Introduction
Gliomas are the most common primary tumors of the
central neural system (CNS), accounting for 31% of all CNS tumors
and 80% of malignant brain tumors in the United States (1). Depending on their severity, glial
tumors are graded from I (benign) to IV (highly malignant)
(2). In general, low-grade tumors
tend to evolve into high-grade glioblastoma multiforme. However,
irrespective of their grading, primary glial tumors almost
invariably display marked infiltrative growth characteristics, and
glioblastoma cells are able to move from the central part of the
tumor far into the surrounding normal brain tissue (3). Cytogenetic studies on glioma cell
lines have shown that they follow an evolutionary pattern in
vitro, having a tendency toward polyploidization and S
karyotype instability. However, these progressive changes appear to
not occur randomly (4). The
infiltrative nature of high-grade glioma is largely responsible the
morbidity and mortality associated with this tumor type (5). Surgical debulking of the tumor may
delay its growth but is inadequate for treatment, as
microscopically small infiltrated tumor foci lead to eventual
recurrence, often in areas that are surgically inaccessible
(6–8). Therefore, patients with high-grade
gliomas face a poor prognosis with <10% of patients surviving
for >2 years. This poor outcome emphasizes the requirement for
the enhancement of the understanding of the underlying mechanisms
of glioma invasion, as this may contribute to the recognition of
novel targets for the therapy of high-grade gliomas (9–11).
Glial tumors are characterized by high
proliferation, aggressive behavior and drug resistance. In spite of
the current therapeutic options, including surgery, chemotherapy,
radiation therapy or other novel modalities, the average survival
of patients still remains short. Thus, novel drugs are constantly
sought after to improve the treatment effects of patients with
malignant gliomas (12–14). Glial tumors are markedly aggressive
in a locally invasive fashion. The goal of surgical resection is to
remove the gadolinium contrast-enhancing component of the tumor,
given the potential morbidity of removing adjacent non-enhancing
normal neuronal tissue. However, following all cases of
glioblastoma resection, non-contrast-enhancing infiltrative tumor
cells remain, which are located away from the main neoplasm. These
invasive tumor cells are responsible for recurrence and,
ultimately, the demise of patients with glioblastoma, despite
radiation therapy and chemotherapy. Recently, glioblastoma stem
cells (GSCs), which are a sub-population of glioblastoma cells,
have been demonstrated to be integral to tumor growth and
perpetuation (9,15,16).
Anthracyclines are important anti-tumor agents used
in the treatment of solid tumors, lymphomas and acute lymphoblastic
as well as myelocytic leukemias. The clinical utility of agents,
including doxorubicin and daunorubicin, and their
well-characterized cardiotoxicity have prompted numerous efforts to
develop analogs that retain the desired spectrum of activity while
being less cardiotoxic (17).
Epirubicin (4′-epidoxorubicin) is an anti-neoplastic agent derived
from doxorubicin. The compounds differ in the configuration of the
hydroxyl group at the 4′ position. Epirubicin, like doxorubicin,
exerts its anti-tumor effects by interfering with the synthesis and
function of DNA and is most active during the S phase of the cell
cycle (18). It acts by
intercalating into DNA strands, resulting in complex formation,
which inhibits DNA and RNA syntheses. It also triggers DNA cleavage
by topoisomerase II, and the generation of oxygen free radicals,
resulting in the activation of mechanisms that lead to cell death.
These mechanisms are also implicated in the cardiac toxicity of
doxurubicin and other anthracyclines (19). Epirubicin has been clinically
applied in treating breast cancer, non-Hodgkin's lymphomas, ovarian
cancer, soft-tissue sarcomas, pancreatic cancer, gastric cancer,
small-cell lung cancer and acute leukemia. As compared to
doxorubicin, epirubicin shows less hematologic or myocardial
toxicity at comparable doses (20). Epirubicin is among the most active
single agents used in the management of patients with breast
cancer. The drug may be administered alone or in combination with
other agents in patients with early breast cancer and those with
metastatic disease (21).
The rapid infiltrative growth of malignant brain
glioma severely destroys normal brain tissue (22). A study has indicated that most
chemotherapeutic drugs are not active against glioblastoma due to
restricted access to the brain tumor site after systemic
administration. To achieve therapeutically effective drug levels in
the brain, significantly higher doses of these drugs require to be
administered, therefore resulting in severe local and systemic
toxicities (23). The use of
anthracyclines in the treatment of soft tissue sarcomas in adults
is well recognized and substantive. At the end of the 1980s, it was
first proven that high doses of anthracyclines, compared with
standard drugs, were able to yield improved response rates
(24), and they are therefore used
in modern clinical settings.
The aim of the present study was to verify that
epirubicin alters the growth, migration and morphological
characteristics of U-87 glioma cells, and to investigate the
underlying molecular mechanisms.
Materials and methods
Cell lines and culture conditions
The human glioma cell line U-87 was received from
the American Type Culture Collection (Manassas, VA, USA). The
primary neuronal cells were isolated as described previously
(25). The cells were cultured in
a standard tissue culture incubator (37°C; 5% CO2; 95%
air; 100% humidity). All tissue culture plastic dishes and flasks
were from Nunc (Roskilde, Denmark). Cells were regularly passaged
by treatment with trypsin (0.05%) and were grown in Eagle's minimum
essential medium (EMEM) supplemented with 10% fetal bovine serum
(FBS), penicillin and streptomycin, non-essential amino acids, 1
mg/ml glucose and 1 mM pyruvate (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA).
Cell viability assay
The U-87 and neuronal cells were plated on 96-well
flat-bottomed microplates at a density of 1×104
cells/well in 100 μl complete growth medium. Prior to drug
treatment, the growth medium was substituted with fresh medium
containing 2% FBS. The U-87 and neuronal cells were exposed to
epirubicin at concentrations of 0.5–100 μM. After 48 h of
incubation at 37°C in a humidified atmosphere of 5% CO2,
the cytotoxic effect of epirubicin was estimated using an MTT
assay. Briefly, the cells were incubated for 3 h with MTT solution
(5 mg/ml; Sigma-Aldrich, St Louis, MO, USA). MTT was metabolized by
viable cells to purple formazan crystals, which were solubilized
overnight in SDS buffer (10% SDS in 0.01 N HCl) and the reaction
product was quantified spectrophotometrically by measuring
absorbance at 570 nm using an Multiskan MK3 microplate reader
(Fisher Thermo Scientific, Waltham, MA, USA). The absorbance of the
control wells was set as 100% and the results were expressed as the
percentage of the control.
