Introduction
In vitro production (IVP), including in
vitro maturation (IVM) and somatic cell nuclear transfer, is an
important technology for producing transgenic cloned pigs. Oocyte
quality influences early embryonic development, fetal growth,
pregnancy and the health of the offspring (1). Thus, a better understanding and
improved IVM are required to generate transgenic cloned pigs
efficiently.
To date, there has been extensive research regarding
the optimal conditions for IVM. Addition of various hormones and
growth factors, including luteinizing hormone, follicle-stimulating
hormone (2), transforming growth
factor, androstenedione (3),
pregnant mare serum gonadotropin, human chorionic gonadotropin
(hCG) (4,5), insulin-like growth factor I (6), and estradiol-17β (5,7), to
IVM media exerts positive effects during meiosis (8). Cumulus cells also have an important
role in oocyte maturation. Cumulus cells regulate the resumption of
meiosis and protect oocytes from oxidative stress (9). Reactive oxygen species (ROS) cause
oxidative damage to oocytes due to an improper in vitro
environment (10,11), while glutathione (GSH) reduces ROS
and oxidative stress (12).
Various factors can increase GSH, including cysteine (13), cysteamine, glutamine (14), vascular endothelial growth factor
(15) and granulocyte-macrophage
colony-stimulating factor (16).
However, the in vitro oocyte quality remains inferior to the
in vivo oocyte quality for transgenic pig production
(17). Numerous aspects of oocyte
maturation have remained to be determined; for example, porcine
follicular fluid (pFF) contains various unknown factors, including
growth factors, cytokines and trance minerals. These factors can
influence oocyte maturation and subsequent embryonal development.
However, the factors or mechanisms have yet to be determined.
The mammalian body contains small amounts of trace
minerals, which are necessary to maintain life and health (18). These minerals are involved in the
formation of bones and teeth, acid-base balance, fluid and moisture
equilibrium, and are used as components of neurotransmitters and
hormones (18). Trace minerals
influence embryonic and fetal survival, as well as other aspects of
reproductive performance and growth in mammals (19). Of these, zinc is an important
factor during reproduction and development.
Zinc is required for normal fetal growth and
development (20). Zinc deficiency
causes fetal teratogenesis, prolonged gestation, difficult labor,
low birth weight and weak offspring (21,22).
In addition, zinc levels are increased 1.7- to 8.7-fold in porcine
conceptuses compared to those in the endometrium or ovary between
days 12 and 30 of gestation (23).
This indicates that the developing conceptus requires increased
uptake and/or utilization of zinc. Another potential pathway for
zinc to influence pregnancy is by its effect on prostaglandin
(PG)F2α synthesis (24–27). Zinc is involved in the formation of
PGs, as the arachidonic acid cascade is regulated by zinc enzymes.
Zinc also has important roles in scavenging free oxygen radicals
(28), DNA synthesis and gene
transcription (29). Thus, zinc is
thought to influence reproduction and development, and it exists in
the oocyte cytoplasm and maturational environment during oocyte
maturation; however, its role and influence during porcine IVM have
not been sufficiently considered by previous studies. Only a
previous study by our group investigated the effects of zinc
supplementation during porcine IVM, showing that supplementation of
0.8 µg/ml zinc during IVM of porcine oocytes improved
embryonic development prior to implantation (30).
The aim of the present study was to clarify the role
of zinc during IVM. For this, zinc deficiency was induced using the
membrane-permeable zinc chelator TPEN, and the effects on cumulus
cell expansion, nuclear maturation, cytoskeletal organization, GSH
and ROS levels, and subsequent embryonic development were
evaluated.
Materials and methods
Chemicals
Unless otherwise indicated, all chemicals and
reagents used in the present study were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Oocyte collection and IVM
The current study was approved by the Committee on
the Ethics of Animal Experiments (permit no. CBNUA-584-13-01;
Chungbuk National University, Cheongju, Republic of Korea). Porcine
ovaries were obtained from 496 Landrace x Duroc crossbreed gilts
(sows) from a local slaughterhouse (Dong-A, Chengju, Korea) and
transported to the laboratory within 2 h in 0.9% (w/v) NaCl
supplemented with penicillin-G (100 IU/ml) and streptomycin sulfate
(100 mg/l) at 30-35°C. Follicular fluid with oocytes was aspirated
from antral follicles (3–6 mm) using an 18-gauge needle connected
to a 10-ml disposable syringe and collected into a 15-ml centrifuge
tube. Cumulus-oocyte complexes (COCs) were recovered under a
stereomicroscope (SZ51; Olympus, Tokyo, Japan), and those with at
least three layers of compact cumulus cells and a homogenous
cytoplasm were selected for IVM. The selected COCs were washed
three times in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid-buffered Tyrode's medium containing 0.05% (w/v) polyvinyl
alcohol (TLH-PVA), and transferred to 500 µl tissue culture
medium 199 (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 0.6 mM cysteine, 0.91 mM sodium pyruvate, 10
ng/ml epidermal growth factor, 75 µg/ml kanamycin, 1
µg/ml insulin and 10% (v/v) pFF. pFF was aspirated from
follicles (3–7 mm) obtained from pre-pubertal gilt ovaries. After
centrifugation at 1,600 xg for 30 min, the supernatants were
collected and filtered sequentially through 1.2- and 0.45-µm
syringe filters (Gelman Sciences, Ann Arbor, MI, USA). The prepared
pFF was then stored at −20°C until use. For maturation, the
selected COCs were washed three times in oocyte maturation medium
containing hormone supplements, and 50–60 oocytes were transferred
to each well of a four-well Nunc dish (Roskilde, Denmark)
containing 500 µl of culture medium and equilibrated for at
least 2 h with 5% CO2 at 39°C in a humidified
atmosphere. During the first 22 h, the COCs were matured with
hormones (10 IU/ml equine chorionic gonadotropin and 10 IU/ml hCG
(Intervet, Boxmeer, the Netherlands). After 22 h of maturation with
hormones, the COCs were washed twice and cultured in hormone-free
IVM medium for an additional 18–20 h.
Assessment of nuclear maturation
After 40–42 h of culture, the oocytes were denuded
by gently pipetting them in IVM medium containing 0.1%
hyaluronidase followed by washing in TLH-PVA. The denuded oocytes
were fixed for 5 min in fixative solution containing 2%
formaldehyde and 0.25% glutaraldehyde and then stained in TLH-PVA
containing 10 µg/ml Hoechst 33342 for 10 min. The stained
oocytes were examined by fluorescence microscopy (Nikon Corp.,
Tokyo, Japan) and classified according to their developmental stage
as follows: Germinal vesicle, metaphase I (MI), anaphase
I/telophase I or MII.
Experimental groups
Zinc deficiency was induced with TPEN. TPEN was
prepared as a 1-mM stock solution and used at a final concentration
of 10 µM. In experiment 1, nuclear maturation, cytoskeletal
component organization, GSH, ROS, and subsequent embryonic
development in the following three groups were evaluated to
investigate the effects of zinc deficiency during IVM: i) Treatment
without TPEN (control); ii) treatment with 10 µM TPEN for 22
h during IVM; and iii) treatment with 10 µM TPEN+10
µM zinc (zinc sulfide) for 22 h during IVM. In experiment 2,
the effects of the zinc deficiency period during IVM on oocyte
maturation and subsequent embryonic development after PA were
determined. TPEN (10 µM) was added to the IVM medium for 0,
7, 15 or 22 h. After TPEN treatment, 10 µM zinc was added to
the IVM medium during the second half of IVM in all but the 0-h
group.
