Introduction
Stroke is a common cause of disability and is the
second leading cause of mortality in adults worldwide (1). One of the common symptoms of stroke
is spasticity, which usually occurs within the first few days or
weeks (2). The presence of
spasticity one year after stroke has been reported in ≤38% of
patients (3,4). Spasticity is associated with pain,
ankylosis, tendon retraction and muscle weakness in patients, all
of which may limit rehabilitation (5,6).
Furthermore, spasticity interferes with the functional recovery of
patients, and may eventually negatively affect the quality of life
of stroke survivors (7).
Therefore, it is essential to identify effective therapeutic
approaches to treat spasticity, and to explore the mechanisms
underlying spasticity, which are currently not well understood.
The possible pathogenesis of stroke-induced
spasticity may be due to an excess of excitatory neurotransmitters,
and a shortage of inhibitory neurotransmitters (8–10).
This imbalance may be mediated by the excitatory neurotransmitter
metabotropic glutamate receptor 1 (mGluR1) and the inhibitory
neurotransmitter γ-amino butyric acid B (γ-GABAB)
receptor (R) (11). The
GABAB R is composed of two subunits: B1 and B2
(GABAB1 and GABAB2) (12), which have an important role in
post-stroke spasticity (13). It
has previously been shown that GABAB R expression is
reduced in the brain stems of rats with post-stroke spasticity
(8), thus indicating that the
GABAB R is closely associated with stroke-induced
spasticity. Furthermore, activation of GABAB R with
baclofen, a GABA agonist, is able to effectively inhibit the muscle
stretch reflex (14), suggesting
that either restoring or preserving normal GABAB R
expression levels may be a potential approach to counteract
stroke-induced spasticity. It has been hypothesized that various
types of intervention, which are able to increase the expression of
GABAB R, may relieve the symptoms of post-stroke
spasticity.
Numerous pharmacological agents have been used to
treat post-stroke spasticity, including baclofen, benzodiazepines
and botulinum toxin (15–16). However, a number of these
therapeutic drugs exhibit considerable side effects, including
muscle weakness, hepatotoxicity, drowsiness and ataxia (15,17,18).
Therefore, there has recently been an increased focus on the use of
natural products, such as traditional Chinese medicines (TCM),
which have relatively low toxicity and fewer side effects, as
compared with modern therapeutics. The classic TCM formula Gua Lou
Gui Zhi decoction (GLGZD) was initially documented in 'Jin Gui Yao
Lue', by Zhongjing Zhang during the Eastern Han Dynasty (~25–220
AD). GLGZD consists of six herbs: Trichosanthes kirilowii
Maxim, Paeonia lactiflora Pall, Cinnamomum cassia
Presl, Glycyrrhiza uralensis Fisch, Zingiber
officinale Rosc and Ziziphus jujuba Mill. As a
well-known TCM compound, it has long been used to treat
stroke-induced spasticity, epilepsy and spinal cord injury
(19–21). The results of previous clinical
experiments have demonstrated that GLGZD exerts a significant
therapeutic effect on post-stroke disabilities, including muscular
spasticity, by improving Fugl-Meyer and Barthel index scores
(22). However, the precise
mechanisms underlying the therapeutic effects of GLGZD remain
largely unknown. In our previous study, GLGZD was shown to exert
neuroprotective and antispastic effects in a cerebral ischemia
model, via modulation of glutamate levels, and AMPA and NMDA
glutamate receptors (23,24). The GABAB R controls
neuronal excitability and glutamate receptor activity, which are
associated with post-stroke spasms (25–27).
The present study used a focal cerebral ischemia/reperfusion (I/R)
injury rat model, which resembles human ischemic stroke, to
evaluate the therapeutic efficacy of GLGZD against spasticity
following cerebral ischemia. In addition, the underlying molecular
mechanisms were investigated, which were hypothesized to be
associated with the GABAB R.
