Introduction
Numerous types of congenital heart defect and
peripheral vascular disease require repairing with vascular
conduits. Autologous vascular grafts often cannot be used for this
purpose due to limited sources, donor site morbidity or even damage
from a pre-existing disease (1).
However, synthetic materials, including expanded
polytetrafluoroethylene (ePTFE) and polyethylene terephthalate
fibre (Dacron) have been widely used in cases of large vessel
lesions (2). However, these
materials are usually non-degradable and lack the potential for
growth. Furthermore, these grafts often suffer from immunologic and
thrombotic complications, including rejection, stenosis,
thrombosis, calcium deposition and infection, as revealed by
long-term follow-up studies (3).
Tissue engineering, in which regenerating vascular
grafts are constructed using cells and degradable scaffolds, allows
for an alternative approach to resolve these problems (4). Promising results have been reported
using tissue-engineered blood vessels (TEBVs) (5), but the repairable vascular defects
are limited to small diameters (<6 mm) under low blood pressure
(6–8). In cases with larger vessels (>6 mm
in diameter), the biomechanical properties of these engineered
grafts cannot sufficiently withstand the powerful impact of blood
flow (9).
In addition, tissue engineering approaches are
limited by the availability of autologous cells. These cells have
often shown a limited replicative capacity, particularly when they
originated from older donors. Therefore, it is necessary to find an
autologous source of progenitor vascular cells with a high
proliferative capacity for the preparation of TEBVs. A possible
solution is the use of bone marrow-derived cells (BMCs), which have
demonstrated multiple cell differentiation abilities and a high
proliferation potential. In previous studies, BMCs have been found
to differentiate into smooth muscle (SM)-like cells or endothelial
cells in vitro (10–15).
Furthermore, when compared with embryonic stem cells, BMCs are not
confounded by ethical considerations and are readily available for
research. In addition, bone marrow aspiration is less invasive and
carries a reduced risk of morbidity at the donor site and may thus
be a promising alternative cell source for vascular tissue
engineering (16–18).
The aim of the present study was to engineer a large
vessel (6 mm in diameter) with canine BMCs and a polyglycolic acid
(PGA) scaffold in a pulsatile flow bioreactor for four weeks of
dynamic cultivation. PGA is a useful scaffold material, as its
rapid degradation prevents the accumulation of degraded fragments
in the body (4). The mechanical
properties of the vessel engineered in the present study were then
assessed in order to test the applicability of the developed method
to the engineering of various organ parts, including blood vessels
and large muscular tubular structures, such as the oesophagus,
intestine or ureter.
Materials and methods
Experimental animals
Twelve adult beagles (6 months old) were purchased
from Chenhang Experimental Animal Raising Farm (Shanghai, China).
The experimental protocol was approved by the Animal Care and
Experimental Committee of Shanghai Jiao Tong University School of
Medicine (Shanghai, China). All the protocols of animal handling
were approved by the Research Ethical Committee of the
hospital.
BMCs isolation and culture
Following sacrifice via intravenous administration
of 50 mg/kg 1% alpha-chloralose (LongDan Pharmaceutical and
Chemical Co., Shanghai, China), bone marrow (10 ml from each dog)
was aspirated from the humeri of the dogs and mixed with heparin
(100 unit heparin/ml bone marrow; Sigma-Aldrich, St. Louis, MO,
USA). Four healthy canine abdominal arteries were donated for use
in the study by the Cardiovascular Research Centre of the Shanghai
9th People's Hospital (Shanghai, China). The bone marrow and
heparin mixture was centrifuged on a Ficoll-Paque density gradient
(Amersham Bioscience, Arlington Heights, IL, USA) for 20 min at 352
× g. Bone marrow mononuclear cells (BMMNCs) were isolated from the
buffy coat layer between the Ficoll-Paque reagent and blood plasma
component and washed three times in phosphate-buffered saline (PBS;
Sigma-Aldrich) solution. The BMMNCs were cultured in low-glucose
Dulbecco's modified Eagle's medium (LG-DMEM; Gibco-BRL, Invitrogen
Life Technologies, Inc., Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA), 100
U/ml penicillin (Sigma-Aldrich), and 100 mg/ml streptomycin
(Sigma-Aldrich) at 37°C with 95% humidity and 5% CO2.
