Introduction
Diosgenin, isolated from Dioscorea
zingiberensis C.H. Wright, is used as a raw material to
synthesize WRC3, and is widely distributed throughout China
(1,2). Diosgenin has been extensively used in
traditional Chinese medicine for the treatment of various diseases
(1,2). Steroidal saponins have recently
attracted attention due to their structural diversity and
significant biological activities (3), including anticancer,
anti-hyperlipidemia, anti-inflammatory (4), hemolytic, antibacterial,
anti-thrombotic (5),
immunomodulatory (6) and anti-HIV
activities. Tong et al (7)
and Rivera et al (8)
reported that seven steroidal saponins isolated from Dioscorea
zingiberensis C.H. Wright were cytotoxic and resulted in the
apoptosis of cancer cells. Kvasnica et al (9) reported that the twelve steroidal
derivatives, platinum (II) complexes with steroidal esters of
l-histidine and l-methionine, exhibited significant effects against
the CEM T-lymphoblastic leukemia cell line with IC50
values in the range of 14–25 µM (10). However, the site of action of the
effective compounds remains to be understood and the precise
mechanisms are not clear.
In recent years, our group has focused on
identifying novel saponins from Dioscorea zingiberensis C.H.
Wright and synthesizing new compounds of diosgenin with glucosyl,
as well as determining the acute toxicity (11) and sub-chronic toxicity, the
structure-activity relationships, and the pharmaceutical activities
of the natural and modified steroidal saponins. Tong et al
(7) reported that the sugar moiety
of steroidal saponins may be crucial in the cytotoxic activity
against cancer cell lines. A saponin derivative, WRC3 synthesized
by our laboratory, has previously been shown to exhibit an
anti-thrombotic effect (12).
However, recently it was identified that this saponin derivative
presented inhibitory effects on cancer cells.
The aim of the present study was to confirm the
anticancer effect of WRC3 and to investigate the possible site of
action using murine cancer cell lines.
Materials and methods
Reagents
Dichloromethane, acetonitrile, acetic anhydride, tin
tetrachloride and sodium methoxide were purchased from Aladdin
Chemical Co., Ltd. (Shanghai, China). Anhydrous dichloromethane was
further purified by distilling with calcium hydride. Anhydrous
acetonitrile was purified by distilling over potassium hydroxide.
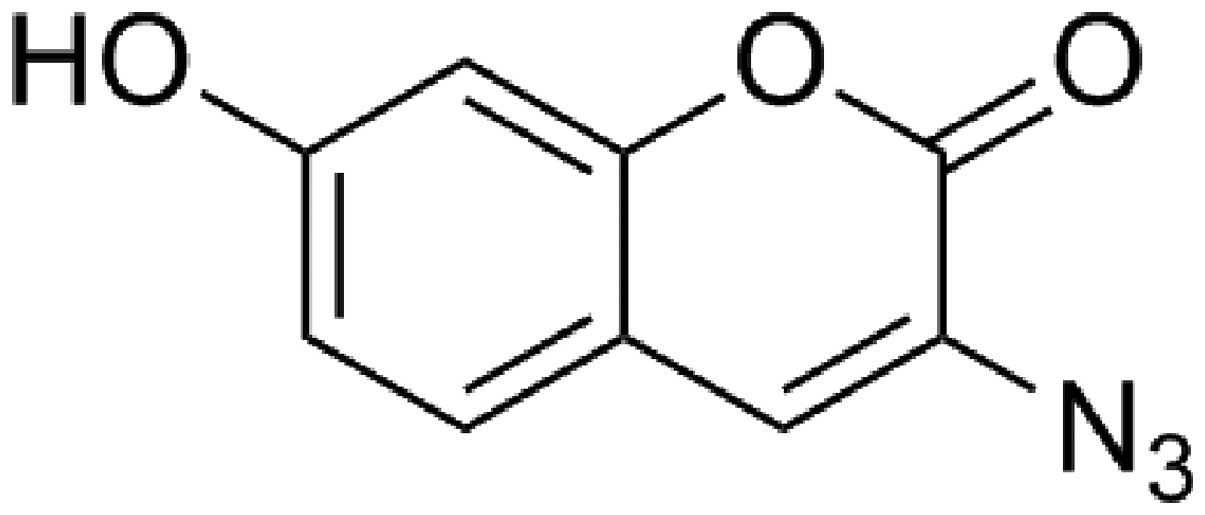
The probe shown in Fig. 1 was
purchased from ABIDING Technology Co., Ltd. (Chengdu, China).
Diosgenin (≥99.0%),
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium (MTT), and
dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
WRC3 synthesis
WRC3 was synthesized as previously described and its
purity was determined by high performance liquid chromatograpgy
(HPLC, >98.0%) (12). WRC4 was
synthesized by our group (purity determined by HPLC, >98%) and
was further modified with alkynyl group (13,14)
for investigating cell distribution of WRC3.