Materials and methods
Reagents
Rabbit anti-rat polyclonal anti-GABAB1 R
(cat. no. 3835; dilution, 1:1,000), rabbit anti-rat polyclonal
anti-GABAB2 R (cat. no. 3839; dilution, 1:1,000), rabbit
anti-rat polyclonal anti-β-actin (cat. no. 4967; dilution,
1:1,000), and goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. no. 7074; dilution,
1:1,000) were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). All other chemicals used in the present study,
unless otherwise stated, were obtained from Sigma-Aldrich (St.
Louis, MO, USA).
Preparation of water extract of
GLGZD
GLGZD consists of six drugs: Trichosanthes
kirilowii Maxim, Paeonia lactiflora Pall, Cinnamomum
cassia Presl, Glycyrrhiza uralensis Fisch, Zingiber
officinale Rosc and Ziziphus jujuba Mill, in a weight
ratio of 10:3:3:2:3:3. Dried crude drugs were purchased from Guo Yi
Tang Chinese Herbal Medicine store (Fujian, China), and the drug
mixture was soaked in double distilled water for 30 min.
Subsequently, the mixture was decocted twice by boiling (1 h each
time). The filtered solution from the two decoctions was then
concentrated using a rotary evaporator (Model RE-2000; Yarong
Biochemistry Instrument Factory, Shanghai, China), in order to
obtain a final concentration of 1.06 g/ml. The GLGZD was
subsequently stored for further analysis.
Animals
Male Sprague-Dawley rats (n=27; initial body weight,
240–280 g) were purchased from Shanghai Laboratory Animal Center
(Shanghai, China), and housed in a temperature- and
humidity-controlled room. The rats underwent a 12 h light/dark
cycle and were provided access to food and water ad libitum.
All animal treatments and experiments were approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
Focal cerebral I/R model and experimental
groups
The cerebral I/R model was established following
middle cerebral artery occlusion (MCAO), as described previously
(28). Briefly, prior to surgery
the rats were fasted for 24 h; during this time the rats were still
provided ad libitum access to water. Following anesthesia
with 10% chloral hydrate by intraperitoneal injection (300 mg/kg),
the left common carotid artery, left external carotid artery (ECA),
and internal carotid artery (ICA) were exposed and isolated by a
midline neck incision. Nylon surgical thread (18–22 mm) was
inserted into the left ICA until the blunted distal end met
resistance, in order to block the middle cerebral artery (MCA). A
total of 2 h after occlusion, reperfusion was achieved by removing
the thread to restore blood supply to the MCA. The rectal
temperature of the rats was maintained at 37°C throughout the
surgical procedure.
The rats were randomly divided into the following
three groups: i) Sham-operated control (SC) group (n=9), which
underwent neck dissection and the exposure of ICA and ECA, without
MCAO; ii) ischemia control (IC) group (n=9), which underwent MCAO
surgery followed by reperfusion 2 h after occlusion; and iii) GLGZD
group (n=9), which underwent MCAO surgery followed by reperfusion 2
h after occlusion, and received GLGZD (14.3 g/kg body weight) by
intragastric administration once a day for a period of 7 days, as
soon as the rats had recovered from reperfusion.
Scoring of neurological defects
A total of 2 h after induction of cerebral ischemia,
and for the following 7 days, neurological defects of the rats were
scored every other day. The neurological defects were scored by two
researchers, who were blinded to the treatment conditions,
according to a standard scoring system on a four-point scale
(28): Score 0, no neurological
defect; score 1, unable to fully extend the right forepaw; score 2,
circling to the right; score 3, falling to the right; and score 4,
loss of walking ability. The rats that scored between 1 and 3
points were considered to be successful models.
Screen tests
In order to measure the muscle tone, strength,
stamina and balance of the rats subjected to cerebral ischemia,
screen tests were performed 2 h after induction of cerebral
ischemia and for the following 7 days, using a net screen
(Dashitong Equipment Co., Ltd., Fuzhou, China). The screen tests
were conducted as described previously (29). The scoring criteria were as
follows: 5, Holding onto the screen and climbing upward; 4, holding
onto the screen with forelimbs and not falling down within 5 sec;
3, holding onto the screen temporarily and slipping off a certain
distance; 2, falling to the ground within 5 sec; 1, falling to the
ground immediately, as soon as the screen was set at a vertical
position.