The medium was replaced twice a week. The characterization of the
BMCs was determined by their CD marker profiles and their ability
to differentiate into osteogenic, adipogenic and chondrogenic
lineages as previously reported (data not shown) (19). BMCs at passage 2 were used for the
vessel construction. Prior to seeding, the identity of the BMCs was
confirmed by immunofluorescent staining with mouse monoclonal
anti-α-smooth muscle actin (α-SMA; cat. no. C6198; Sigma-Aldrich),
rabbit monoclonal anti-α-calponin (cat. no. ab46794; Abcam,
Cambridge, UK) and mouse monoclonal anti-smooth muscle myosin heavy
chain (SM-MHC; cat. no. M7786; Sigma-Aldrich), all at a dilution of
1:100.
Culture of BMC-PGA sheets in dishes
A total of 30 mg unwoven PGA fibres (Albany
International Research Company Inc., Albany, NY, USA) were
constructed into a 35×30×2 mm mesh. The scaffold was first soaked
in 75% ethanol (Sigma-Aldrich) for 2 h. Subsequently, it was washed
three times with PBS and incubated in DMEM for 10 min. The medium
was removed, and the scaffold was incubated at 37°C prior to being
used. The BMCs were collected and re-suspended in the culture
medium at a density of 6×107 cells/ml. Finally,
3×107 cells were evenly seeded onto the PGA mesh in
100-mm culture dishes (Falcon, Oxnard, CA, USA). To accomplish the
complete adhesion of the BMCs to the fibres, the cell scaffold
constructs were then incubated at 37°C with 95% humidity and 5%
CO2 for 4 h. Thereafter, sufficient DMEM with 10% FBS
was added to the dish to cover the construct. The cell-PGA sheet
was incubated for another five days prior to being placed into the
bioreactor.
Scanning electron microscopic (SEM)
observation
For the observation of the growth conditions of the
cells on the PGA, parts of the cell-PGA sheets were harvested for
an SEM examination after five days of culture. The samples were
first pre fixed with 2.5% glutaraldehyde (Sigma-Aldrich) for 2 h at
4°C and then washed three times with PBS. Thereafter, the samples
were post fixed with 1% osmic acid (Sigma-Aldrich) for 2 h at 4°C.
After washing with PBS three more times, the samples were
dehydrated through an ethanol gradient (Sigma-Aldrich), mounted,
sputter-coated with gold and then viewed under a scanning electron
microscope (Quanta 200; FEI, Hillsboro, OR, USA).
Vessel reactor preparation
The blood vessel reactor (Hongli, Shanghai, China)
was a closed-loop perfusion system that primarily consisted of a
culture chamber, silicone tubes, a media reservoir, a peristaltic
pump, a magnetic valve and a pressure meter. The culture chamber
including the four culture dishes was composed of transparent
polycarbonate with four lids for gas exchange (Fig. 1). The 6-mm outer diameter
medical-grade silicone tube was threaded through the side arms of
the chamber and secured. Sterile PBS was pumped from the media
reservoir through the silicone tubes and back into the reservoir.
The circulation was managed by a peristaltic pump and a magnetic
valve. The former provided continuous fluid flow at a rate of
0.01–1,000 ml/min. The latter was periodically closed under the
control of a custom-made computer. The periodic closing of the
valve produced a pulsed flow in the bioreactor that attempted to
mimic the circulation system in vivo (blood vessel
bioreactor: Designed and manufactured by Professor Hong Li;
Department of Life Information and Instrument Engineering, Hangzhou
Electronic Science and Technology University, Hangzhou, Zhejiang,
China). Therefore, it was possible to adjust the corresponding
radial distension rate of the silicone tube by modifying the flow
rate. The pressure meter showed the changes in the media pressure,
which was necessary for the modification of the circulation system.
Prior to use, the culture chamber was sterilised with ethylene
oxide.
Dynamic culture in the vessel
reactor
After being cultured in dishes for five days, the
cell PGA sheets were wrapped around the silicone tubes and further
fixed by biodegradable sutures (Ethicon, Inc., Somerville, NJ, USA;
Fig. 2B) in the culture chamber of
the vessel reactor. The sheets were covered with DMEM containing
10% FBS in the chamber. Thereafter, the culture chamber was
connected to the entire vessel reactor. Sterile PBS flowed through
the silicone tubes in a pulsatile manner at a frequency of 75
beats/min. The flow rate was gradually increased and adjusted
(70–80 ml/min) to reach a radial distension of ~5% of the original
diameter of the constructs. The culture chamber was placed in the
incubator at 37°C for four weeks, and the culture media was changed
twice a week. Cell-PGA constructs cultured under static conditions
without pulsatile radial stress were used as controls.
