples from the Sm group were lower than in the W group.
Simvastatin treatment of rats exposed to cigarette smoke
significantly increased the levels of VEGF, almost to the same
level as the CtL group (control group). The mRNA expression levels
of VEGF were similar in the lung tissues and BALF samples. In the
SmSt group, simvastatin increased the expression of VEGF in the
AECs compared with the Sm group. These results suggested that
simvastatin treatment may prevent the cigarette smoke-induced
decrease of VEGF in lung tissue and may upregulate the expression
of VEGF in the AECs.
Statins are involved in improving endothelial cell
function and promoting angiogenesis. They increase the production
of vasodilators, including nitric oxide and VEGF, and decrease the
production of vasoconstrictors, including endothelin-1, triggering
oxidative stress (35). Takahashi
et al (25) demonstrated
that the concentration gradient of VEGF between the lungs and the
circulatory system increases in response to simvastatin treatment
in an emphysema model. BALF consists of an airway epithelial-cell
lining fluid (ELF), which was diluted with saline in each rat. The
concentration of VEGF in the ELF is suggested to be higher than in
the plasma, since the measured BALF concentrations were similar to
those in the plasma.
Asahara et al (36) and Brown et al (37) demonstrated that VEGF induces the
mobilization of endothelial progenitor cells, and stimulation of
resident AECs. In the present study, treatment with 5 mg/kg
day−1 simvastatin induced epithelial cell proliferation.
Therefore, VEGF may directly and indirectly promote tissue-specific
proliferation of AECs.
The present study had several limitations. Firstly,
only the direct morphological impact of simvastatin on rats was
measure, and was not a mechanistic investigation. Another
limitation was the use of a dose of 5 mg/kg simvastatin and 16 week
time-period only. Therefore, it was not possible to investigate
whether there were dose- or time-dependent effects of simvastatin.
In addition, no physiological data regarding lung function were
assessed and no in vitro data were available. Statins may
exert pleiotropic effects in COPD via multiple pathways, and the
pathogenesis of COPD is complicated. It would, therefore, be
beneficial to investigate the mechanisms underlying statin
involvement with COPD, in order to understand their clinical
relevance and applicability.
In conclusion, the results of the present study
demonstrated that simvastatin exhibits a significant impact on the
expression of VEGF and attenuates cigarette smoke-induced emphysema
in rats. It was hypothesized that simvastatin, at least in part,
may exert beneficial effects in patients with COPD. Further
investigation into the mechanisms of statins is required in order
to improve the pathophysiology and to alleviate the symptoms of
COPD.
Acknowledgments
This study was supported by the National Scientific
Foundation of China (grant no. 2007BAI24804).
References
|
1
|
World Health Statistics. 2008, Geneva:
World Health Organization. Available from URL: http://www.who.int/whosis/whostat/2008/en/index.html.
|
|
2
|
Zhong N, Wang C, Yao W, Chen P, Kang J,
Huang S, Chen B, Wang C, Ni D, Zhou Y, et al: Prevalence of chronic
obstructive pulmonary disease in China: A large, population-based
survey. Am J Respir Crit Care Med. 176:753–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogg JC, Chu F, Utokaparch S, Woods R,
Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson
HO and Paré PD: The nature of small-airway obstruction in chronic
obstructive pulmonary disease. N Engl J Med. 350:2645–2653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hogg JC: Pathophysiology of airflow
limitation in chronic obstructive pulmonary disease. Lancet.
364:709–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mead J, Turner JM, Macklem PT and Little
JB: Significance of the relationship between lung recoil and
maximum expiratory flow. J Appl Physiol. 229:95–108. 1967.
|
|
6
|
Liebow AA: Pulmonary emphysema with
special reference to vascular changes. Am Rev Respir Dis. 80:67–93.
1959.PubMed/NCBI
|
|
7
|
Kasahara Y, Tuder RM,
Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK,
Waltenberger J and Voelkel NF: Inhibition of VEGF receptors causes
lung cell apoptosis and emphysema. J Clin Invest. 106:1311–1319.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki M, Betsuyaku T, Nagai K, Fuke S,
Nasuhara Y, Kaga K, Kondo S, Hamamura I, Hata J, Takahashi H and
Nishimura M: Decreased airway expression of vascular endothelial
growth factor in cigarette smoke-induced emphysema in mice and COPD
patients. Inhal Toxicol. 20:349–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Young RP, Hopkins R and Eaton TE:
Pharmacological actions of statins: Potential utility in COPD. Eur
Respir Rev. 18:222–232. 2009. View Article : Google Scholar
|
|
10
|
Janda S, Park K, FitzGerald JM, Etminan M
and Swiston J: Statins in COPD: A systematic review. Chest.
136:734–743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao JK and Laufs U: Pleiotropic effects
of statins. Annu Rev Pharmacol Toxicol. 45:89–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Veillard NR, Braunersreuther V, Arnaud C,
Burger F, Pelli G, Steffens S and Mach F: Simvastatin modulates
chemokine and chemokine receptor expression by geranylgeranyl
isoprenoid pathway in human endothelial cells and macrophages.
Atherosclerosis. 188:51–58. 2006. View Article : Google Scholar
|
|
13
|
Wolfrum S, Jensen KS and Liao JK:
Endothelium-dependent effects of statins. Arterioscler Thromb Vasc
Biol. 23:729–736. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frick M, Dulak J, Cisowski J, Józkowicz A,
Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O and
Weidinger F: Statins differentially regulate vascular endothelial
growth factor synthesis in endothelial and vascular smooth muscle
cells. Atherosclerosis. 170:229–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taraseviciene-Stewart L, Scerbavicius R,
Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M and Voelkel
NF: Simvastatin causes endothelial cell apoptosis and attenuates
severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol.