Bromodeoxyuridine (BRDU) cell
proliferation assay
DNA synthesis in proliferating cells was evaluated
by measuring Bromodeoxyuridine (BRDU) incorporation using a
commercial Cell Proliferation ELISA System (11669915001; Roche
Diagnostics, Basel Switzerland). The U-87 cells were seeded into
96-well microplates at a density of 1×104 cells/ml in a
proliferation medium containing 10% FBS. On the following day, the
medium was replaced and the cells were exposed to 1–50 μM
epirubicin for 48 h. Subsequently, the cells were incubated for 2 h
with a BRDU labeling solution containing 10 μM BRDU. The
assay was performed according to the manufacturer's instructions.
The optical density values were evaluated at 450 nm using an ELISA
reader. The culture medium alone was applied as a control for
non-specific binding.
Western blot analysis
The U-87 cells were grown in six-well plates
(3×105 cells/ml with 3 ml/well) in a medium containing
10% FBS for 24 h at 37°C. On the following day, the medium was
replaced with fresh 2% FBS-EMEM and the cells were treated with
epirubicin at concentrations of 1, 5 or 10 μM. After 24 h of
incubation, the cells were washed in cold phosphate-buffered saline
(PBS) and lysed in radioimmunoprecipitation assay buffer
[containing 50 μM Tris-HCl (pH 7.4), 150 mM NaCl, 1% sodium
deoxycholate, 0.1% SDS, 10 mM NaF, 1 mM sodium orthovanadate, 1 mM
EDTA, 1% Triton X-100 and protease inhibitor cocktail] at 4°C for
30 min. In parallel, the lysates were centrifuged at 10,000 ×g for
15 min at 4°C. Laemmli buffer was added to 50 mg sample and the
mixture was boiled for 5 min, followed by separation using 9%
SDS-PAGE. The proteins were transferred onto an Immobilon P
membrane (Merck, Darmstadt, Germany). After the transfer, the
membrane was blocked with blocking buffer (5% non-fat dried milk in
Tris-buffered saline/0.1% Tween 20) for 1 h at room temperature and
then examined with appropriate dilutions of primary antibodies from
the Cell Proliferation ELISA and VEGF Enzyme Immunoassay (KHG0111;
Strathmann Biotec, Hamburg, Germany) kits overnight at 4°C. The
membranes were washed three times for 10 min with PBS containing
0.05% Triton X-100 and incubated for 1 h with horseradish
peroxidase-labelled anti-mouse antibody. The membranes were
visualized using Western Blot Chemiluminescence Reagent (Amersham
Biosciences, Amersham, UK) using Kodak Biomax film (Kodak,
Rochester, NY, USA). The blots were re-probed with antibodies
against β-actin to ensure equal loading and transport of
proteins.
Determination of matrix metalloproteinase
(MMP)-9 and vascular endothelial growth factor (VEGF) concentration
by ELISA
MMP-9 levels in the conditioned media of the U-87
cells were determined using a commercial human MMP-9 immunoassay
kit (RayBiotec, Norcross, GA, USA), and VEGF protein released into
the conditioned medium was assessed using the VEGF Enzyme
Immunoassay kit. The U-87 cells (3×105 cells/ml) were
seeded in 24-well plates with 1 ml EMEM containing 10% FBS and
incubated for 24 h. The growth medium was then replaced with EMEM
containing 2% FBS and the U-87 cells were treated with epirubicin
(0.5, 2.5 or 5 μM). After 48 h of incubation, the culture
medium was collected, centrifuged and immediately frozen at −80°C
until quantification of MMP-9 or VEGF using the kits according to
the manufacturer's instructions.
Cell migration (wound healing) assay
Tumor cell migration was assessed using the wound
healing assay. Tumor cells were plated at 1×106 cells on
4-cm culture dishes (Nunc). The cell monolayer was scratched with a
pipette tip (P300) and the plates were rinsed twice with PBS to
wash off the dislodged cells. Next, epirubicin dilutions in fresh
medium (0.1–1 μM) supplemented with 2% FBS were applied and
the number of cells migrated into the wound area after 24 h was
estimated and compared to that in control cultures. Plates were
stained using the May-Grunwald-Giemsa method and observed using an
Olympus BX51 System microscope (Olympus, Tokyo, Japan). Results are
expressed as the percentage of the number of cells migrated into
the wound area compared with that in the control group.
Assessment of morphological changes and
cytoskeleton staining
The U-87 cells (5±104 cells/ml) were
grown in Leighton tubes containing rectangular glass coverslips for
24 h. The medium was replaced with fresh medium containing 2% FBS
and different concentrations of epirubicin (1, 5 or 10 μM),
and the cells were incubated for 48 h at 37°C. Control cells were
cultured in medium without epirubicin. After the treatment, the
cells were fixed and stained according to the standard
hematoxylin-eosin (Poly Scientific R&D Corp., Bayshore, NY,
USA) method. Observation was performed under the Olympus BX51
System microscope and the micrographs were processed using analySiS
software, version 5.0 (Soft Imaging System GmbH, Münster, Germany).
Part of the cell samples were fixed in a 3% formaldehyde solution
in PBS, (pH 7.4), for 30 min at room temperature, incubated with
0.5% Triton X-100 in PBS for 15 min at room temperature, blocked in
PBS containing 3% bovine serum albumin (BSA; Sigma-Aldrich) for 1 h
at room temperature and incubated with tetramethylrhodamine-labeled
phalloidin (0.5 mg/ml) in PBS for 1 h at room temperature in the
dark. Representative fluorescence micrographs of these cells were
captured using the Olympus BX51 System microscope.
Statistical analysis
Values are expressed as the mean ± standard error.
Statistical analyses were performed using GraphPAD Prism 5
(GraphPAD Software Inc., La Jolla, CA, USA). The data were analyzed
by one-way analysis of variance, followed by Dunnett's test.
P<0.01 was considered to indicate a statistically significant
difference between values.