Histological analyses
After four weeks of culture, the engineered vessel
walls were harvested, fixed in 10% formalin (Sigma-Aldrich) and
embedded in paraffin (Sigma-Aldrich). The vessels were then
sequentially cut into 4-µm sections. The sections were then
subjected to haematoxylin and eosin (HE) or Masson's trichrome and
Gomori staining (all stains were from Sigma-Aldrich).
Biomechanical testing and hydroxyproline
assay
A tensile tester (4456; Instron, Norwood, MA, USA)
was used in the biomechanical tests. As previously reported
(20), the engineered vessel wall
was placed between the jaws of the tester with a constant
elongation (3 mm/min) applied along the longitudinal axis until
rupture occurred to determine the maximum tensile force. To
determine the suture-holding strength, 5–0 polypropylene sutures
(Ethicon, Inc.) were placed in four quadrants of the vessel wall at
a location of 1 mm from the vessel edge, followed by constant
elongation (3 mm/min) along the longitudinal axis of the vessel in
the same tester until the sutures pulled through the edge of the
vessel (21). For the
hydroxyproline assay, the vessel wall was dried and weighed. The
total hydroxyproline content of each vessel was determined by a
colorimetric assay, as described by Reddy and Ewemeka (22). In the current study, a
Sigma-MAK008, Hydroxyproline Assay Kit (Sigma-Aldrich) and Genesys
20 Spectrophotometer (Z376027; Sigma-Aldrich) were used. Native
canine abdominal arteries (6 mm in diameter) served as the normal
controls in all of the above tests.
Statistical analysis
Each experiment was repeated at least three times.
As the original data were normally distributed, the results were
expressed as the mean ± standard deviation. Significant differences
between values were determined using Student's t-test. A
P-value less than 0.05 was considered to indicate a statistically
significant difference. All of the statistical analyses were
performed using SPSS version 16 (SPSS, Inc., Chicago, IL, USA).
Results
Culture of BMCs
After 4–5 days of culture in the plates, the BMCs
were elongated and showed a spindle-like shape. Within another 2 3
days, the cells reached confluency and were subsequently passaged
onto a fresh plate. Immunofluorescent staining of the cells at
passage 2 showed that nearly half of the cells were positive for
anti-alpha-SMA, calponin and SM-MHC staining (Fig. 2).
Culture of BMC-PGA constructs in
dishes
Passage-2 BMCs were seeded onto the PGA mesh. After
24 h of culture, the cells began to spread and extended along the
length of the fibres. After another five days of culture in the
dishes, a cell-PGA sheet was formed (Fig. 3A). Micrographs showed that the
abundant BMCs had adhered to the PGA fibres, with secreted
extracellular matrix (ECM) filling the space between the fibres
(Fig. 3B), which was further
confirmed by SEM observation (Fig.
3C).
Dynamic culture in the vessel
reactor
After being placed into the bioreactor, the BMC-PGA
constructs were incubated for four weeks (Fig. 3D). In the dynamic group, the
constructs demonstrated a glossy and tubular structure with a round
lumen of 6 mm in diameter (Fig.
4A). The engineered vessel in the dynamic group already
exhibited excellent elasticity after four weeks of culture. The
vessel wall had the capacity to repeatedly rebound to its original
shape after deformation to a flat shape with tweezers. By contrast,
the vessel walls in the static culture group showed a collapsed
lumen and rough surface (Fig. 4B).
In addition, the control vessels grown for 4 weeks in static
culture failed to show any elasticity and easily collapsed upon the
application of pressure.
Histological observation
After four weeks of culture in the bioreactor,
several layers of smooth muscle-like cells and a few collagenous
fibres were revealed by histological examination (Fig. 5A and B). The PGA fibres did not
degrade completely, and a few elastic fibres were observed at this
time-point (Fig. 5C). By contrast,
disorganised and randomly distributed cells, randomly organized
collagenous fibres and small elastic fibres were observed in the
static group (Fig. 5D–F). The
above results were further confirmed by immunohistochemical
staining for α-SMA and calponin (data not shown), using a canine
abdominal artery as a positive control (Fig. 5G–I).
Biomechanical testing and hydroxyproline
assay
There were significant differences in the
biomechanical properties between the dynamic and static groups.