Immunofluorescence imaging
Oocytes were collected after IVM, fixed in 4%
paraformaldehyde for 40 min and then permeabilized with 1% Triton
X-100 for 30 min. After incubation in am Image-iTTM FX Signal
Enhancer (I36933; Invitrogen Life Technologies) for 30 min, the
oocytes were blocked with 1% bovine serum albumin (BSA; A9418;
Sigma-Aldrich) in phosphate-buffered saline (PBS) for 1 h and then
incubated with fluorescein isothiocyanate-conjugated monoclonal
anti-α-tubulin antibodies (F2168, 1:100; Sigma-Aldrich) or
tetramethylrhodamine-conjugated phalloidin (P1951, 1:200;
Sigma-Aldrich) for 1 h at 37°C. After nuclear staining with 10
µg/ml Hoechst 33342, The oocytes were mounted on slides with
1,4-diazabicyclo-(2.2.2) octane (P0126; Beyotime Institute of
Biotechnology, Shanghai, China) and observed with a laser-scanning
confocal microscope (TCS SP2 AOBS; Leica Microsystems, Wetzlar,
Germany).
Measurement of intracellular ROS and GSH
levels
The oocytes were sampled after 44 h of IVM to
determine their intracellular ROS and GSH levels as described
previously (31). Briefly,
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen
Life Technologies) and CellTracker Blue
(4-chloromethyl-6.8-difluoro-7-hydroxycoumarin; Invitrogen Life
Technologies) were used to detect intracellular ROS as green
fluorescence and the GSH level as blue fluorescence. Ten oocytes
from each treatment group were incubated in the dark for 30 min in
TLH-PVA supplemented with 10 µM H2DCFDA and 10 µM
CellTracker. After incubation, the oocytes were washed with
Dulbecco's PBS (Invitrogen Life Techonologies) containing 0.1%
(w/v) PVA and placed in 10-µl microdrops, and fluorescence
was detected under an epifluorescence microscope (TE300; Nikon)
with ultraviolet filters (460 nm for ROS and 370 nm for GSH).
Fluorescence images were saved as graphic files in TIFF format. The
fluorescence intensities of the oocytes were analyzed with ImageJ
software v. 1.41o (National Institutes of Health, Bethesda, MD,
USA) and normalized to a control.
Parthenogenetic activation (PA)
For PA, mature oocytes were activated with two
pulses of 120 V/mm of DC for 60 µsec in 280 mM mannitol
containing 0.01 mM CaCl2 and 0.05 mM MgCl2.
Following electrical activation, the PA embryos were treated with 5
µg/ml cytochalasin B in IVC medium for 6 h at 39°C in a
humidified atmosphere of 5% CO2 in air.
In vitro embryo culture
The PA embryos were washed three times with IVC
medium (porcine zygote medium 3) and cultured in 30-µl
microdrops of IVC medium. Embryos in culture medium were covered
with pre-warmed mineral oil and incubated at 39°C for 7 days under
a humidified atmosphere of 5% O2, 5% CO2 and
90% N2.
Embryo evaluation and total cell
count
The day when PA was performed was designated as day
0. The embryos were evaluated under a stereomicroscope for cleavage
on day 2 (48 h). Blastocyst formation was assessed on day 7 (168
h). To determine the total cell number in the blastocysts on day 7,
blastocysts were collected and the zona pellucida of (if unhatched)
was dissolved with 0.5% protease. The zona-free blastocysts were
washed in PBS containing 1% (w/v) BSA and stained with 10
µg/ml Hoechst 33342 for 5 min. Following a final wash in
PBS-BSA, the blastocysts were fixed briefly (10 min) in 4%
paraformaldehyde in PBS. The blastocysts were mounted on glass
slides in a drop of 100% glycerol, gently squashed under a cover
slip and observed by fluorescence microscopy (Nikon) at ×400
magnification.
Statistical analysis
Each experiment consisted of at least three
replicates. All statistical analyses were performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). The GSH and ROS levels and
embryonic development data (e.g., rate of cleavage, blastocyst
formation and number of nuclei) were compared by a one-way analysis
of variance, followed by Duncan's multiple range test. All values
are expressed as the mean or mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Zinc deficiency inhibits oocyte
maturation and subsequent embryo development
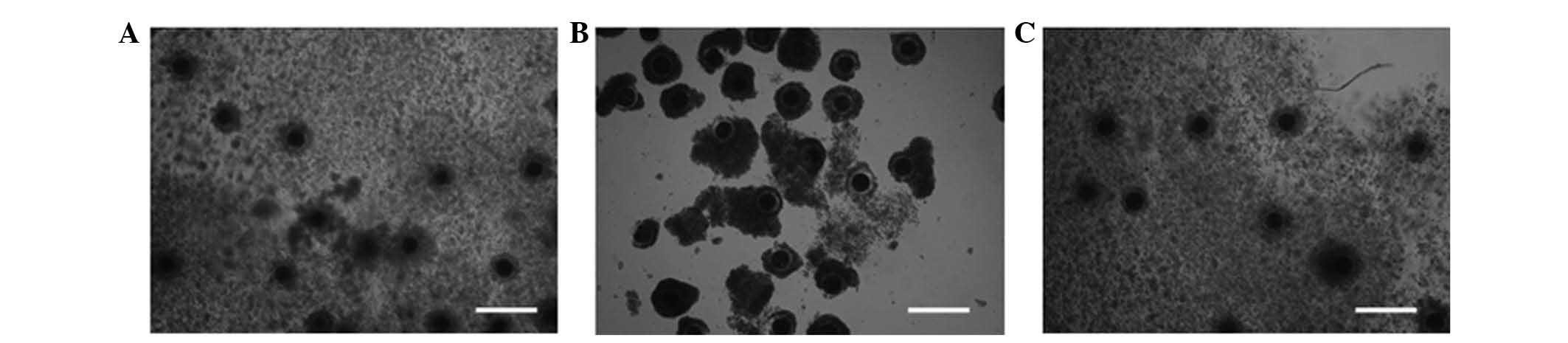
Cumulus cell expansion was observed after IVM. The
control and TPEN+zinc treatment groups showed normal cumulus cell
proliferation, while in the TPEN-treated group, cumulus cell
proliferation did not proceed (Fig.
1).
Almost all oocytes in the TPEN-treated groups were
arrested at MI. Only 0.61% of the oocytes matured to MII. By
contrast, >90% of the oocytes reached MII in the control and
TPEN+zinc-treated groups (Table
I).
 | Table IEffect of zinc deficiency during
in vitro maturation on nuclear maturation of porcine
oocytes. |
Table I
Effect of zinc deficiency during
in vitro maturation on nuclear maturation of porcine
oocytes.