291:L668–L676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC
and Song IS: Simvastatin induces apoptosis in human colon cancer
cells and in tumor xenografts, and attenuates colitis-associated
colon cancer in mice. Int J Cancer. 123:951–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Punturieri A, Szabo E, Croxton TL, Shapiro
SD and Dubinett SM: Lung cancer and chronic obstructive pulmonary
disease:needs and opportunities for integrated research. J Natl
Cancer Inst. 101:554–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueda K, Jinbo M, Li TS, Yagi T, Suga K and
Hamano K: Computed tomography-diagnosed emphysema, not airway
obstruction, is associated with the prognostic outcome of
early-stage lung cancer. Clin Cancer Res. 12:6730–6736. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Liu X, Chen J, Zacharek A, Cui X,
Savant-Bhonsale S, Liu Z and Chopp M: Simvastatin enhances bone
marrow stromal cell differentiation into endothelial cells via
notch signaling pathway. Am J Physiol Cell Physiol. 296:C535–C543.
2009. View Article : Google Scholar :
|
|
20
|
Architectural and technical code for
laboratory animal facility (GB50447-2008): National Standard of the
People's Republic of China. China Architecture & Building
Press; Beijing, China: 2008
|
|
21
|
Lee JH, Lee DS, Kim EK, Choe KH, Oh YM,
Shim TS, Kim SE, Lee YS and Lee SD: Simvastatin inhibits cigarette
smoking-induced emphysema and pulmonary hypertension in rat lungs.
Am J Respir Crit Care Med. 172:987–993. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HY, Pang BS, Niu SJ, Ma L, Xin P and
Weng XZ: Expression of matrix metalproteinases in lung tissue and
levels of some cytokines in bronchoalveolar lavage fluid in the
obstructive emphysema rat models. Chin J Inter Med. 42:181–185.
2003.
|
|
23
|
Vernooy HJ, Dentener MA, van Suylen RJ,
Buurman WA and Wouters EF: Long-term intratracheal
lipopolysaccharide exposure in mice results in chronic lung
inflammation and persisient pathology. Am J Respir Cell Mol Biol.
26:152–159. 2002. View Article : Google Scholar
|
|
24
|
Kasagi S, Seyama K, Mori H, Souma S, Sato
T, Akiyoshi T, Suganuma H and Fukuchi Y: Tomato juice prevents
senescence-accelerated mouse P1 strain from developing emphysema
induced by chronic exposure to tobacco smoke. Am J Physiol Lung
Cell Mol Physiol. 290:L396–L404. 2006. View Article : Google Scholar
|
|
25
|
Takahashi S, Nakamura H, Seki M, Shiraishi
Y, Yamamoto M, Furuuchi M, et al: Reversal of elastase –induced
pulmonary emphysema and promotion of alveolar epithelial cell
proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol
Physiol. 294:L882–L890. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patel BJ, Marchetti N, Kim V, Gaughan JP
and Criner GJ: Airway wall thickness correlates with dynamic
hyperinflation in severe COPD. Proc Am Thorac Soc (abstract).
3:A7122006.
|
|
27
|
Wright JL, Cosio M and Churg A: Animal
models of chronic obstructive pulmonary disease. Am J Physiol Lung
Cell Mol Physiol. 295:L1–L15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wright JL: The importance of
ultramicroscopic emphysema in cigarette smoke-induced lung disease.
Lung. 179:71–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy DM, Forrest IA, Corris PA, Johnson
GE, Small T, Jones D, Fisher AJ, Egan JJ, Cawston TE, Ward C and
Lordan JL: Simvastatin attenuates release of neutrophilic and
remodeling factors from primary bronchial epithelial cells derived
from stable lung transplant recipients. Am J Physiol Lung Cell Mol
Physiol. 294:L592–L599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ou XM, Wen FQ, Uhal BD, Feng YL, Huang XY,
Wang T, Wang K, Liu DS, Wang X and Chen L: Simvastatin attenuates
experimental small airway remodelling in rats. Respirology.
14:734–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davis BB, Zeki AA, Bratt JM, Wang L,
Filosto S, Walby WF, Kenyon NJ, Goldkorn T, Schelegle ES and
Pinkerton KE: Simvastatin inhibits smoke-induced airway epithelial
injury: Implications for COPD therapy. Eur Respir J. 42:350–361.
2013. View Article : Google Scholar
|
|
32
|
Takeda N, Kondo M, Ito S, Ito Y, Shimokata
K and Kume H: Role of RhoA inactivation in reduced cell
proliferation of human airway smooth muscle by simvastatin. Am J
Respir Cell Mol Biol. 35:722–729. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaner RJ, Ladetto JV, Singh R, Fukuda N,
Matthay MA and Crystal RG: Lung overexpression of the vascular
endothelial growth factor gene induces pulmonary edema. Am J Respir
Cell Mol Biol. 22:657–664. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marwick JA, Stevenson CS, Giddings J,
MacNee W, Butler K, Rahman I and Kirkham PA: Cigarette smoke
disrupts VEGF165-VEGFR-2 receptor signaling complex in rat lungs
and patients with COPD: Morphological impact of VEGFR-2 inhibition.
Am J Physiol Lung Cell Mol Physiol. 290:L897–L908. 2006. View Article : Google Scholar
|
|
35
|
Bayorh MA, Ganafa AA, Eatman D, Walton M
and Feuerstein GZ: Simvastatin and losartan enhance nitric oxide
and reduce oxidative stress in salt-induced hypertension. Am J
Hypertens. 18:1496–1502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown KR, England KM, Goss KL, Snyder JM
and Acarregui MJ: VEGF induces airway epithelial cell proliferation
in human fetal lung in vitro. Am J Physiol Lung CellMol Physiol.
281:L1001–L1010. 2001.
|