Results
Selective cytotoxic and
anti-proliferative effects of epirubicin
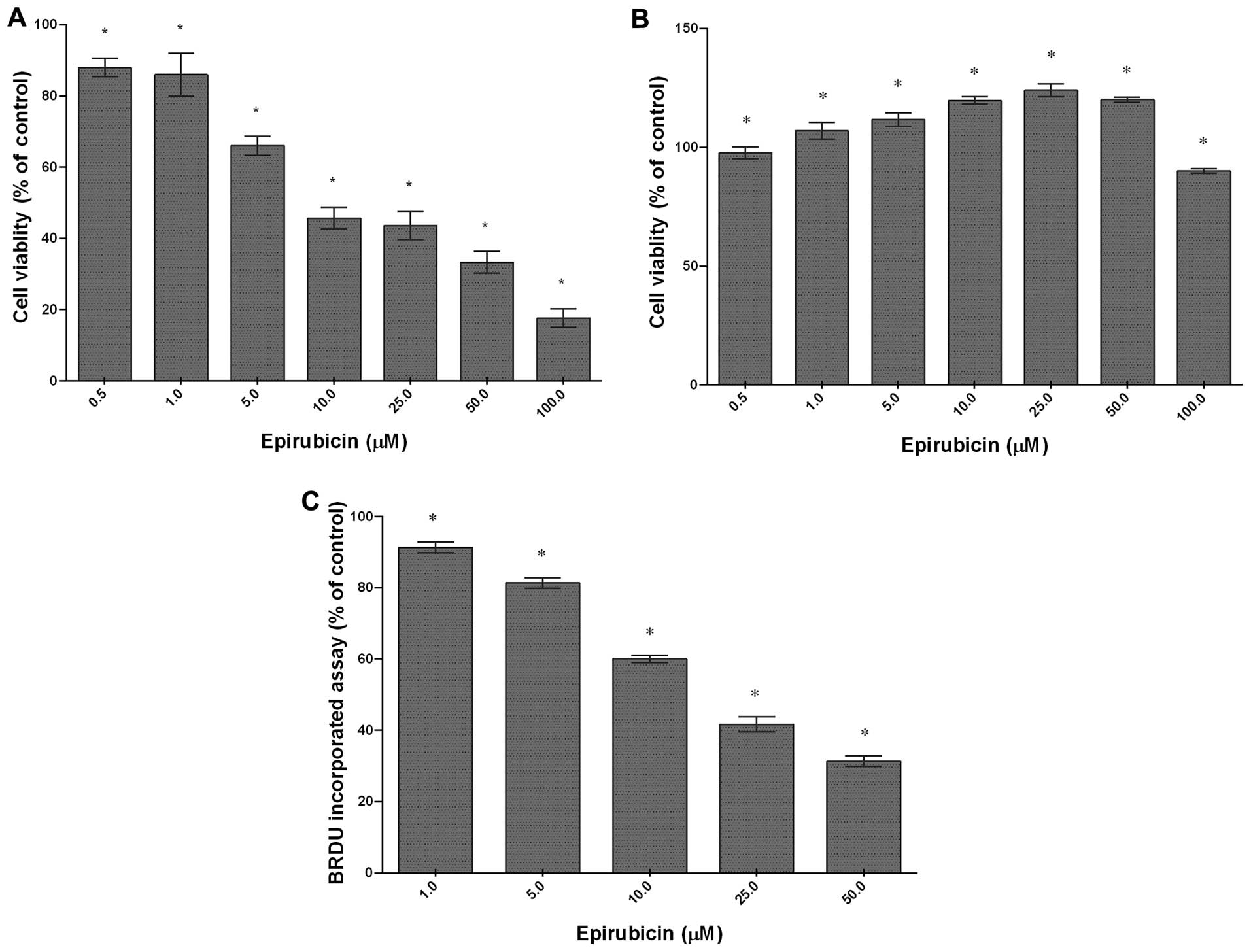
The cytotoxic effect of epirubicin was determined in
the U-87 glioma cells and neuronal primary culture by using an MTT
cell viability assay. Epirubicin significantly decreased the
viability of rat U-87 cells in a concentration-dependent manner
with an IC50 of 6.3 μM (Fig. 1A). At the highest concentration
used (100 μM), epirubicin reduced cell viability to
approximately 14% in comparison to that of the control. In contrast
to the U-87 glioma cells, the rat normal neuronal cells were
resistant to epirubicin. When they were incubated with epirubicin
at 1–50 μM, the number of live cells in the cultures was
markedly higher compared with that of untreated cells (Fig. 1B). These results clearly indicate
selective, dose-dependent cytotoxicity of epirubicin against tumor
cells. To examine the anti-proliferative potential of epirubicin,
the U-87 glioma cells were treated with 1, 5, 10, 25 or 50
μM epirubicin for 48 h and the BRDU assay was performed.
Epirubicin effectively reduced BRDU incorporation during DNA
synthesis (Fig. 1C). A significant
decrease in cell division was found even at 1 μM. The most
pronounced effect was observed following treatment with 50
μM epirubicin, which suppressed proliferation of the U-87
cells by 70%. These results were in agreement with the results of
the MTT cell viability assay and indicated a dose-dependent
anti-proliferative activity of epirubicin in the cells
examined.