Measurement of infarct volume
A total of 7 days after induction of cerebral
ischemia, the rats were sacrificed with 10% chloral hydrate, and
perfused transcardially with 0.9% NaCl. The brain of each rat was
harvested and dissected into six coronal blocks (2 mm/slice). The
fresh slices were incubated in 2% (w/v) 2,3,5-triphenyltetrazolium
chloride solution in phosphate-buffered saline for 20 min at 37°C
in the dark. Normal areas of the brain were stained dark red based
on intact mitochondrial function, whereas the infarct areas
remained unstained. Images of the stained slices were captured
using a high-resolution digital camera (Canon Sx20; Canon, Inc.,
Tokyo, Japan). The Motic Med 6.0 Digital Medical Image Analysis
system (Motic Microscopes, Xiamen, China) was used to quantify the
infarct volume by integration of the areas from the sections.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cortical infarct
region of the rats using TRIzol® reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. Purified RNA (3 µg) was reverse
transcribed into cDNA using the RevertAid™ H Minus First Strand
cDNA Synthesis kit (Fermentas, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's instructions.
The obtained cDNA was used to determine the mRNA expression levels
of GABAB1 R and GABAB2 R by PCR using
Taq DNA polymerase (Fermentas); β-actin served as an
internal control. An S1000 PCR thermal cycler (Bio-rad
Laboratories, Inc., Hercules, CA, USA) was used and the PCR
conditions were as follows: 94°C for 3 min, denaturation for 30 sec
at 94°C (35 cycles), annealing for 30 sec at 58°C, polymerization
for 45 sec at 72°C and finally extension for 10 min at 72°C. The
primers and the annealing temperatures (°C) used for the
amplification were as follows: Sense, 5′-CGG GTG GTA TGC TGA CAA
CTG GT-3′ (23 bp, 58°C) and antisense, 5′-ATG TTG GAA ATG CTT CGG
GTG TT-3′ (23 bp, 58°C) for GABAB1 R; sense, 5′-GGA CTT
CAA CTA CAC AGA CCA CAC GC-3′ (26 bp, 58°C) and antisense, 5′-TTG
TAT TCG CCG ACC TTC ACC TCT CT-3′ (26 bp, 58°C) for
GABAB2 R; and sense, 5′-CTA TCG GCA ATG AGC GGT TC-3′
(20 bp, 58°C) and antisense, 5′-ACT GTG TTG GCA TAG AGG TCT-3′ (21
bp, 58°C) for β-actin. Samples were analyzed by 1.5% agarose gel
electrophoresis (Biowest, Hong Kong, China). The DNA bands were
then examined using a Gel Documentation system (Gel Doc 2000;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
The cortical infarct region of the rats was
homogenized in non-denaturing lysis buffer (EMD Millipore, Boston,
MA, USA) and centrifuged at 12,000 × g for 10 min. The protein
concentration in the supernatants was then determined. Protein
lysates were separated by 10% SDS-PAGE and then electrophoretically
transferred to polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology, Shanghai, China). The membranes were
blocked for 2 h with 5% non-fat dry milk, and were then incubated
with primary antibodies targeting GABAB1 R,
GABAB2 R and β-actin (1:1,000 dilution) overnight at
4°C. The membranes were then incubated with appropriate
HRP-conjugated secondary antibodies for 1 h. The blots were
visualized using enhanced chemiluminescence, and images were
captured using a Bio-Image Analysis system (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are presented as the means of ≥3
determinations. Statistical analysis of the data was performed with
Student's t-test and one-way analysis of variance using the SPSS
package for Windows version 16.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
GLGZD ameliorates neurological defects
and cerebral infarction
The neuroprotective effects of GLGZD were examined
by evaluating the neurological defect scores and cerebral infarct
volume of the rats. As shown in Fig.