| Group | Oocytes cultured
for maturation (n)a | Number of oocytes
(n)
|
|---|
| Germinal
vesicle | Metaphase I | Anaphase and
telophase I | Metaphase II |
|---|
| Control | 150 | 0 | 3.3±0.7b | 4.0±1.2 | 92.7±1.8b |
| TPEN | 157 | 1.9±1.1 | 96.2±1.0c | 1.3±0.6 | 0.6±0.6c |
| TPEN+Zn | 156 | 0 | 5.1±1.2b | 1.9±0.0 | 93.0±1.2b |
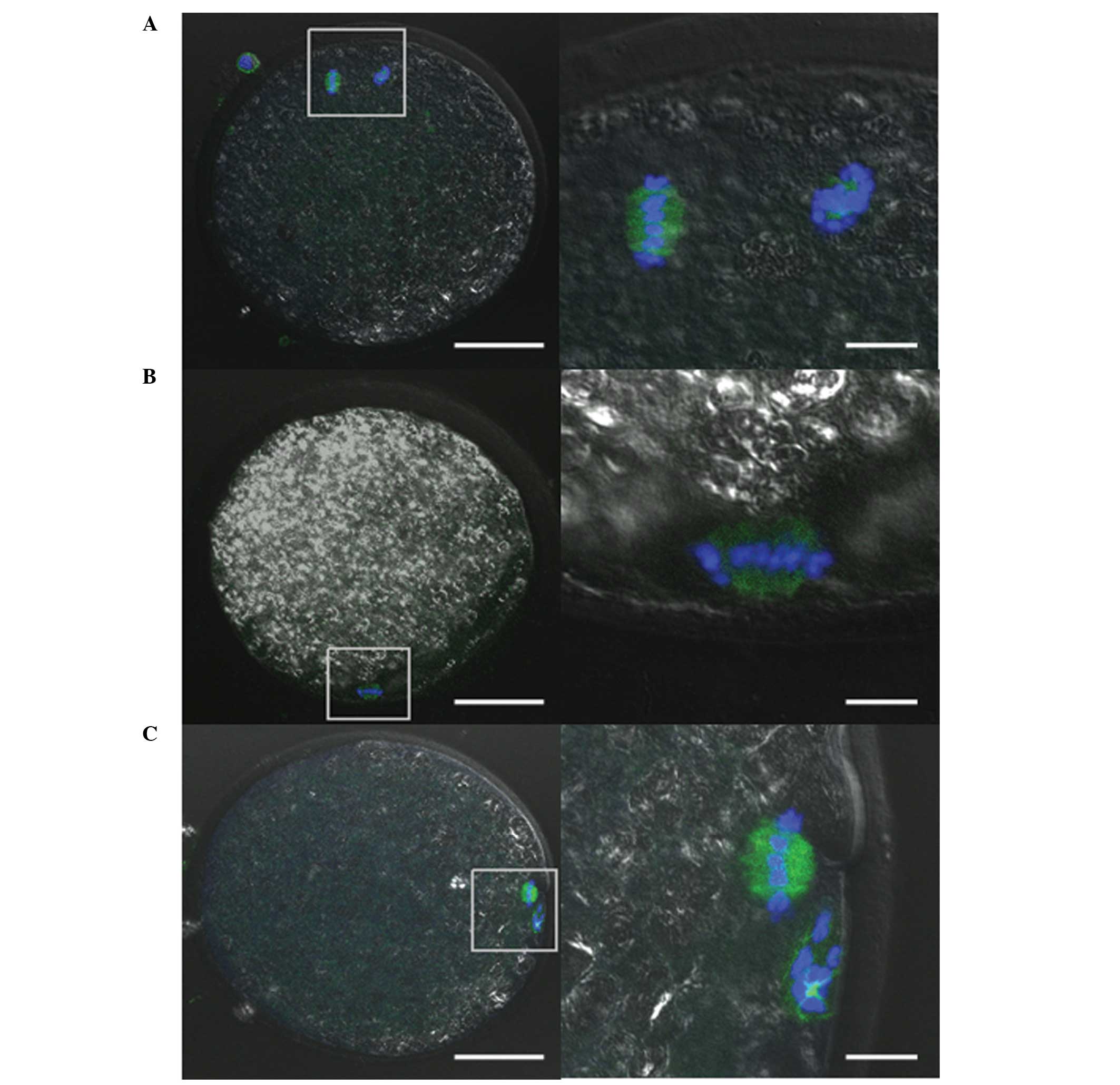
Cytoskeletal organization was investigated by
immunofluorescence staining to determine an accurate meiotic rate.
Almost all oocytes in the control and TPEN+zinc-treated groups
displayed a metaphase spindle and polar body (Fig. 2). By contrast, almost all of the
TPEN-treated oocytes displayed only a metaphase spindle (Fig. 2). Although the meiotic stages were
different, the shapes of the chromatin and spindle were normal in
all groups. Only a few oocytes in the TPEN-treated group did not
have a spindle signal.
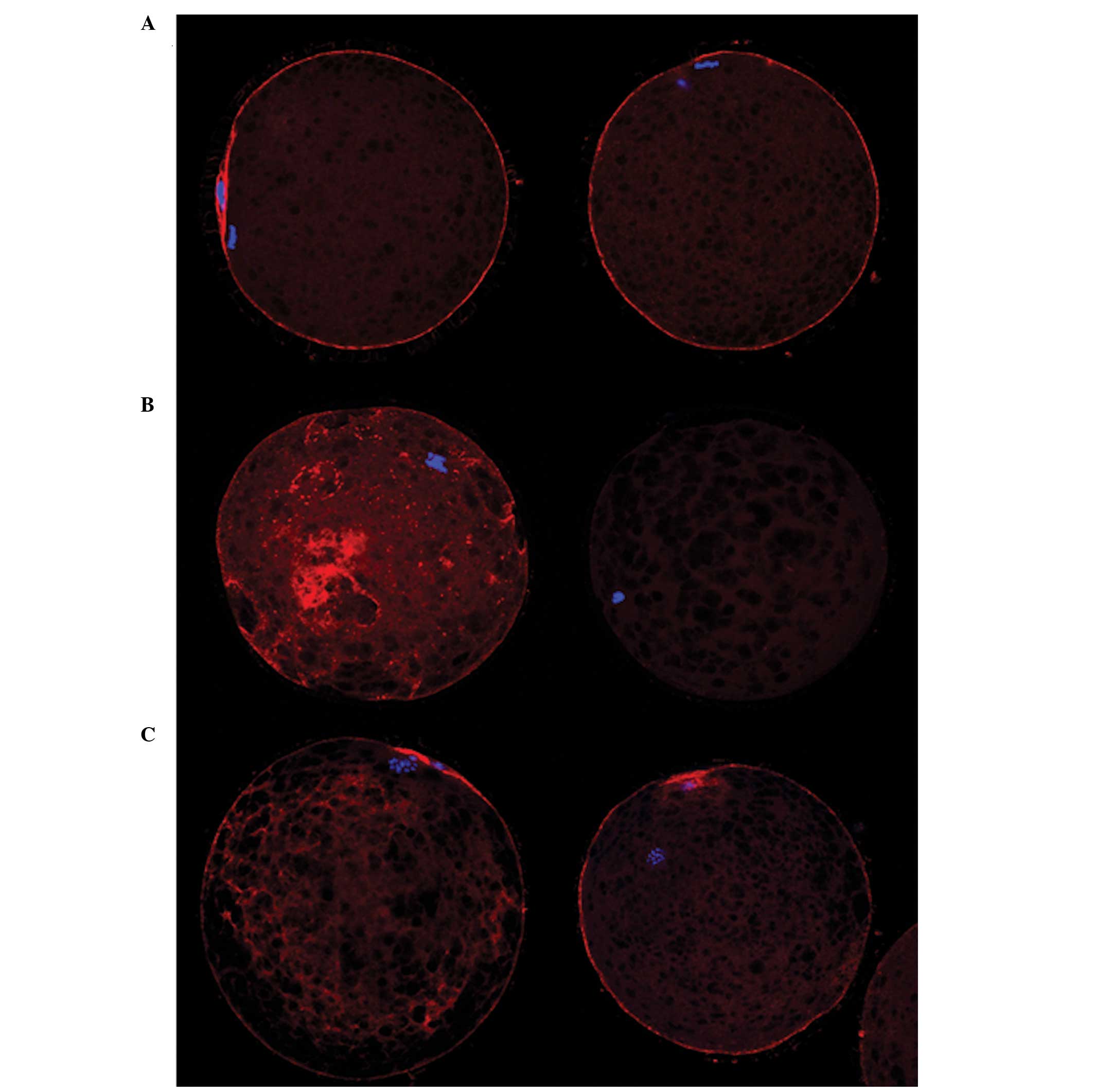
In the control, a typical M II-stage distribution of
microfilaments was observed. Strong signals were detected at the
cortex (Fig. 3). The microfilament
distribution was abnormal in the TPEN-treated group; the
microfilaments were irregularly distributed in the cytoplasm and
cortex, which were unevenly shaped. Certain oocytes in the
TPEN-treated group did not have a microfilament signal (Fig. 3). Most of the oocytes in the
TPEN+zinc-treated group showed a relatively normal microfilament
distribution. Weak microfilament signals were detected in the
cytoplasm of certain oocytes.