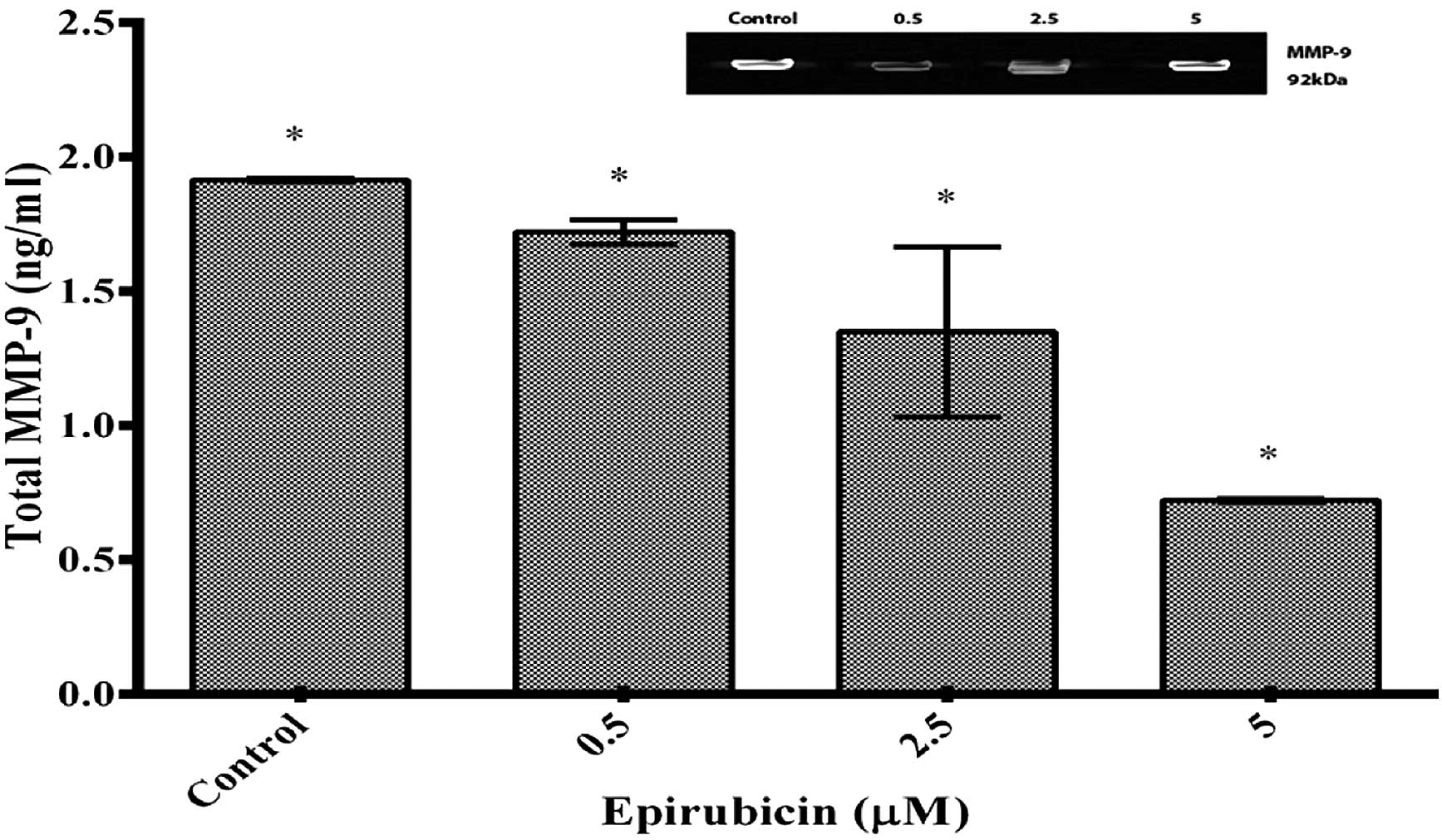
Epirubicin decreases MMP-9 secretion by
U-87 cells
The most pronounced inhibitory effect on MMP-9
secretion was detected at the epirubicin concentration of 5
μM according to the ELISA assay. As shown in Fig. 2, epirubicin reduced the capacity of
the U-87 cells to secrete total MMP-9 after 48-h incubation in a
dose-dependent manner. The amount of MMP-9 released into the
culture medium after the treatment with epirubicin at the
concentrations of 2.5 and 5 mM was reduced by 1.43±2.1 and 0.7±1.3
ng/ml, respectively, compared with that in the control.
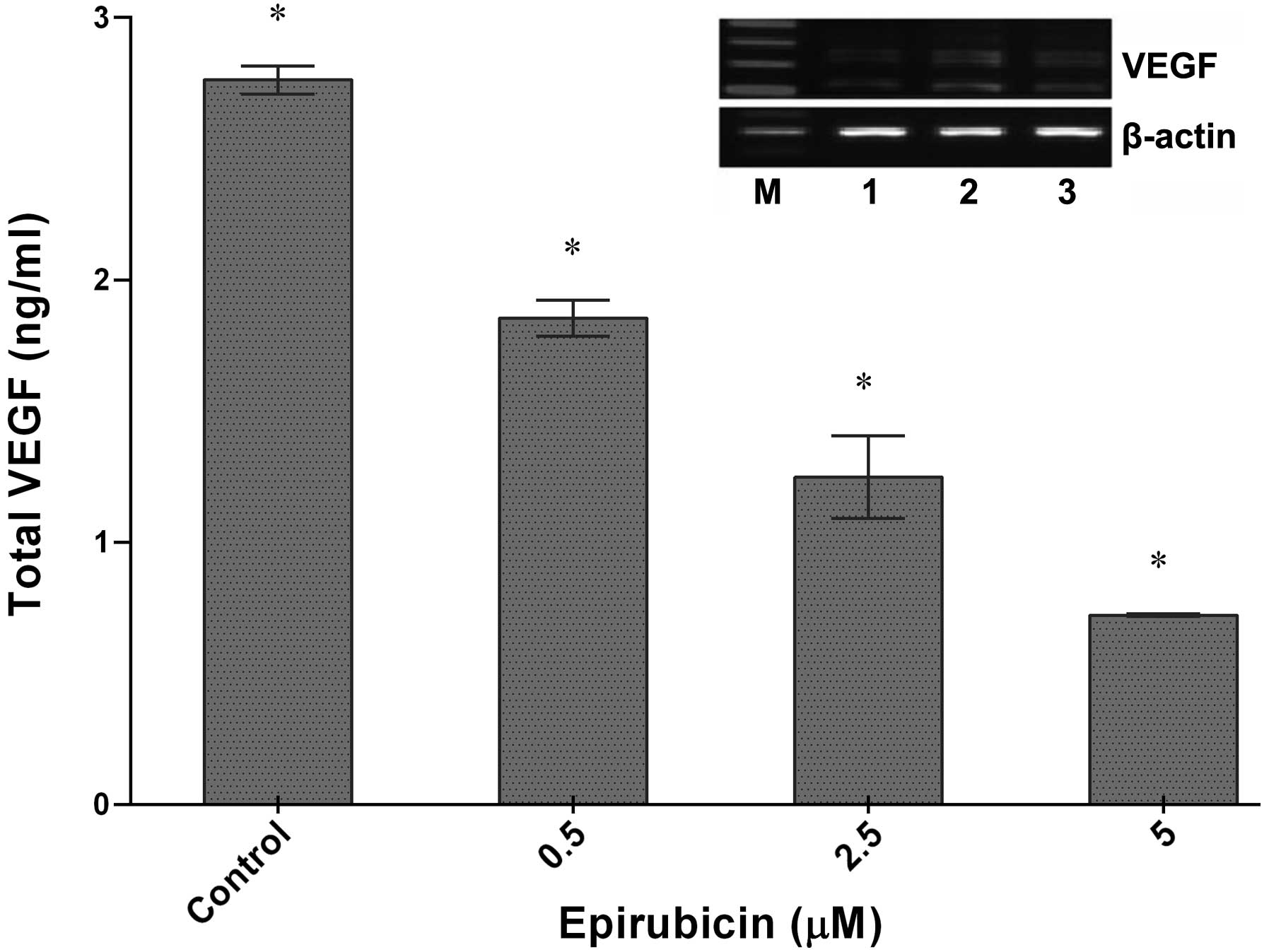
Epirubicin markedly reduces secretion of
VEGF by U-87 glioma cells
The effect of epirubicin on VEGF release into the
U-87 culture medium was quantified by ELISA. There is a demand for
novel chemotherapeutics targeting VEGF and they are promising to be
potent (26), as VEGFs have a
significant role in angiogenesis and lymphangiogenesis (27). As indicated in Fig. 3, VEGF secretion of U-87 cells was
markedly decreased following treatment of the cells with increasing
epirubicin concentrations. After 48 h of incubation of the U-87
cells with epirubicin, the VEGF levels were diminished from 2.56
ng/ml (untreated cells) to 1.22 ng/ml and 0.80 ng/ml at 2.5
μM and 5 μM epirubicin, respectively.