After being cultured for four weeks, the tensile strength (Fig. 6) and the suture-holding retention
strength (Fig. 7) of the dynamic
group were much higher (P<0.05) than those in the static group.
In addition, the biomechanical properties of the dynamic group
attained nearly 40% of those of the native canine abdominal
arteries.
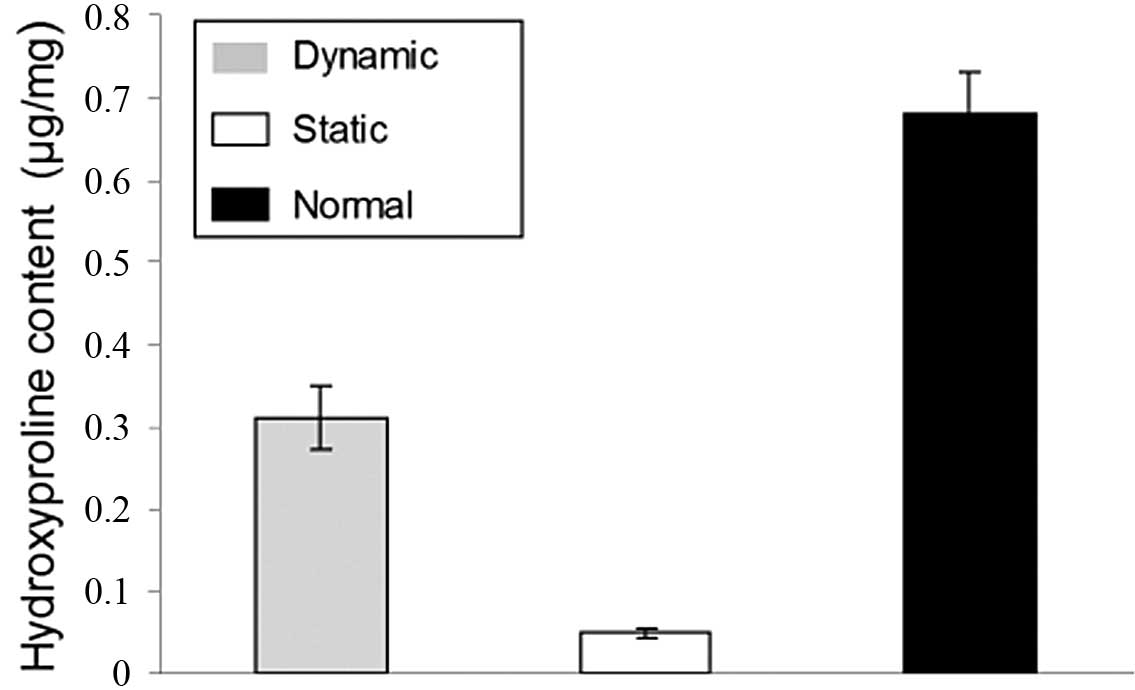
The hydroxyproline content of the dynamic group was
significant higher (P<0.05) than that in the static group at the
same time-points (Fig. 8). The
hydroxyproline concentration in the dynamic group reached ~40% of
that in the native canine abdominal arteries.
Discussion
Numerous studies have demonstrated the potential
ability of BMCs to differentiate into vascular cell types and serve
as 'seed cells' in vitro (14,23–28).
In such applications, BMCs may require an extended period during
which they are seeded and cultured in vitro prior to being
implanted in vivo. In the present study, the cultured BMCs,
without any requirement for additional cytokines or transgenic
technology, were used to construct an elastic large muscular vessel
wall under pulsatile stimulation in a bioreactor. After culture,
the newly formed tissues showed SM cell-like characteristics,
including morphological indicators, the expression of SM cell
specific cytoskeletal markers (α SMA and calponin) and fine
biomechanical properties. These results demonstrated that BMCs are
able to differentiate into SM or SM-like cells in vitro and
may be utilised as a potential cell source for the tissue
engineering of SMs, particularly that of large-diameter aortas.
The ideal vascular substitute should be
non-thrombogenic and resistant to infections as well as possess
good biocompatibility, biological characteristics, sufficient
mechanical properties and compliance that match those of the native
vessels, as well as be able to respond to physiological stimuli
with vasomotion and remodelling (29). As to the repair of large vessels
under high blood pressure, strong mechanical and haemodynamic
properties are of great importance for the success of the
substitute, which has been shown in the successful application of
synthetic materials, including Dacron and ePTFE, even in the
absence of endothelial cell sheeting (30,31).