The GSH and ROS levels were significantly altered in
the TPEN-treated group; the GSH levels decreased significantly,
while the ROS levels increased significantly (Fig. 4). The GSH and ROS levels changed
slightly in the TPEN+zinc-treated group, but not significantly.
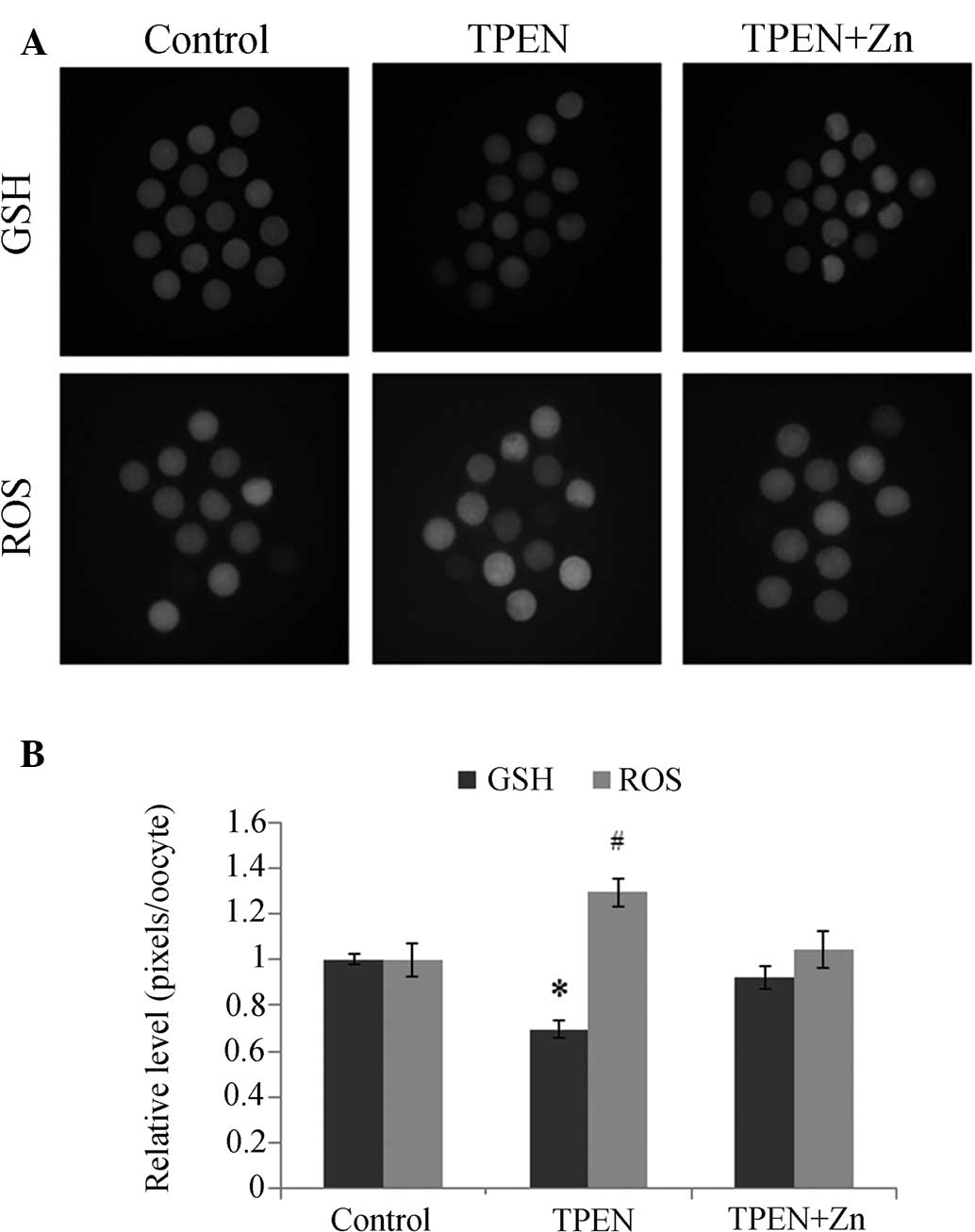 | Figure 4Epifluorescent photomicrographic
images of in vitro matured porcine oocytes. (A) Oocytes were
stained with (top row) CellTracker Blue and (bottom row)
2′,7′-dichlorodihydrofluorescein diacetate to detect intracellular
levels of GSH and reactive ROS, respectively. Metaphase-II oocytes
derived from the control group, TPEN-treated group and
TPEN+Zn-treated group. (B) Effect of zinc deficiency on
intracellular GSH and ROS levels on porcine oocytes during in
vitro maturation. Values are expressed as the mean ± standard
error of the mean (n=3). *, #P<0.05 vs. control. ROS,
reactive oxygen species; GSH, glutathione; TPEN, zinc chelator
N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylendiamine. |
Subsequent development of PA embryos was also
decreased in the TPEN-treated group. On day 2 of IVC, almost all
oocytes in the TPEN-treated group were arrested at the one-cell
stage (Fig. 5). Although the
cleavage patterns on day 2 were slightly different between the
control and TPEN+zinc-treated groups, the two groups showed normal
cleavage patterns. A total of 40.0 and 42.0% of the embryos
developed to blastocysts in the control and TPEN+zinc-treated
groups, respectively (Table II).
No cleaved embryos or blastocysts were observed in the TPEN-treated
group.
 | Table IIEffect of zinc deficiency on
embryonic development after parthenogenetic activation during in
vitro maturation. |
Table II
Effect of zinc deficiency on
embryonic development after parthenogenetic activation during in
vitro maturation.
| Group | Embryos cultured
(n)a | Embryos developed
to
| Total cell number
in blastocyst |
|---|
| ≥2-cell (%) | Blastocyst (%) |
|---|
| Control | 157 | 72.9±2.8 | 40.0±7.5 | 51.0±6.2 |
| TPEN | 147 | 0 | 0 | – |
| TPEN+Zn | 144 | 75.5±2.2 | 42.0±6.7 | 47.2±4.8 |
Zinc withdrawal inhibits oocyte
maturation and subsequent embryonic development in a time-dependent
manner
The cumulus cells in the group treated for 7 h were
slightly expanded subsequent to IVM, whereas no expansion was
observed in the groups treated for 15 and 22 h (Fig. 6).