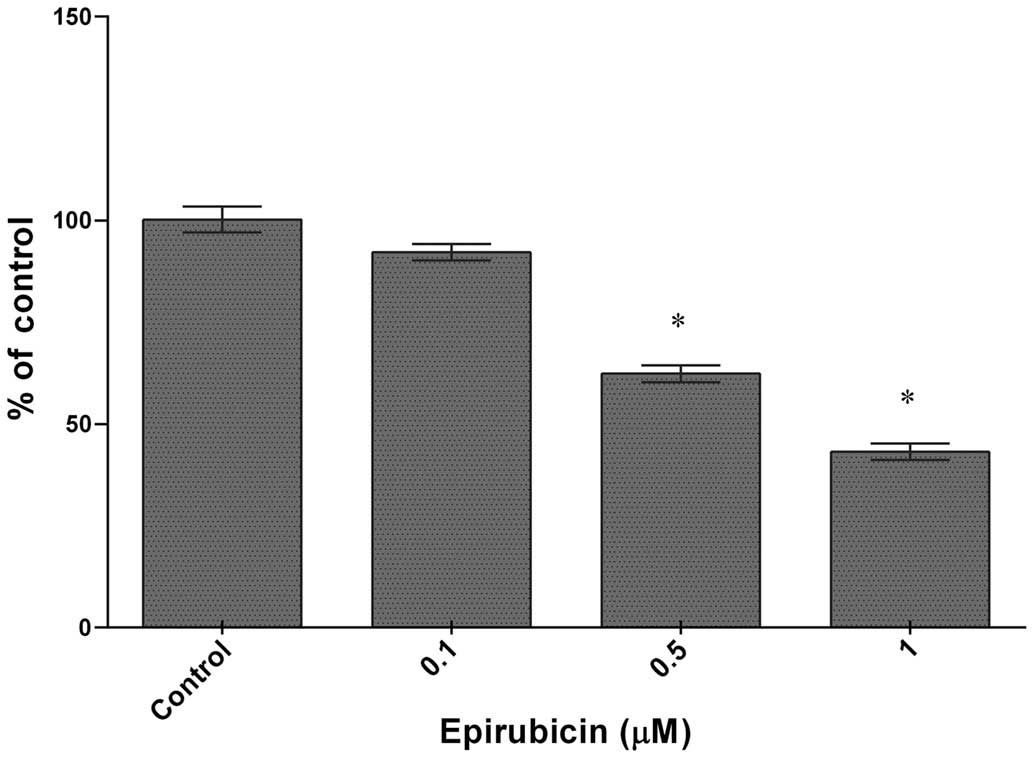
Epirubicin inhibits the migration of U-87
cells
The influence of epirubicin on cell migration was
estimated using a wound healing assay. As shown in Fig. 4, epirubicin caused a significant
decrease in cell migration ranging from 28% at 0.1 μM to
~59% when the cells were exposed to 1 μM of epirubicin
(IC50=0.5 μM).
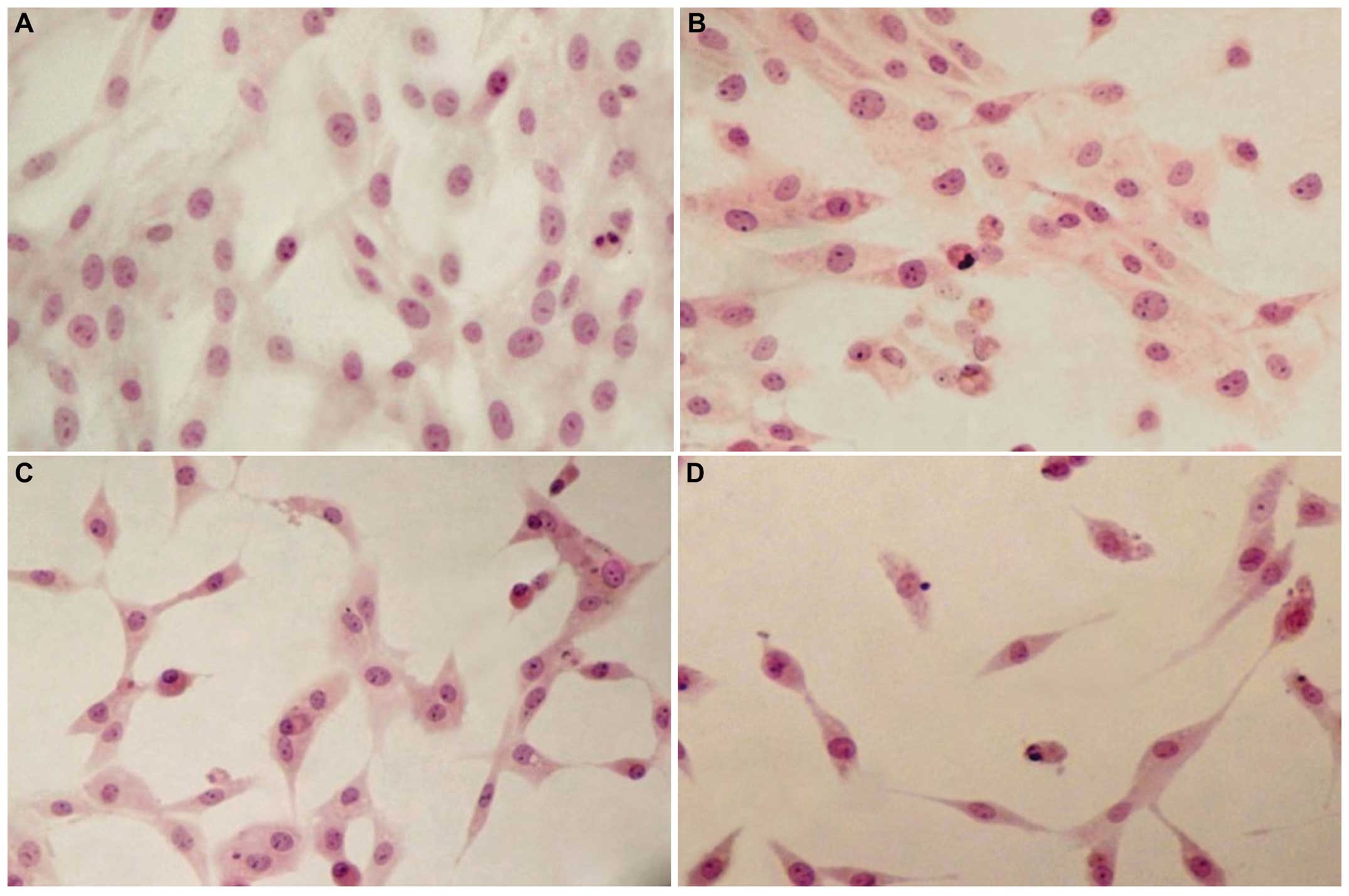
Epirubicin alters the morphology and the
cytoskeletal assemblyof U-87 cells
To assess the effect of epirubicin on cell
morphology, the treated cells were stained with hematoxylin-eosin
and observed under a light microscope. The morphological study
revealed that the control cells had a fibroblastic or
dendritic-like morphology with abundant cytoplasm and presented
extensive prolongations. Their nuclei were round with a vast number
of nucleoli (Fig. 5A). The
untreated cells also demonstrated a high proliferative activity, as
a number of them underwent mitotic division. The cells exposed to 1