Previous studies have indicated that good mechanical properties are
most important in the engineering of large blood vessels (1,4,29).
To create novel and functional TEBVs in
vitro, cells were seeded onto biodegradable polymer scaffolds
in the present study. An optimal polymer scaffold supports
biological tissue growth by providing the proper surface for cell
attachment, proliferation, differentiation and the secretion of
extracellular ECM proteins, as well as by directing new tissue
formation. PGA is one of the most widely used polymer scaffold
materials in the engineering of numerous types of tissue, including
blood vessels, as it undergoes rapid degradation to soluble, safe
by-products through the chemical hydrolysis of the chain ester
linkages and supports cellular adhesion and collagenous matrix
deposition (32,33). However, rapidly degrading PGA may
substantially damage the tissue mechanics. This effect was apparent
in the control group of the present study.
To overcome this limitation, several vessel
bioreactors have been developed and utilised for vascular tissue
engineering with the aim of mimicking a suitable physiological
environment for the arterial vessel wall (6,34–37).
The blood's mechanical force is an important physiological
component of the environment experienced by cells: It promotes the
circumferential orientation of the cells as well as the deposition
of the extracellular matrix and likely contributes to the survival
of the implanted substitute. Several studies reported on the
effects of the cyclic strain observed in smooth muscle cells, whose
responses demonstrated an increased production of growth factors,
enhanced synthesis or proliferation of the matrix and an increased
content of contractile protein (38–40).
Kim and Mooney found more elastin and contractile SMCs in the
constructs cultured under a cyclic strain than in the controls (no
strain) (41). Stegemann et
al (42) showed that four days
of exposure to periodic mechanical strain increased aortic SMC
proliferation in rats. Hamilton et al (43) demonstrated that cyclic strain can
be used to stimulate the expression of SMC specific genes in
primary BMCs. Similarly, the results of the present study
demonstrated that the engineered vessel walls exhibited improved
biomechanical properties following pulsatile stimulation with
cyclic mechanical training in the bioreactor compared with those of
vessels cultured under static conditions. Furthermore, the present
study confirmed that vessels grown in dynamic culture exhibited
good orientation of smooth muscle layers and even organization of
the collagenous fibres according to histological observation.
In elastic and muscular arteries, elastin is a
critical structural and regulatory matrix protein that has an
important and dominant role in conferring elasticity to the vessel
wall (44). Elastin also regulates
vascular smooth muscle cell activity and phenotype (40,45).
Several scaffolds have been studied to promote elastin
biosynthesis. Long and Tranquillo (46) demonstrated that more elastin was
secreted by SMCs on fibrin gels than on collagen gels. According to
a study by Ramamurthi and Vesely (47), a scaffold composed of hyaluron gels
cross-linked with divinyl sulfone had the ability to increase the
amount of elastin secreted by the SMCs. Thomas and Nair (48) showed promising results according to
which the SMCs exhibited increased elastin production on a
co-polymer of natural biodegradable gelatin with vinyl acetate
(GeVAc) after mechanical stimulation. These studies indicated that
a scaffold can have a significant impact on the synthesis of
elastin. Therefore, further studies focusing on modifying PGA
fibres with fibrin or cross-linking it with other promising
materials may be beneficial for engineering improved vessel walls
with a higher elastin content.
In the present study, Masson staining of artery
sections for the identification of SMCs revealed red staining of
native arteries, while the staining was faint in the construct
cells, indicating that the nutrient supply in the engineered vessel
was poor. As the vessel grew while being wrapped around a silicone
tube, the nutrients in the culture media are required to pass
through the thick vessel wall to reach the inner part. This implies
that the thickness of the engineered vessel wall was limited in the
culture system of the present study. Further improvements in the
culture system are required in future studies.
Several previous studies have demonstrated that the
mechanical stimulation of cells has significant effects on the cell
phenotype, ECM deposition and mechanical properties of the graft
(48–52). An optimal culture strategy should
facilitate the efficient regeneration of vascular tissue. The
stimulation of BMCs with a 10% strain for 7 days was reported to
obviously upregulate the expression of α-SMA and h1-calponin
(48). In addition, Oluwole et
al (53) demonstrated that the
cyclic deformation of a 350- and 2,500-µ strain applied at 1
Hz to 10-day-old bone marrow cultures resulted in elevated levels
of alkaline phosphatase and prostacyclins. In the present study, a
5% radial distension of the vessels was achieved using a rate of 75
beats/min, which is similar to the circulation conditions in native
adult vessels in vivo. The mechanical properties of the
vessel walls engineered under the dynamic culture were
significantly higher than those cultured under the static
conditions but did not attain the same parameters as the native
arteries. Therefore, it is necessary to plan an advanced control
strategy and optimise the regeneration process of the vascular
tissue inside the vessel bioreactor. Specifically, future studies
should address the pulse rate, deformation rate, pulsatile pressure
and culture duration.