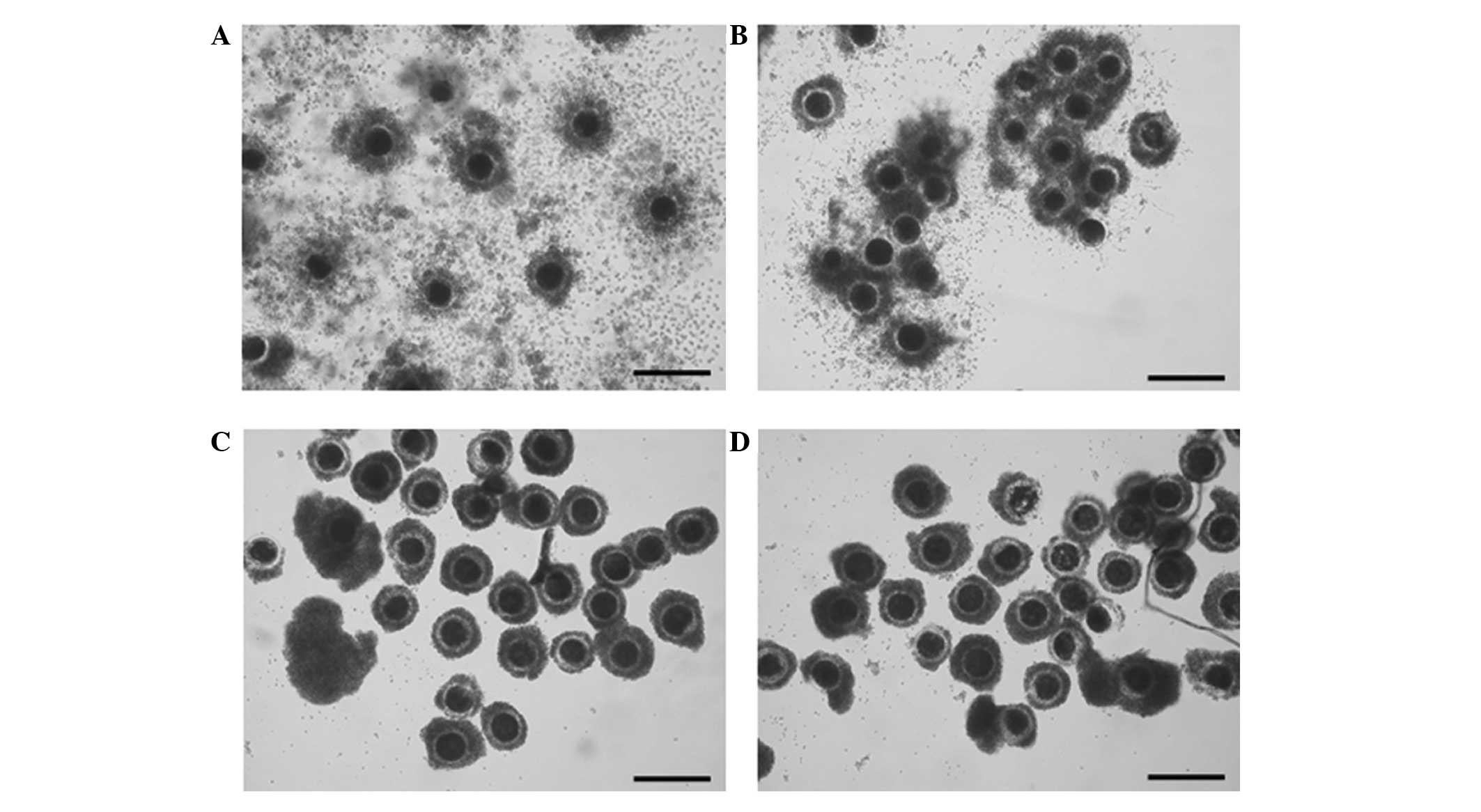 | Figure 6Effect of zinc-deficient time during
IVM on cumulus cell expansion at 42 h after IVM. (A) Control (no
treatment), (B) 7 h TPEN treatment, (C) 15 h TPEN treatment and (D)
22 h TPEN treatment (magnification, ×50; scale bar, 250 µm).
The control group shows abundant cumulus cell expansion. Slight
cumulus cell expansion was observed in B. However, cumulus cell
expansion was inhibited in C and D. IVM, in vitro
maturation; TPEN, zinc chelator
N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylendiamine. |
An assessment of nuclear maturation produced results
that were similar to those for cumulus cell expansion. Nuclear
maturation rates decreased with increasing TPEN treatment duration.
The MI and MII rates in the control group were 11.5 and 83.9%,
respectively. The group treated for 7 h had MI and MII rates of
50.4 and 44.8%, respectively. No MII oocytes were observed in the
groups treated for 15 and 22 h (Table III). Approximately 98.0 and 97.2%
of the oocytes in the groups treated for 15 and 22 h, respectively,
were in MI.
 | Table IIIEffect of zinc deficiency for various
durations on nuclear maturation during in vitro
maturation. |
Table III
Effect of zinc deficiency for various
durations on nuclear maturation during in vitro
maturation.
| TPEN treatment
time | Oocytes cultured
for maturation (n)a | Oocytes at various
stages (%)
|
|---|
| Germinal
vesicle | Metaphase I | Anaphase and
telophase I | Metaphase II |
|---|
| 0 h | 105 | 0.9±0.9 | 11.5±2.9b | 3.8±0.8d | 83.9±3.9d |
| 7 h | 101 | 1.9±1.9 | 50.4±1.3c | 3.0±0.1c,d | 44.8±3.0c |
| 15 h | 98 | 2.0±1.0 | 98.0±1.0d | 0.0b | 0.0b |
| 22 h | 103 | 1.9±0.9 | 97.2±1.7d | 1.0±1.0b,c | 0.0b |
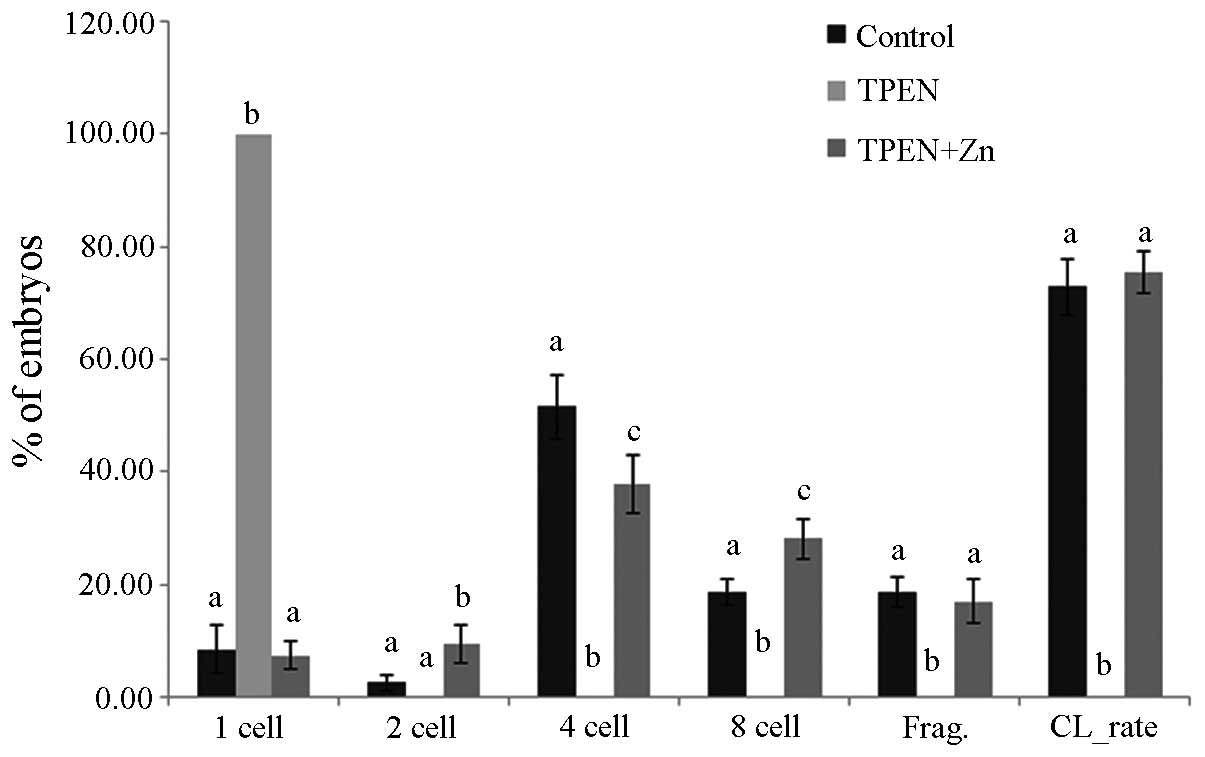
Subsequent development was also different with TPEN
treatment time. The number of one-cell stage embryos on day 2
increased by increasing TPEN treatment duration. In the group
treated for 7 h, the rate of cleaved embryos decreased
significantly compared to that in the control group. The numbers of
two- and four-cell stage embryos decreased, while the numbers of
one-cell stage and fragmented embryos increased. Most embryos did
not cleave in the groups treated for 15 and 22 h (Fig. 7). The blastocyst formation rate and
total cell number in the blastocysts were highest in the control
group. The blastocyst formation rate and total cell number in the
blastocysts in the group treated for 7 h decreased significantly
(blastocyst formation rate, 10.4%; total cell number, 23.2). Only
2.6 and 3.0% of the embryos developed beyond the two-cell stage in
the groups treated for 15 and 22 h, and no blastocysts formed
(Table IV).