or 5 μM epirubicin for 48 h showed typical features of late
apoptosis, including shrinkage and chromatin condensation (Fig. 5B and C). The most marked
morphological alterations were noted at a concentration of 10
μM epirubicin (Fig. 5D). In
this group, the cells were rounded and shrunken, with irregular and
pycnotic nuclei and a reduced length of cytoplasmic protrusions. In
this group, the number of vacuolized, non-proliferating and
detached cells was also markedly higher in comparison to those in
the groups exposed to the lower dosages of epirubicin. These
findings suggested that epirubicin effectively induced apoptosis in
glioma cells.
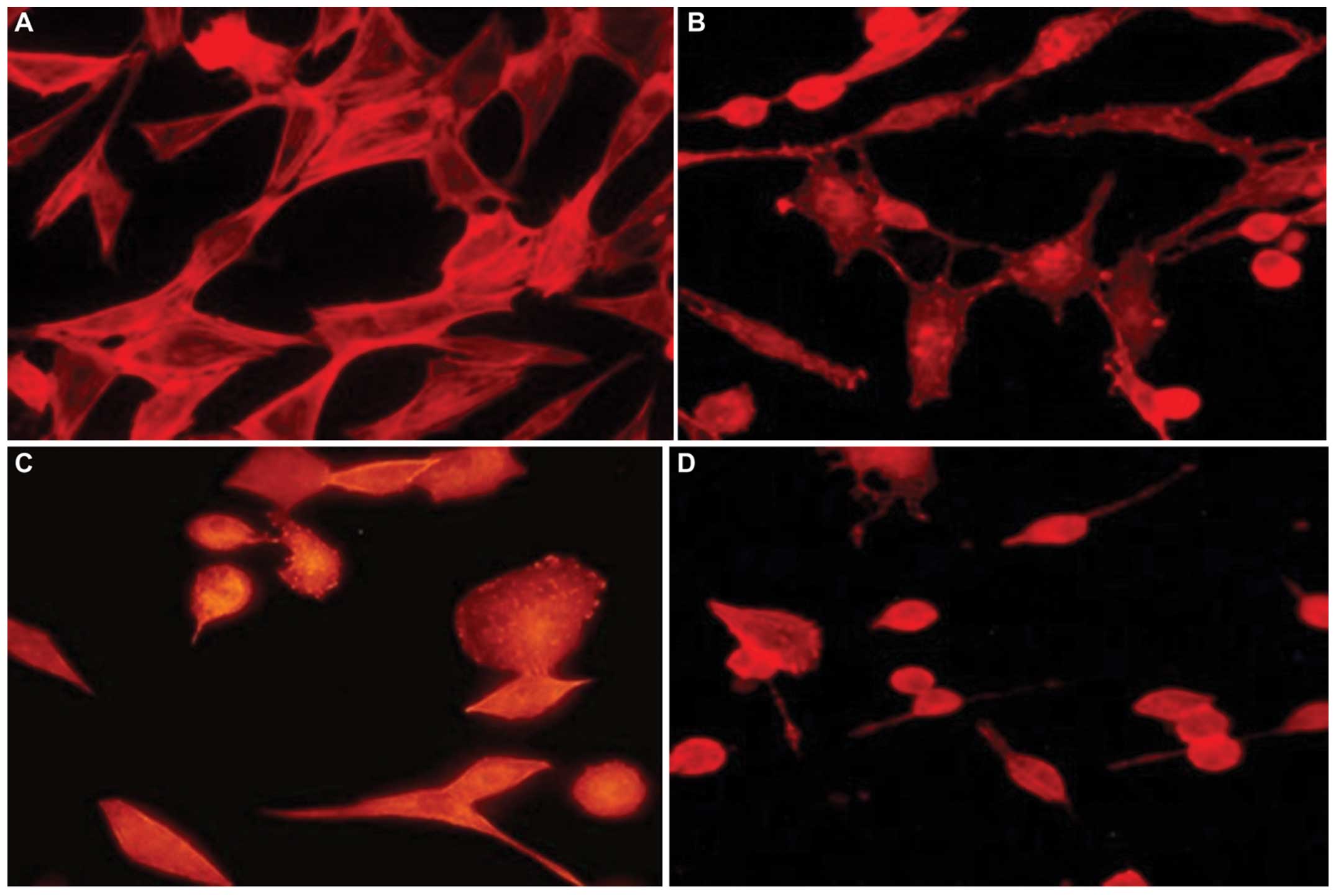
In order to study the constitution of the actin
cytoskeleton, the control and treated cells were stained using
rhodamine-labelled phalloidin and visualized under a fluorescence
microscope. The control cells showed highly organized, dense actin
structures extending from the cell surface throughout the cytosol
and along broad lamellipodia (Fig.