In conclusion, an elastic large vessel wall (6 mm in
diameter) was constructed in the present study using a PGA seeded
with BMCs under pulsatile flow conditions in a vessel bioreactor.
The dynamically cultured constructs exhibited a superior tissue
constitution according to histological examination, as well as
enhanced mechanical properties compared with the constructs that
were grown in stationary culture. Further studies will focus on
seeding endothelial cells on the surface of the lumen to engineer
composite vascular conduits and to repair canine aortic
defects.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81000842). The
authors would like to thank Professor Hong Li (Department of Life
Information and Instrument Engineering, Hangzhou Electronic Science
and Technology University, Hangzhou, China), and Demin Ying, Lijuan
Zong and Bing Zhong (laboratory technicians of the Department of
Plastic and Reconstructive Surgery, Shanghai 9th People's Hospital,
School of Medicine, Shanghai Jiaotong University, Shanghai, China)
for their technical support.
References
|
1
|
Naito Y, Shinoka T, Duncan D, Hibino N,
Solomon D, Cleary M, Rathore A, Fein C, Church S and Breuer C:
Vascular tissue engineering: Towards the next generation vascular
grafts. Adv Drug Deliv Rev. 63:312–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khait L and Birla RK: Bypassing the
patient: Comparison of biocompatible models for the future of
vascular tissue engineering. Cell Transplant. 21:269–283. 2012.
View Article : Google Scholar
|
|
3
|
Wang F, Mohammed A, Li C, Ge P, Wang L and
King MW: Degradable/non-degradable polymer composites for in-situ
tissue engineering small diameter vascular prosthesis application.
Biomed Mater Eng. 24:2127–2133. 2014.PubMed/NCBI
|
|
4
|
Woods I and Flanagan TC: Electrospinning
of biomimetic scaffolds for tissue-engineered vascular grafts:
Threading the path. Expert Rev Cardiovasc Ther. 12:815–832. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinberg CB and Bell E: A blood vessel
model constructed from collagen and cultured vascular cells.
Science. 231:397–400. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao
C, Chang LJ, Chen YE, Ma PX and Yang B: Engineering vascular tissue
with functional smooth muscle cells derived from human iPS cells
and nanofibrous scaffolds. Biomaterials. 35:8960–8969. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niklason LE, Gao J, Abbott WM, Hirschi KK,
Houser S, Marini R and Langer R: Functional arteries grown in
vitro. Science. 284:489–493. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe M, Shin'oka T, Tohyama S, Hibino
N, Konuma T, Matsumura G, Kosaka Y, Ishida T, Imai Y, Yamakawa M,
et al: Tissue-engineered vascular autograft: Inferior vena cava
replacement in a dog model. Tissue Eng. 7:429–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nerem RM and Seliktar D: Vascular tissue
engineering. Ann Rev Biomed Eng. 3:225–243. 2001. View Article : Google Scholar
|
|
10
|
Galmiche MC, Koteliansky VE, Brière J,
Hervé P and Charbord P: Stromal cells from human long-term marrow
cultures are mesenchymal cells that differentiate following a
vascular smooth muscle differentiation pathway. Blood. 82:66–76.
1993.PubMed/NCBI
|
|
11
|
Kashiwakura Y, Katoh Y, Tamayose K,
Konishi H, Takaya N, Yuhara S, Yamada M, Sugimoto K and Daida H:
Isolation of bone marrow stromal cell-derived smooth muscle cells
by a human SM22alpha promoter: In vitro differentiation of putative
smooth muscle progenitor cells of bone marrow. Circulation.