 | Table IVEffect of zinc deficiency for various
durations on embryonic development after parthenogenetic activation
during in vitro maturation. |
Table IV
Effect of zinc deficiency for various
durations on embryonic development after parthenogenetic activation
during in vitro maturation.
| TPEN Treatment
time | Embryos cultured
(n)a | Embryos developed
to
| Cell number in
blastocyst |
|---|
| ≥2-cell (%) | Blastocyst (%) |
|---|
| 0 h | 101 | 65.3±1.4b | 29.7±1.2b | 51.4±4.5c |
| 7 h | 107 | 42.6±4.8c | 10.4±1.4c | 23.2±1.6d |
| 15 h | 114 | 2.6±0.1d | 0.0d | – |
| 22 h | 103 | 3.0±1.6d | 0.0d | – |
Discussion
The IVM environment has a prominent effect on oocyte
maturation and early embryonic development. Various factors are
known to affect oocyte and embryonic development. However, the
contribution of zinc to porcine oocyte maturation has yet to be
determined. In the present study, the involvement of zinc in
porcine oocyte maturation was investigated by inducing zinc
deficiency during porcine IVM.
A decrease in the zinc concentration of oocytes was
induced by TPEN treatment. TPEN is a lipid-soluble zinc metal
chelator that decreases the intracellular levels of zinc (32). Although TPEN has a strong affinity
for transition metals, including iron, copper (33) and zinc, while the content of other
metals is unchanged relative to that of control cells, TPEN
treatment only significantly reduces the zinc content compared with
that in the control group (34).
TPEN is usually used as a zinc-specific chelator in in vitro
studies (35,36). The concentration of TPEN was set at
10 µM in accordance with the protocols of previous studies.
According to Kim et al (34), concentrations <10 µM did
not have any effect on meiotic maturation, whereas concentrations
≥20 µM had toxic effects on mice oocytes.
In the present study, a decrease in cumulus cell
expansion was observed as the first effect of TPEN treatment.
Cumulus cell expansion occurred normally in the control and
TPEN+zinc-treated groups but not in the TPEN-treated group.
TPEN-induced zinc deficiency was previously shown to markedly
increase apoptosis induced by cytokines, lipids and oxidative
stress in somatic cells (37–39).
Apoptosis caused by zinc deficiency may also inhibit cumulus cell
expansion. Cumulus cells have an important role in oocyte
maturation. Cumulus cells surround each individual oocyte and are
functionally associated to the nuclear or cytoplasmic maturation of
oocytes (40). Cumulus cells
control nuclear maturation by maintaining a meiotic block at the
germinal vesicle stage (41) and
trigger the resumption of meiosis by secreting a meiosis-inducing
substance (8). Cumulus cells are
required for cytoplasmic maturation and developmental competence
during IVM as they synthesize and transport GSH to oocytes
(42). Thus, poor expansion of
cumulus cells has a negative impact on oocyte maturation.
In the present study, nuclear maturation was also
affected by TPEN-induced zinc deficiency. MI oocytes increased in
number, while MII oocytes decreased in number only in the
TPEN-treated group. TPEN treatment causes a meiotic block at
telophase I during mouse IVM (34); however, in the present study, the
rates of anaphase and telophase did not change in any of the
groups, possibly due to differences in the meiotic process or the
role of zinc in meiosis in different species. In conventional IVM
of porcine oocytes, most incompetent oocytes are arrested at MI
(43), and zinc deficiency may be
a factor leading to MI arrest. As described above, poor cumulus
cell expansion would also have affected nuclear maturation.
Meiotic spindle morphology accurately reflects an
oocyte's meiotic status (44,45).
Therefore, spindle morphology was investigated by α-tubulin
immunofluorescence. The control and TPEN+zinc-treated groups
developed a metaphase spindle with a polar body, whereas the
TPEN-treated group only developed a metaphase spindle. According to
Ueno et al (46), spindle
morphology indicates oocyte quality. However, in the present study,
the shape and size of the spindles were similar in all groups. The
spindle length was ~10 µm, the spindle was round, and no
significant differences were observed between the experimental
groups. Thus, zinc deficiency did not directly affect the meiotic
spindle.
Microfilaments are cytoskeletal components with an
important role during cell division (47). In the present study, microfilament
abnormalities were observed in the TPEN-treated group. A previous
study showed that the microfilament area exists at the cortex
during metaphase in porcine oocytes (48). However, microfilaments occurred
randomly in the cytoplasm and cortex of TPEN-treated oocytes.
Abnormal microfilament distribution would affect meiotic
maturation. Longo and Chen (49)
reported on the role of microfilaments during meiosis in mouse
oocytes. Their study showed that the meiotic spindle with chromatin
failed to move to the oocyte cortex, and extrusion of the polar
body was inhibited by treatment with the microfilament-disrupting
agent cytochalasin B. This result provided an explanation for the
meiotic block and failure of polar body extrusion in TPEN-treated
oocytes. It also suggested t hat zinc is involved in microfilament
distribution during porcine oocyte maturation.
In the TPEN-treated group, the decreased GSH and
increased ROS levels indicated poor cytoplasmic maturity. The GSH
concentration increases during IVM, reaching its highest level at
MII (50,51). Decreased GSH in the TPEN-treated
oocytes can be explained by reduced cumulus cell expansion or
decreased synthesis due to improper cytoplasmic maturation. The
increased ROS levels in the TPEN-treated oocytes implied a lack of
anti-oxidant activity of GSH and zinc. The main functions of GSH in
oocytes include anti-oxidant activity and protection against the
harmful effects of ROS (52).
Although the underlying mechanisms have yet to be elucidated, zinc
is also involved in protecting oocytes from oxidative stress
(53). Zinc acts as an
anti-oxidant at multiple cell levels (54) and can induce the synthesis of
metallothionein, a protein that chelates redox-active metals and
scavenges hydroxyl radicals via its cysteine groups (55). Zinc is an important constituent of
Cu/Zn superoxide dismutase (56),
which scavenges free oxygen radicals. Numerous studies have
reported that a zinc deficiency induces oxidative stress in in
vitro-cultured cells (57–59).
Improper cytoplasmic maturation is a major problem
in IVM of porcine oocytes. The low developmental potential of
porcine oocytes during IVM is the result of improper cytoplasmic
maturation. In the present study, improper cytoplasmic maturation
due to zinc deficiency led to decreased embryonic development.