6A). Direct observation of the U-87 cells exposed to epirubicin
for 48 h revealed dose-dependent alterations in actin cytoskeletal
organization (Fig. 6B–D).
Anthracycline treatment resulted in rapid F-actin depolymerization
in the cell body, loss of bundles of actin filaments and stress
fibers, as well as suppressed formation of long cellular
protrusions. The epirubicin-induced cytoskeletal disruption may
have contributed to the structural changes described above.
Discussion
Glioblastoma is the most frequent (65%) and most
malignant histological type of CNS tumor in the USA (26). Anthracycline treatment is
associated with an increased risk of dose-dependent heart failure,
which limits their clinical application. Epirubicin (EPI) has been
demonstrated to inhibit tumor cell growth in numerous tumor cell
lines and is currently applied to treat solid and hematologic
malignancies, including breast cancer (28,29).
A study has reported the efficacy of epirubicin in vitro in
the C6 glioma cell line (30). In
a study by Cantoni et al (31), exposure of a human tumor cell line
(HeLa) to epirubicin and doxorubicin for either 1 h or 24 h at a
scope of concentrations resulted in greater cytotoxicity of
epirubicin over doxorubicin at the 1-h exposure, while the toxicity
of the two anthracyclines was similar following 24 h of incubation.
The authors linked this to the greater uptake rate of epirubicin
and the earlier achievement of cytotoxic levels within the cells,
while not excluding other, additional, mechanisms.
The present study showed that epirubicin markedly
diminished U-87 cell viability following 48-h exposure. Of note,
epirubicin did not kill normal neuronal cells in culture and even
increased the activity of mitochondrial dehydrogenase at high
concentrations, as indicated by increased rates of formazan
production in the MTT assay. Additional investigations are required
in order to fully elucidate the mechanisms by which statins mediate
distinct effects on neuronal cells. The results of the present
study are in line with the results reported by Hershman and Shao
(32) as well as O'Shaughnessy
et al (33), who reported
that anthracyclines decreased cellular proliferation. Cytostatic
effects of anthracycline antibiotics resulted from their binding to
nucleic acids, which contributes to the inhibition of protein
synthesis in cells as reported earlier by Vitvitsky (34). Anthracycline drugs that act as
topoisomerase-II inhibitors induce DNA damage in cells. The quinone
group of anthracyclines may undergo a one-electron reduction to
yield a semiquinone, thus producing free radicals and contributing
to cancer formation (35).
Malignant cell transformation results in a loss of tyrosine kinase
(TK) regulation with spontaneously increased TK activity, even in
the absence of external stimuli, resulting in uncontrolled cell
reproduction, which is encountered in nearly 70% of tumors
(36). Finally, TKs are also
involved in tumor angiogenesis, as their stimulation by VEGF,
platelet-derived growth factor and transforming growth factor-α
promote tumor growth-associated neovascularization. Epidermal
growth factor receptor is present in multiple tumor types and
enables cancer cell proliferation, invasion and migration (37,38).
It has been recognized that anthracyclines also
exert anti-cancer activities through inhibition of factors driving
characteristic hallmarks of malignant cells, including fast
proliferation, enhanced locomotion, aggressiveness, angiogenesis
and dissemination to various sites in the body (39). Thus, the present study assessed
which aspects of tumor invasion may be affected by epirubicin.
Addition of epirubicin to the culture medium impaired the mobility
of U-87 cells as determined by the wound-healing assay. The
inhibitory effect was apparent at low concentrations, such as 6.3
μM epirubicin. The processes of cell invasion and metastasis
are mediated not only by MMPs, but also by other molecules,
including VEGF. VEGF is the most important stimulator of
angiogenesis in numerous types of cancer (40,41).
In conclusion, the present study indicated that
epirubicin showed anti-proliferative and anti-metastatic activity.
The findings further indicated that the VEGF may be an appropriate
target to suppress invasive behavior and progression of brain tumor
cells as demonstrated using the U-87 cell line. The activity of
epirubicin against glioma cells was associated with the alteration
of motility and suppression of the secretion of VEGF and MMP-9.
These findings indicated that epirubicin is a potential therapeutic
agent for the treatment of gliomas; however, further studies are
required.
References
|
1
|
García-Claver A, Lorente M, Mur P, et al:
Gene expression changes associated with erlotinib response in
glioma cell lines. Eur J Cancer. 49:1641–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleihues P, Louis DN, Scheithauer BW, et
al: The WHO classification of tumors of the nervous system. J
Neuropathol Exp Neurol. 61:215–225. 2002.PubMed/NCBI
|
|
3
|
de Vries NA, Beijnen JH, Boogerd W and van
Tellingen O: Blood-brain barrier and chemotherapeutic treatment of
brain tumors. Expert Rev Neurother. 6:1199–1209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mark J, Westermark B, Pontén J and
Hugosson R: Banding patterns in human glioma cell lines. Hereditas.
87:243–260. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Couldwell WT, de Tribolet N, Antel JP,
Gauthier T and Kuppner MC: Adhesion molecules and malignant
gliomas: implications for tumorigenesis. J Neurosurg. 76:782–791.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khong A, Cleaver AL, Alatas MF, Wylie BC,
Connor T, Fisher SA, Broomfield S, Lesterhuis WJ, Currie AJ and
Lake RA: The efficacy of tumor debulking surgery is improved by
adjuvant immunotherapy using imiquimod and anti-CD40. BMC cancer.