107:2078–2081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu K, Sugiyama S, Aikawa M, Fukumoto
Y, Rabkin E, Libby P and Mitchell RN: Host bone-marrow cells are a
source of donor intimal smooth-muscle-like cells in murine aortic
transplant arteriopathy. Nat Med. 7:738–741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong Z, Calkins G, Cheng EC, Krause D and
Niklason LE: Influence of culture medium on smooth muscle cell
differentiation from human bone marrow derived mesenchymal stem
cells. Tissue Eng Part A. 15:319–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang N, Ren GD, Zhou Z, Xu Y, Qin T, Yu RF
and Zhang TC: Cooperation of myocardin and smad2 in inducing
differentiation of mesenchymal stem cells into smooth muscle cells.
IUBMB Life. 64:331–339. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kusuma S, Facklam A and Gerecht S:
Characterizing human pluripotent stem cell-derived vascular cells
for tissue engineering applications. Stem Cells Dev. 24:451–458.
2015. View Article : Google Scholar
|
|
16
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colter DC, Sekiya I and Prockop DJ:
Identification of a subpopulation of rapidly self-renewing and
multipotential adult stem cells in colonies of human marrow stromal
cells. Proc Natl Acad Sci USA. 98:7841–7845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujiwara H, Oda K, Saiki Y, Sakamoto N,
Ohashi T, Sato M, Tabata Y and Tabayashi K: The wrapping method
using biodegradable felt strips has a preventive effect on the
thinning of the aortic wall: Experimental study in the canine
aorta. J Vasc Surg. 43:349–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaner PJ, Martin ND, Tulenko TN, Shapiro
IM, Tarola NA, Leichter RF, Carabasi RA and Dimuzio PJ:
Decellularized vein as a potential scaffold for vascular tissue
engineering. J Vasc Surg. 40:146–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reddy GK and Ewemeka CS: A simplified
method for the analysis of hydroxyproline in biological tissues.
Clin Biochem. 29:225–229. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho SW, Kim IK, Lim SH, Kim DI, Kang SW,
Kim SH, Kim YH, Lee EY, Choi CY and Kim BS: Smooth muscle-like
tissues engineered with bone marrow stromal cells. Biomaterials.
25:2979–2986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong Z and Niklason LE: Small-diameter
human vessel wall engineered from bone marrow-derived mesenchymal
stem cells (hMSCs). FASEB J. 22:1635–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang G, Drinnan CT, Geuss LR and Suggs
LJ: Vascular differentiation of bone marrow stem cells is directed
by a tunable three-dimensional matrix. Acta Biomater. 6:3395–3403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sekiguchi H, Ii M, Jujo K, Yokoyama A,
Hagiwara N and Asahara T: Improved culture-based isolation of
differentiating endothelial progenitor cells from mouse bone marrow
mononuclear cells. PloS One. 6:e286392011. View Article : Google Scholar
|
|
27
|
van den Akker NM, Kolk FF, Jeukens F,
Verbruggen S, Gagliardi M, Dullens S, Heschel I, Post MJ, Molin DG
and Waltenberger J: Vascular potency of Sus scrofa bone
marrow-derived mesenchymal stem cells: A progenitor source of
medial but not endothelial cells. Tissue Eng Part A. 18:828–839.
2012. View Article : Google Scholar
|
|
28
|
Roh JD, Brennan MP, Lopez-Soler RI, Fong
PM, Goyal A, Dardik A and Breuer CK: Construction of an autologous
tissue-engineered venous conduit from bone marrow-derived vascular
cells: Optimization of cell harvest and seeding techniques. J
Pediatr Surg. 42:198–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu H, Su J, Sun J and Ren T: Preparation
and characterization of new nano-composite scaffolds loaded with
vascular stents. Int J Mol Sci. 13:3366–3381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedman SG, Lazzaro RS, Spier LN, Moccio
C and Tortolani AJ: A prospective randomized comparison of Dacron
and polytetrafluoroethylene aortic bifurcation grafts. Surgery.
117:7–10. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peck M, Gebhart D, Dusserre N, McAllister
TN and L'Heureux N: The evolution of vascular tissue engineering
and current state of the art. Cells Tissues Organs. 195:144–158.
2012. View Article : Google Scholar :
|
|
32
|
Pankajakshan D and Agrawal DK: Scaffolds
in tissue engineering of blood vessels. Can J Physiol Pharmacol.
88:855–873. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gui L, Zhao L, Spencer RW, Burghouwt A,
Taylor MS, Shalaby SW and Niklason LE: Development of novel
biodegradable polymer scaffolds for vascular tissue engineering.