Thus, the role of zinc in the maturation process is important.
The duration of zinc deficiency also affected
porcine oocyte maturation. TPEN treatment fir >7 h inhibited
cumulus cell expansion and meiotic maturation. Furthermore, oocytes
did not recover with zinc supplementation after >15 h of TPEN
treatment. In previous studies on mouse oocytes, normal oocyte
meiotic maturation occurred with TPEN treatment for <7 h,
whereas meiotic maturation was inhibited by TPEN exposure for
>12 h. Although 9 h-exposed oocytes reached MII, they had a
large spindle and polar body (34). These results were not observed in
porcine oocytes. The effect of zinc on microfilaments may therefore
be different between species. In mice, meiotically blocked oocytes
due to TPEN treatment displayed a normal microfilament distribution
and cleavage, whereas an abnormal microfilament distribution was
present and no cleavage occurred in TPEN-treated pig oocytes.
In conclusion, the results of the present study
showed that nuclear and cytoplasmic maturation were decreased in
zinc-deficient porcine oocytes. Furthermore, zinc deficiency
decreased subsequent embryonic development. In addition, zinc
deficiency for >1 h caused irreversible damage to oocytes. These
results indicated that zinc regulates the meiotic process and has
important roles in oocyte maturation. Additional studies are
required to identify the mechanism of action of zinc during oocyte
maturation.
Acknowledgments
This work was supported, in part, by a grant from
the Next-Generation Bio Green 21 Program (no. PJ00956901), Rural
Development Administration, and the National Research Foundation of
Korea Grant funded by the Korean Government (nos.
NRF-2012R1A1A4A01004885 and NRF-2013R1A2A2A04008751), Republic of
Korea.
References
|
1
|
Sirard MA, Richard F, Blondin P and Robert
C: Contribution of the oocyte to embryo quality. Theriogenology.
65:126–136. 2006. View Article : Google Scholar
|
|
2
|
Mattioli M, Bacci ML, Galeati G and Seren
E: Developmental competence of pig oocytes matured and fertilized
in vitro. Theriogenology. 31:1201–1207. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh B, Barbe GJ and Armstrong DT:
Factors influencing resumption of meiotic maturation and cumulus
expansion of porcine oocyte-cumulus cell complexes in vitro. Mol
Reprod Dev. 36:113–119. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Funahashi H, Cantley T and Day BN:
Different hormonal requirements of pig oocyte-cumulus complexes
during maturation in vitro. J Reprod Fertil. 101:159–165. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Funahashi H and Day BN: Effects of the
duration of exposure to hormone supplements on cytoplasmic
maturation of pig oocytes in vitro. J Reprod Fertil. 98:179–185.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Illera MJ, Lorenzo PL, Illera JC and
Petters RM: Developmental competence of immature pig oocytes under
the influence of EGF, IGF-I, follicular fluid and gonadotropins
during IVM-IVF processes. Int J Dev Biol. 42:1169–1172. 1998.
|
|
7
|
Bing YZ, Naga T and Rodriguez-Martinez H:
Effects of cysteamine, fsh and estradiol-17beta on in vitro
maturation of porcine oocytes. Theriogenology. 55:867–876. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia GL, Kikuchi K, Noguchi J and Izaike Y:
Short time priming of pig cumulus-oocyte complexes with FSH and
forskolin in the presence of hypoxanthine stimulates cumulus cells
to secrete a meiosis-activating substance. Theriogenology.
53:1807–1815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tatemoto H, Sakurai N and Muto N:
Protection of porcine oocytes against apoptotic cell death caused
by oxidative stress during in vitro maturation: Role of cumulus
cells. Biol Reprod. 63:805–810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cetica PD, Pintos LN, Dalvit GC and Beconi
MT: Antioxidant enzyme activity and oxidative stress in bovine
oocyte in vitro maturation. IUBMB Life. 51:57–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nasr-Esfahani MH, Aitken JR and Johnson
MH: Hydrogen peroxide levels in mouse oocytes and early cleavage
stage embryos developed in vitro or in vivo. Development.
109:501–507. 1990.PubMed/NCBI
|
|
12
|
Luberda Z: The role of glutathione in
mammalian gametes. Reprod Biol. 5:5–17. 2005.PubMed/NCBI
|
|
13
|
Yoshida M, Ishigaki K, Nagai T, Chikyu M
and Pursel VG: Glutathione concentration during maturation and
after fertilization in pig oocytes: Relevance to the ability of
oocytes to form male pronucleus. Biol Reprod. 49:89–94. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong BS and Yang X: Cysteine, glutathione
and Percoll treatments improve porcine oocyte maturation and
fertilization in vitro. Mol Reprod Dev. 59:330–335. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biswas D, Jeon Y, Kim GH, Jeung EB and
Hyun SH: Effect of vascular endothelia growth factor on in vitro
porcine oocyte maturation and subsequent developmental competence
after parthenogenesis. J Anim Vet Adv. 9:2924–2931. 2010.
View Article : Google Scholar
|
|
16
|
Kwak SS, Jeung SH, Biswas D, Jeon YB and
Hyun SH: Effects of porcine granulocyte-macrophage
colony-stimulating factor on porcine in vitro-fertilized embryos.
Theriogenology. 77:1186–1197. 2012. View Article : Google Scholar
|
|
17
|
Walker SC, Shin T, Zaubrecher GM, Romano
JE, Johnson GA, Bazer FW and Piedrahita JA: A highly efficient
method for porcine cloning by nuclear transfer using in
vitro-matured oocytes. Cloning Stem Cells. 4:105–112. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nielsen FH: Ultratrace minerals mythical
elixirs or nutrients of concern? Bol Asoc Med P R. 83:131–133.
1991.PubMed/NCBI
|
|
19
|
Hostetler CE, Kincaid RL and Mirando MA:
The role of essential trace elements in embryonic and fetal
development in livestock. Vet J. 166:125–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vallee BL and Falchuk KH: The biochemical
basis of zinc physiology. Physiol Rev. 73:79–118. 1993.PubMed/NCBI
|
|
21
|
Favier AE: The role of zinc in
reproduction. Hormonal mechanisms. Biol Trace Elem Res. 32:363–382.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bedwal RS and Bahuguna A: Zinc, copper and
selenium in reproduction. Experientia. 50:626–640. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hostetler CE, Cronrath JD, Becker WC and
Mirando MA: Dietary supplementation of proteinated trace minerals
(OPTiMIN) in sows and replacement gilts increases mineral
concentrations in reproductive tissues. Abstracts 14th
International Congress Animal Reproduction. 1:2722000.
|
|
24
|
Wauben IP, Xing HC and Wainwright PE:
Neonatal dietary zinc deficiency in artificially reared rat pups
retards behavioral development and interacts with essential fatty
acid deficiency to alter liver and brain fatty acid composition. J
Nutr. 129:1773–1781. 1999.PubMed/NCBI
|
|
25
|
Sakuma S, Fujimoto Y, Miyata Y, Ohno M,
Nishida H and Fujita T: Effects of Fe (2+), Zn (2+), Cu (2+) and Se
(4+) on the synthesis and catabolism of prostaglandins in rabbit
gastric antral mucosa. Prostaglandins Leukot Essent Fatty Acids.
54:193–197. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakuma S, Fujimoto Y, Kitao A, Sakamoto H,
Nishida H and Fujita T: Simultaneous measurement of prostaglandin
and arachidonoyl CoA formed from arachidonic acid in rabbit kidney
medulla microsomes: The roles of Zn2+ and Cu2+ as modulators of
formation of the two products. Prostaglandins Leukot Essent Fatty
Acids. 61:105–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chanmugam P, Wheeler C and Hwang DH: The
effect of zinc deficiency on prostaglandin synthesis in rat testes.