1, all of the rats in the IC and GLGZD groups exhibited marked
signs of cerebral infarction and neurological defects, whereas the
rats in the SC group did not show any manifestation of cerebral
injury. However, following treatment with GLGZD for 7 days, the
cerebral infarct volumes were significantly reduced (Fig. 1A and B), and the neurological
defect scores were improved (Fig.
1C). These results indicate that GLGZD may have therapeutic
efficacy against cerebral I/R injury.
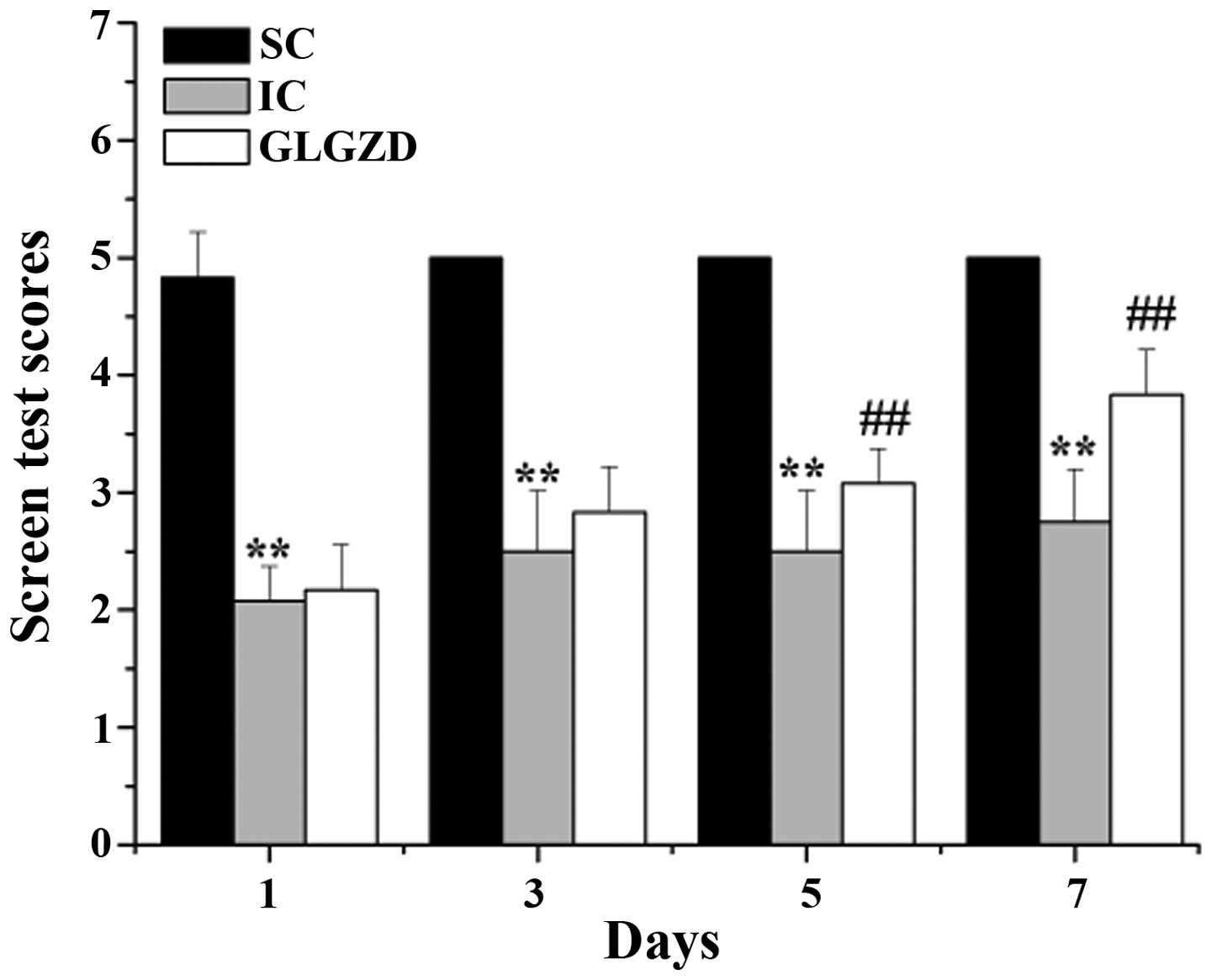
GLGZD improves motor performance
To determine the motor function of the rats
subjected to cerebral I/R, screen tests were performed on the rats
from all of the experimental groups. As shown in Fig. 2, the screen test scores of the IC
rats were significantly decreased, as compared with the rats in the
SC group. However, disordered motor function was significantly
ameliorated following treatment with GLGZD between days 5 and 7.