Tissue Eng Part A. 17:1191–1200. 2011. View Article : Google Scholar :
|
|
34
|
Engbers-Buijtenhuijs P, Buttafoco L, Poot
AA, Dijkstra PJ, de Vos RA, Sterk LM, Geelkerken RH, Vermes I and
Feijen J: Biological characterization of vascular grafts cultured
in a bioreactor. Biomaterials. 27:2390–2397. 2006. View Article : Google Scholar
|
|
35
|
Arrigoni C, Chittò A, Mantero S and
Remuzzi A: Rotating versus perfusion bioreactor for the culture of
engineered vascular constructs based on hyaluronic acid. Biotechnol
Bioeng. 100:988–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Y, Wennink JW, Kamphuis MM, Sterk LM,
Vermes I, Poot AA, Feijen J and Grijpma DW: Dynamic culturing of
smooth muscle cells in tubular poly (trimethylene carbonate)
scaffolds for vascular tissue engineering. Tissue Eng Part A.
17:381–387. 2011. View Article : Google Scholar
|
|
37
|
Couet F and Mantovani D: A new bioreactor
adapts to materials state and builds a growth model for vascular
tissue engineering. Artif Organs. 36:438–445. 2012. View Article : Google Scholar
|
|
38
|
Seliktar D, Nerem RM and Galis ZS: The
role of matrix metalloproteinase-2 in the remodeling of cell-seeded
vascular constructs subjected to cyclic strain. Ann Biomed Eng.
29:923–934. 2001. View Article : Google Scholar
|
|
39
|
Stegemann JP and Nerem RM: Phenotype
modulation in vascular tissue engineering using biochemical and
mechanical stimulation. Ann Biomed Eng. 31:391–402. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nikolovski J, Kim BS and Mooney DJ: Cyclic
strain inhibits switching of smooth muscle cells to an
osteoblast-like phenotype. FASEB J. 17:455–457. 2003.PubMed/NCBI
|
|
41
|
Kim BS and Mooney DJ: Scaffolds for
engineering smooth muscle under cyclic mechanical strain
conditions. J Biomech Eng. 122:210–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stegemann JP, Hong H and Nerem RM:
Mechanical, biochemical and extracellular matrix effects on
vascular smooth muscle cell phenotype. J Appl Physiol (1985).
98:2321–2327. 2005. View Article : Google Scholar
|
|
43
|
Hamilton DW, Maul TM and Vorp DA:
Characterization of the response of bone marrow-derived progenitor
cells to cyclic strain: Implications for vascular
tissue-engineering applications. Tissue Eng. 10:361–369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patel A, Fine B, Sandig M and Mequanint K:
Elastin biosynthesis: The missing link in tissue-engineered blood
vessels. Cardiovasc Res. 71:40–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Opitz F, Schenke-Layland K, Cohnert TU,
Starcher B, Halbhuber KJ, Martin DP and Stock UA: Tissue
engineering of aortic tissue: Dire consequence of suboptimal
elastic fiber synthesis in vivo. Cardiovasc Res. 63:719–730. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Long JL and Tranquillo RT: Elastic fiber
production in cardiovascular tissue-equivalents. Matrix Biol.
22:339–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramamurthi A and Vesely I: Evaluation of
the matrix-synthesis potential of crosslinked hyaluronan gels for
tissue engineering of aortic heart valves. Biomaterials.
26:999–1010. 2005. View Article : Google Scholar
|
|
48
|
Thomas LV and Nair PD: The effect of
pulsatile loading and scaffold structure for the generation of a
medial equivalent tissue engineered vascular graft. Biores Open
Access. 2:227–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kanda K, Matsuda T and Oka T: Mechanical
stress induced cellular orientation and phenotypic modulation of
3-D cultured smooth muscle cells. ASAIO J. 39:M686–M690. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chiquet M, Matthisson M, Koch M,
Tannheimer M and Chiquet-Ehrismann R: Regulation of extracellular
matrix synthesis by mechanical stress. Biochem Cell Biol.
74:737–744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim BS and Mooney DJ: Scaffols for
engineering smooth muscle under cyclic mechanical strain
conditions. J Biomech Eng. 122:210–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi ZD and Tarbell JM: Fluid flow
mechanotransduction in vascular smooth muscle cells and
fibroblasts. Ann Biomed Eng. 39:1608–1619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Oluwole BO, Du W, Mills I and Sumpio BE:
Gene regulation by mechanical forces. Endothelium. 5:85–93. 1997.
View Article : Google Scholar : PubMed/NCBI
|