J Nutr. 114:2066–2072. 1984.PubMed/NCBI
|
|
28
|
de Haan JB, Tymms MJ, Cristiano F and Kola
I: Expression of copper/zinc superoxide dismutase and glutathione
peroxidase in organs of developing mouse embryos, fetuses and
neonates. Pediatr Res. 35:188–196. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chesters JK: Trace element-gene
interactions with particular reference to zinc. Proc Nutr Soc.
50:123–129. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeon Y, Yoon JD, Cai L, Hwang SU, Kim E,
Zheng Z, Lee E, Kim DY and Hyun SH: Supplementation of zinc on
oocyte in vitro maturation improves preimplatation embryonic
development in pigs. Theriogenology. 82:866–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeon Y, Kwak SS, Cheong SA, Seong YH and
Hyun SH: Effect of trans-epsilon-viniferin on in vitro porcine
oocyte maturation and subsequent developmental competence in
preimplantation embryos. J Vet Med Sci. 75:1277–1286. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Treves S, Trentini PL, Ascanelli M, Bucci
G and Di Virgilio F: Apoptosis is dependent on intracellular zinc
and independent of intracellular calcium in lymphocytes. Exp Cell
Res. 211:339–343. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith RM and Martell AE: NIST critically
selected stability constants of metal complexes database. National
Institute of Standards and Technology; 2004, http://www.nist.gov/srd/upload/46_8.htm.
Accessed June 22nd, 2015.
|
|
34
|
Kim AM, Vogt S, O'Halloran TV and Woodruff
TK: Zinc availability regulates exit from meiosis in maturing
mammalian oocytes. Nat Chem Biol. 6:674–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY
and Yoon YH: Depletion of intracellular zinc and copper with TPEN
results in apoptosis of cultured human retinal pigment epithelial
cells. Invest Ophthalmol Vis Sci. 42:460–465. 2001.PubMed/NCBI
|
|
36
|
Nakatani T, Tawaramoto M, Opare Kennedy D,
Kojima A and Matsui-Yuasa I: Apoptosis induced by chelation of
intracellular zinc is associated with depletion of cellular reduced
glutathione level in rat hepatocytes. Chem Biol Interact.
125:151–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pang W, Leng X, Lu H, Yang H, Song N, Tan
L, Jiang Y and Guo C: Depletion of intracellular zinc induces
apoptosis of cultured hippocampal neurons through suppression of
ERK signaling pathway and activation of caspase-3. Neurosci Lett.
552:140–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mendivil-Perez M, Velez-Pardo C and
Jimenez-Del-Rio M: TPEN induces apoptosis independently of zinc
chelator activity in a model of acute lymphoblastic leukemia and ex
vivo acute leukemia cells through oxidative stress and mitochondria
caspase-3- and AIF-dependent pathways. Oxid Med Cell Longev.
2012:3132752012. View Article : Google Scholar
|
|
39
|
Meerarani P, Ramadass P, Toborek M, Bauer
HC, Bauer H and Hennig B: Zinc protects against apoptosis of
endothelial cells induced by linoleic acid and tumor necrosis
factor alpha. Am J Clin Nutr. 71:81–87. 2000.PubMed/NCBI
|
|
40
|
Abeydeera LR: In vitro production of
embryos in swine. Theriogenology. 57:256–273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanghe S, Van Soom A, Nauwynck H, Coryn M
and de Kruif A: Minireview: Functions of the cumulus oophorus
during oocyte maturation, ovulation and fertilization. Mol Reprod
Dev. 61:414–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maedomari N, Kikuchi K, Ozawa M, Noguchi
J, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T and Kashiwazaki N:
Cytoplasmic glutathione regulated by cumulus cells during porcine
oocyte maturation affects fertilization and embryonic development
in vitro. Theriogenology. 67:983–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kikuchi K, Somfai T, Nakai M and Nagai T:
Appearance, fate and utilization of abnormal porcine embryos
produced by in vitro maturation and fertilization. Soc Reprod
Fertil Suppl. 66:135–147. 2009.PubMed/NCBI
|
|
44
|
Buendia B, Clarke PR, Félix MA, Karsenti
E, Leiss D and Verde F: Regulation of protein kinases associated
with cyclin A and cyclin B and their effect on microtubule dynamics
and nucleation in Xenopus egg extracts. Cold Spring Harb Symp Quant
Biol. 56:523–532. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Albertini DF: Cytoplasmic reorganization
during the resumption of meiosis in cultured preovulatory rat
oocytes. Dev Biol. 120:121–131. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ueno S, Kurome M, Ueda H, Tomii R, Hiruma
K and Nagashima H: Effects of maturation conditions on spindle
morphology in porcine MII oocytes. J Reprod Dev. 51:405–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roberts K, Raff M, Alberts B, Walter P,
Lewis J and Johnson A: Molecular Biology of the Cell. 5th edition.
Routledge; London: 2002
|
|
48
|
Kim NH, Funahashi H, Prather RS, Schatten
G and Day BN: Microtubule and microfilament dynamics in porcine
oocytes during meiotic maturation. Mol Reprod Dev. 43:248–255.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Longo FJ and Chen DY: Development of
cortical polarity in mouse eggs: involvement of the meiotic
apparatus. Dev Biol. 107:382–394. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Funahashi H, Cantley TC, Stumpf TT,
Terlouw SL and Day BN: Use of low-salt culture medium for in vitro
maturation of porcine oocytes is associated with elevated oocyte
glutathione levels and enhanced male pronuclear formation after in
vitro fertilization. Biol Reprod. 51:633–639. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zuelke KA, Jeffay SC, Zucker RM and
Perreault SD: Glutathione (GSH) concentrations vary with the cell
cycle in maturing hamster oocytes, zygotes and pre-implantation
stage embryos. Mol Reprod Dev. 64:106–112. 2003. View Article : Google Scholar
|
|
52
|
Brad AM, Bormann CL, Swain JE, Durkin RE,
Johnson AE, Clifford AL and Krisher RL: Glutathione and adenosine
triphosphate content of in vivo and in vitro matured porcine
oocytes. Mol Reprod Dev. 64:492–498. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Powell SR: The antioxidant properties of
zinc. J Nutr. 130(5S Suppl): 1447S–1454S. 2000.PubMed/NCBI
|
|
54
|
Bray TM and Bettger WJ: The physiological
role of zinc as an antioxidant. Free Radic Biol Med. 8:281–291.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sato M and Bremner I: Oxygen free radicals
and metallothionein. Free Radic Biol Med. 14:325–337. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Olin KL, Golub MS, Gershwin ME, Hendrickx
AG, Lonnerdal B and Keen CL: Extracellular superoxide dismutase
activity is affected by dietary zinc intake in nonhuman primate and
rodent models. Am J Clin Nutr. 61:1263–1267. 1995.PubMed/NCBI
|
|
57
|
Ho E and Ames BN: Low intracellular zinc
induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA
binding, and affects DNA repair in a rat glioma cell line. Proc
Natl Acad Sci USA. 99:16770–16775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Oteiza PI, Clegg MS, Zago MP and Keen CL:
Zinc deficiency induces oxidative stress and AP-1 activation in 3T3
cells. Free Radic Biol Med. 28:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song Y, Leonard SW, Traber MG and Ho E:
Zinc deficiency affects DNA damage, oxidative stress, antioxidant
defenses, and DNA repair in rats. J Nutr. 139:1626–1631. 2009.
View Article : Google Scholar : PubMed/NCBI
|