These results indicate that treatment with GLGZD may ameliorate
cerebral I/R-induced spasticity in rats.
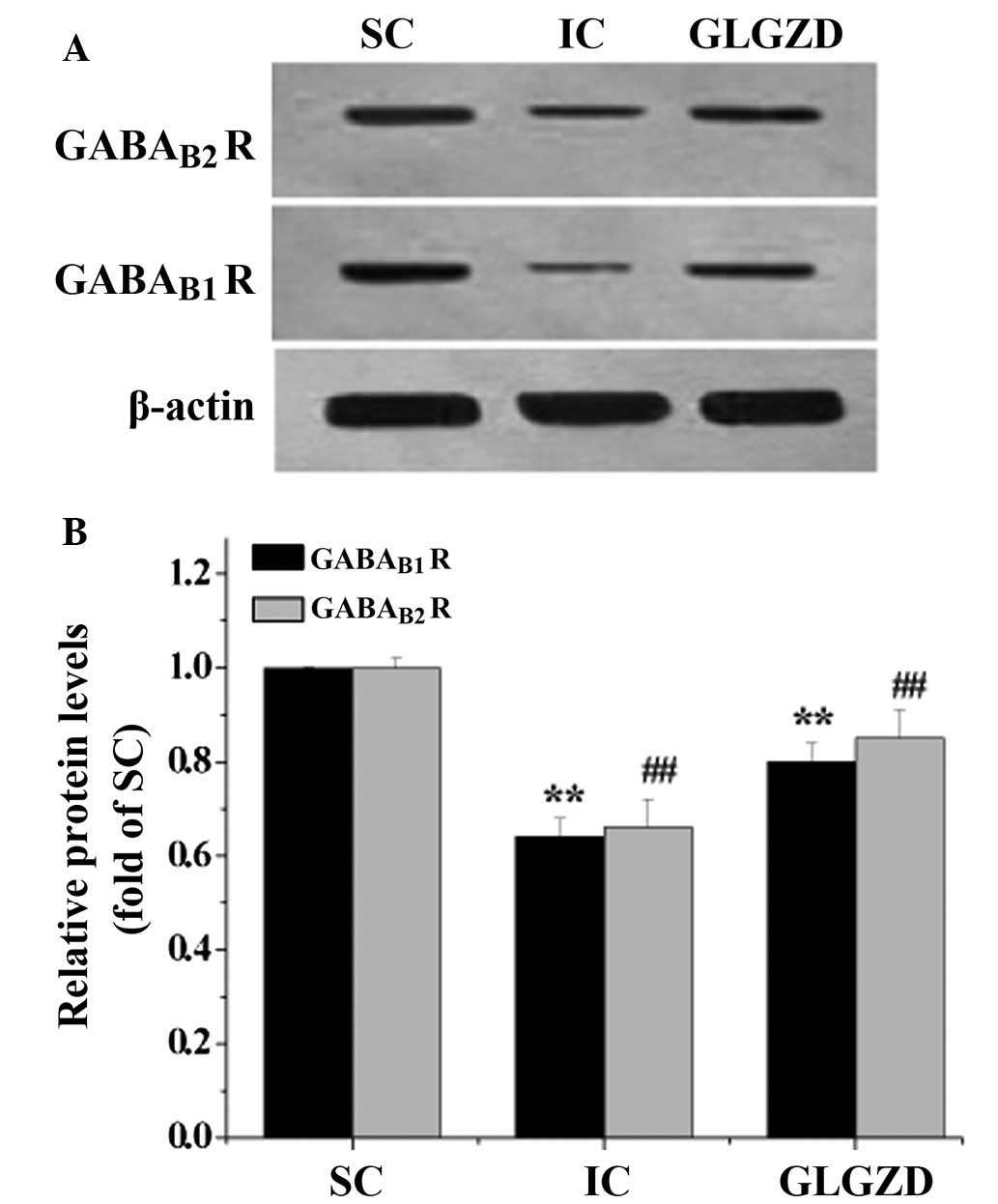
GLGZD increases the expression levels of
GABAB R subunits following cerebral I/R
To further explore the mechanisms underlying the
GLGZD-induced amelioration of post-cerebral I/R spasm, RT-PCR and
western blot analysis were performed to detect the mRNA and protein
expression levels of GABAB R subunits in the ischemic
cortex, respectively. The mRNA and protein expression levels of the
GABAB1 R and GABAB2 R were decreased in the
IC group, as compared with the SC group. However, following
treatment with GLGZD for 7 days, the expression levels of the
GABAB1 R and GABAB2 R were increased
(Fig. 3 and 4). These data suggest that treatment with
GLGZD may promote the activation of the GABAB R in the
ischemic cortex.
Discussion
Post-stroke spasticity is one of the most physically
debilitating conditions in aged populations, due to muscle
hyperactivity, which results in limb stiffness and muscle spasm
(30). It has been hypothesized
that post-stroke spasticity is induced by an imbalance between
excitatory and inhibitory neurotransmitters, which may be mediated
by the expression of mGlu R1 and GABAB R. This imbalance
may induce the abnormal transmission of sensory information and
motion instruction, and subsequently increase the muscle activity
and the active tonic stretch reflex (31). Direct evidence regarding the
involvement of the GABAB R in post-stroke spasticity has
yet to be reported; however, numerous lines of research have
suggested that the GABAB R may affect the clinical signs
of spasticity, and participate in an experimental animal model of
cerebral ischemia-induced limb spasms (8,32). A
previous clinical study demonstrated that baclofen, a GABA agonist,