14:9692014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao LC, Juan WS, Chang CC, Tai SH, Chuang
MT, Sze CI and Lee EJ: Surgical debulking plus adjuvant
chemoradiotherapy of a huge basal ganglion nongerminomatous germ
cell tumor with long term survival. Int J Pediatr. 2:312014.
|
|
8
|
Bush S, Williams H, Aksu C, Rungruang B,
Macfee M and Ghamande S: PET probe-assisted surgical debulking in
patients with recurrent gynecologic tumors. Gynecol Oncol.
130:e111–e112. 2013. View Article : Google Scholar
|
|
9
|
Hochberg FH and Pruitt A: Assumptions in
the radiotherapy of glioblastoma. Neurol. 30:907–911. 1980.
View Article : Google Scholar
|
|
10
|
Berens ME, Rutka JT and Rosenblum ML:
Brain tumor epidemiology, growth and invasion. Neurosurg Clin N Am.
1:1–18. 1990.PubMed/NCBI
|
|
11
|
Cho KK, Mikkelsen T, Lee YJ, et al: The
role of protein kinase Calpha in U-87 glioma invasion. Int J Dev
Neurosci. 17:447–461. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demuth T and Berens ME: Molecular
mechanisms of glioma cell migration and invasion. J Neurooncol.
70:217–228. 2004. View Article : Google Scholar
|
|
13
|
Holland EC: Glioblastoma multiforme: the
terminator. Proc Natl Acad Sci USA. 97:6242–6254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slawińska-Brych A, Zdzisińska B and
Kandefer-Szerszeń M: Fluvastatin inhibits growth and alters the
malignant phenotype of the C6 glioma cell line. Pharmacol Rep.
66:121–129. 2014. View Article : Google Scholar
|
|
15
|
Hadjipanayis CG and Van Meir EG: Brain
cancer propagating cells: biology, genetics and targeted therapies.
Trends Mol Med. 15:519–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hentschel SJ and Lang FF: Current surgical
management of glioblastoma. Cancer J. 9:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuffel MJ, Reid JM and Ames MM:
Anthracyclines and their C-13 alcohol metabolites: growth
inhibition and DNA damage following incubation with human tumor
cells in culture. Cancer Chemother Pharmacol. 30:51–57. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cersosimo RJ and Hong WK: Epirubicin: a
review of the pharmacology, clinical activity and adverse effects
of an adriamycin analogue. J Clin Oncol. 4:425–439. 1986.PubMed/NCBI
|
|
19
|
Bonadonna G, Gianni L, Santoro A, Bonfante
V, Bidoli P, Casali P, Demicheli R and Valagussa P: Drugs ten years
later: epirubicin. Ann Oncol. 4:359–369. 1993.PubMed/NCBI
|
|
20
|
Birtle AJ: Anthracyclines and
cardiotoxicity. Clin Oncol. 12:146–152. 2000.
|
|
21
|
Coukell AJ and Faulds D: Epirubicin. An
updated review of its pharmacodynamic and pharmacokinetic
properties and therapeutic efficacy in the management of breast
cancer. Drugs. 53:453–482. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Astner ST, Pihusch R, Nieder C, Rachinger
W, Lohner H, Tonn JC, Molls M and Grosu AL: Extensive local and
systemic therapy in extraneural metastasized glioblastoma
multiforme. Anticancer Res. 26:4917–4920. 2006.
|
|
23
|
Dhanikula RS, Argaw A, Bouchard JF and
Hildgen P: Methotrexate loaded polyether-copolyester dendrimers for
the treatment of gliomas: enhanced efficacy and intratumoral
transport capability. Mol Pharm. 5:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parney IF and Chang SM: Current
chemotherapy for glioblastoma. Cancer J. 9:149–156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reynolds B and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirano A, Shimizu T, Imamura H, et al: The
combination of epirubicin plus docetaxel as neoadjuvant
chemotherapy in locally-advanced breast cancer. Anticancer Res.
26:581–584. 2006.PubMed/NCBI
|
|
29
|
Tokudome N and Ito Y: Adjuvant
chemotherapy based on evidence-based medicine for breast cancer
patients. Japan J Cancer Chemother. 33:318–323. 2006.In
Japanese.
|
|
30
|
Schott B and Robert J: Comparative
activity of anthracycline 13-dihydrometabolites against rat
glioblastoma cells in culture. Biochem Pharmacol. 38:4069–4074.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cantoni O, Sestili P, Cattabeni F, Geroni
C and Giuliani F: Comparative effects of doxorubicin and
4′-epi-doxorubicin on nucleic acid metabolism and cytotoxicity in a
human tumor cell line. Cancer Chemo Pharmacol. 27:47–5. 1990.
View Article : Google Scholar
|
|
32
|
Hershman DL and Shao T: Anthracycline
cardiotoxicity after breast cancer treatment. Oncology (Williston
Park). 23:227–234. 2009.
|
|
33
|
O'Shaughnessy J, Twelves C and Aapro M:
Treatment for anthracycline-pretreated metastatic breast cancer.
Oncologist. 7(Suppl 6): 4–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vitvitsky VM: Erythrocytes as carriers of
anthracycline antibiotics in vitro and in vivo. erythrocyte
engineering for drug delivery and targeting. pp. 992002
|
|
35
|
Salvatorelli E, Guarnieri S, Menna P, et
al: Defective one- or two-electron reduction of the anticancer
anthracycline epirubicin in human heart. Relative importance of
vesicular sequestration and impaired efficiency of electron
addition. J Biol Chem. 281:10990–11001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dulak J and Józkowicz A: Regulation of
vascular endothelial growth factor synthesis by nitric oxide: facts
and controversies. Antioxidants and redox signaling. 5:123–132.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gerber DE: Targeted therapies: a new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
38
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hortobágyi G: Anthracyclines in the
treatment of cancer. An overview Drugs. 54(Suppl 4): 1–7. 1997.
|
|
40
|
Shibata T, Tamura M, Kabashima N, et al:
Fluvastatin attenuates IGF-1-induced ERK1/2 activation and cell
proliferation by mevalonic acid depletion in human mesangial cells.
Life Sci. 84:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takenaka M, Hirade K, Tanabe K, et al:
Simvastatin stimulates VEGF release via p44/p42 MAP kinase in
vascular smooth muscle cells. Biochem Biophys Res Commun.
301:198–203. 2003. View Article : Google Scholar : PubMed/NCBI
|