was able to cross the blood-brain barrier and bind to the
GABAB R at the presynaptic terminal, suggesting that it
may be used for the treatment of spasticity (14). In addition, catgut implantation at
acupoints has been shown to relieve limb spasticity in the brain
stem of rats after stroke via upregulation of the GABAB
R expression (8). These studies
suggest that the GABAB R may have an important role in
the mechanisms underlying post-stroke spasticity. The
GABAB R is a heterodimeric G-protein-coupled receptor,
which is composed of two subunits: GABAB1 R and
GABAB2 R. The GABAB R is abundantly expressed
post- and presynaptically throughout the central nervous system in
virtually all types of neurons (33). It mediates slow inhibitory
neurotransmission, and/or hyperpolarizes the neuronal membrane,
thereby controlling the excitability of neurons and consequently
participating in post-stroke spasticity (34–36).
GLGZD is a classical TCM formula that has previously
been used to treat post-stroke spasticity. Recent clinical trials
have demonstrated that GLGZD had a significant therapeutic effect
on limb spasm in patients following cerebral stroke (21,22).
However, the mechanisms underlying its neuroprotective and
antispastic effects remain largely unknown. The present study aimed
to further elucidate the mechanisms of GLGZD, using a focal
cerebral ischemia rat model. The results of the present study
demonstrated that treatment with GLGZD exerted neuroprotective
effects by improving neurological defects and reducing cerebral
infarct volume. In addition, GLGZD was shown to improve motor
performance, as determined using a screen test. Furthermore, as
expected, GLGZD was able to significantly upregulate the mRNA and
protein expression levels of both GABAB1 R and
GABAB2 R in the cerebral ischemic cortex.
In conclusion, the present study demonstrated that
treatment with GLGZD exerts neuroprotective effects and improves
spasticity in an ischemic stroke model via upregulation of the
GABAB R. These findings suggest that GLGZD may be a
potential therapeutic agent for the treatment of cerebral ischemia
and spasticity.
Acknowledgments
The present study was supported by the Ministry of
Science and Technology of the International Science and Technology
Cooperation Projects (grant no. 2011DFG33240), and the Nature
Science Foundation of Fujian Province of China (grant no.
2014J01358).
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayer NH and Esquenazi A: Muscle
overactivity and movement dysfunction in the upper motoneuron
syndrome. Phys Med Rehabil Clin N Am. 14:855–883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sommerfeld DK, Eek EU, Svensson AK,
Holmqvist LW and von Arbin MH: Spasticity after stroke: Its
occurrence and association with motor impairments and activity
limitations. Stroke. 35:134–139. 2004. View Article : Google Scholar
|
|
4
|
Lundström E, Terént A and Borg J:
Prevalence of disabling spasticity 1 year after first-ever stroke.
Eur J Neurol. 15:533–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duncan PW, Zorowitz R, Bates B, Choi JY,
Glasberg JJ, Graham GD, Katz RC, Lamberty K and Reker D: Management
of Adult Stroke Rehabilitation Care: A clinical practice guideline.
Stroke. 36:e100–e143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown P: Pathophysiology of spasticity. J
Neurol Neurosurg Psychiatry. 57:773–777. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doan QV, Brashear A, Gillard PJ, Varon SF,
Vandenburgh AM, Turkel CC and Elovic EP: Relationship between
disability and health-related quality of life and caregiver burden
in patients with upper limb poststroke spasticity. PM R. 4:4–10.
2012. View Article : Google Scholar
|
|
8
|
Liu CM, Li RQ, Song XL and Feng XD: Effect
of catgut implantation at acupoints on GABAB and mGluR1 expressions
in brain stem of rats with spasticity after stroke. J Tradit Chin
Med. 34:566–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellenberger C, Mevissen M, Doherr M,
Scholtysik G and Jaggy A: Inhibitory and excitatory
neurotransmitters in the cerebrospinal fluid of epileptic dogs. Am
J Vet Res. 65:1108–1113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramanathan M, Babu CS, Justin A and
Shanthakumari S: Elucidation of neuroprotective role of endogenous
GABA and energy metabolites middle cerebral artery occluded model
in rats. Indian J Exp Biol. 50:391–397. 2012.PubMed/NCBI
|
|
11
|
Yang JP, Zhang XQ, Wang XD and Wang KW:
Inhibitory and excitatory amino acids in cerebral spinal fluid in
children with cerebral palsy. Zhong Hua Xiao Er Wai Ke Za Zhi.
19:282–284. 1998.In Chinese.
|
|
12
|
Galvez T, Parmentier ML, Joly C,
Malitschek B, Kaupmann K, Kuhn R, Bittiger H, Froestl W, Bettler B
and Pin JP: Mutagenesis and modeling of the GABAB receptor
extracellular domain support a venus flytrap mechanism for ligand
binding. J Biol Chem. 274:13362–13369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benke D: GABAB receptor trafficking and
interacting proteins: targets for the development of highly
specific therapeutic strategies to treat neurological disorders?
Biochem Pharmacol. 86:1525–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein EM: Spasticity management: An
overview. J Child Neurol. 16:16–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lapeyre E, Kuks JB and Meijler WJ:
Spasticity: Revisiting the role and the individual value of several
pharmacological treatments. NeuroRehabilitation. 27:193–200.
2010.PubMed/NCBI
|
|
16
|
Simon O and Yelnik AP: Managing spasticity
with drugs. Eur J Phys Rehabil Med. 46:401–410. 2010.PubMed/NCBI
|
|
17
|
Sun X: Research on formula treating
paralysis and spasticity from 'treatise on febrile and
miscellaneous diseases'. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.In Chinese.
|
|
18
|
Yelnik AP, Simon O, Bensmail D,
Chaleat-Valayer E, Decq P, Dehail P, Quentin V, Marque P, Parratte
B, Pellas F, et al Agence française de sécurité sanitaire des
produits de santé: Drug treatments for spasticity. Ann Phys Rehabil
Med. 52:746–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satkunam LE: Rehabilitation medicine: 3.
Management of adult spasticity. CMAJ. 169:1173–119. 2003.PubMed/NCBI
|
|
20
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi decotion on c-fos and c-jun in epileptic rats. Chuan Hua Xi
Zhong Yi Yao Yan Jiu Suo. 23:21–22. 2005.In Chinese.
|
|
21
|
Yang CM, Chen LD and Tao J: New usage of a
classical formula-Gua Lou Gui Zhi decotion. Liao ning Zhong Yi Za
Zhi. 8:166–167. 2012.In Chinese.
|
|
22
|
Chen YL, Chen LD and Tao J: Clinical
research on treating limbs spam from cerebral apoplexy with Gualou
Guizhi decoction. Clin J Chin Med. 5:7–9. 2013.In Chinese.
|
|
23
|
Huang J, Tao J, Xue XH, Yang S, Han P, Lin
Z, Xu W, Lin J, Peng J and Chen L: Gua Lou Gui Zhi decoction exerts
neuroprotective effects on post-stroke spasticity via the
modulation of glutamate levels and AMPA receptor expression. Int J
Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
24
|
Chen X, Li H, Huang M, Huang M, Xu W, Chu
K, Chen L and Zhang Y: Effect of Gua Lou Gui Zhi decoction on focal
cerebral ischemia-reperfusion injury through regulating the
expression of excitatory amino acids and their receptors. Mol Med
Rep. 10:248–254. 2014.PubMed/NCBI
|
|
25
|
Chalifoux JR and Carter AG: GABAB receptor
modulation of synaptic function. Curr Opin Neurobiol. 21:339–344.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oshiro M, Hefferan MP, Kakinohana O,
Lukacova N, Sugahara K, Yaksh TL and Marsala M: Suppression of
stretch reflex activity after spinal or systemic treatment with
AMPA receptor antagonist NGX424 in rats with developed baclofen
tolerance. Br J Pharmacol. 161:976–985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gómez-Soriano J, Goiriena E and Taylor J:
Spasticity therapy reacts to astrocyte GluA1 receptor upregulation
following spinal cord injury. Br J Pharmacol. 161:972–975. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo J, Liu L, Ma C, Xu B, Duan X and Wang
B: Effect of restraint stress on depression-like behaviors in rats
after transient focal cerebral ischemic injury. Neural Regen Res.
2:390–394. 2007. View Article : Google Scholar
|
|
30
|
Mayer NH, Esquenazi A and Childers MK:
Common patterns of clinical motor dysfunction. Muscle Nerve. (Suppl
6): S21–S35. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doble A: The role of excitotoxicity in
neurodenenerative disease: Implications for therapy. Pharmacol
Ther. 81:163–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bowery NG: GABAB receptor: a site of
therapeutic benefit. Curr Opin Pharmacol. 6:37–43. 2006. View Article : Google Scholar
|
|
33
|
Bettler B, Kaupmann K, Mosbacher J and
Gassmann M: Molecular structure and physiological functions of
GABA(B) receptors. Physiol Rev. 84:835–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gassmann M and Bettler B: Regulation of
neuronal GABA(B) receptor functions by subunit composition. Nat Rev
Neurosci. 13:380–394. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chalifoux JR and Carter AG: GABAB receptor
modulation of synaptic function. Curr Opin Neurobiol. 21:339–344.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benarroch EE: GABAB receptors: Structure,
functions, and clinical implications. Neurology. 78:578–584. 2012.
View Article : Google Scholar : PubMed/NCBI
|