Cell lines and culture
Mouse B16 melanoma cells (BCRC 60031) were purchased
from the Bioresources Collection and Research Center (BCRC,
Hsinchu, Taiwan) and cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco Life Technologies, Carlsbad, CA, USA). The cells were
supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies), 100 U/ml penicillin and 100 µg/ml
streptomycin, at 37°C in a 5% CO2 atmosphere (15).
Animals
All animal experiments were approved by the ethics
committee of the West China Hospital, West China Medical School,
Sichuan University (Sichuan, China), and conducted according to the
Institutional Animal Care and Use Committee Guidelines of the West
China Hospital, West China Medical School, Sichuan University. Male
Kunming mice (36±2 g) were provided by the West China Hospital
Experiment Animal Center (Chengdu, China). The animals were housed
in a comfortable environment under appropriate temperature (22±1°C)
and humidity (55±5%) control with a 12 h light/12 h dark cycle, and
allowed free access to food and water.
Cell viability measurement
B16 cells, purchased from the Cell Bank of the
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China), were cultured in RPMI-1640
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 U/ml streptomycin, at 37°C and
95% relative humidity with 5% CO2. The cell viability
assay was performed using MTT. Briefly, cells were plated in
96-well plates at a density of 5×103 cells/well,
following incubation overnight. A series of diosgenyl derivative
(WRC3) were dissolved in DMSO, and diluted with culture media to a
series of concentrations (10, 20, 40, 60 and 80 µM). When
the cells were exposed to diosgenyl derivatives for 48 h, 10% MTT
was added, and cells were incubated for an additional 4 h at 37°C.
The absorbance was then measured with a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) at 540 nm. All
experiments were repeated three times.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining of WRC3 treated B16
cells
Annexin V-FITC/PI double-staining assay was used to
quantify apoptosis, according to the manufacturer's instructions
(KeyGEN, Nanjing, China). B16 cells were treated with WRC3 (2.5
µM) for 24 h and collected. Cells from each well were
centrifuged for 5 min at 2000 × g at 4°C, washed twice with
phosphate-buffered saline and suspended in 300 µl binding
buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Annexin V-FITC conjugate (3 µl) and 3
µl PI solution was added to each cell suspension and
incubated for 15 min at room temperature in the dark. The samples
were analyzed on a flow cytometer (MoFlo™ Cytomation, Modular Flow
Cytometer) and Cell-Quest software (version 3.1; BD Biosciences,
Franklin Lakes, NJ, USA). Double staining of cells with
FITC-Annexin V and PI permits the discrimination between live cells
(FITC−PI−), early apoptotic
(FITC+PI−), late apoptotic
(FITC+PI+) and necrotic cells
(FITC−PI+) (16,17).
Microscopy analysis distribution of
fluorescence in cells
B16 mouse melanoma cells were seeded in 6-well
plates (3×105/2 ml per well) containing glass
coverslips, and were cultivated in 10% FCS/RPMI-1640 medium at
37°C. Growth medium was supplemented with alkynyl-modified WRC4
(100 µM). After growing for 3 days, the cells were washed
with 10% PBS on cover slips, and cells were fixed and permeabilized
with paraformaldehyde for 10 min, then subjected to the probe
labeling reaction as follows: 0.1 mM probe/1 mM CuSO4/2
mM sodium ascorbate were mixed in PBS at room temperature for 30
min. Subsequently, the fixed and labeled cells were rinsed with PBS
and stained with PI (2 µg/ml in 5% BSA/PBS) at room
temperature for 30 min (13).
Fluorescent images were captured by Nikon ECLIPSE Ti (Chengdu,
China) laser scanning confocal microscopy system.
Acute toxicity studies in mice
Twelve Kunming mice were administered WRC3 (2.5, 5.0
or 7.5 g/kg) dissolved in 0.9% normal saline and were observed for
a week. After 1 week, the mice were sacrificed by decapitation
under pentobarbital anaesthesia (60 mg/kg body weight), and blood
and main viscera samples were obtained, prior to biochemical
analysis. Serum was collected to measure alanine transaminase
(ALT), aspartate transaminase (AST), UREA and creatine (CREA).
Liver tissue recovered from the necropsy was divided into two
sections, one was used for visual inspection of any morphological
changes in the organ tissue samples, and the other was fixed with
10% formalin, embedded in paraffin, sectioned and stained with
hematoxylin and eosin (HE) (Aladdin Chemical Co., Ltd., Shanghai,
China) for histological examination using standard techniques.
Following HE staining, the slides were observed and the images were
captured using a BX51TF BX51 optical microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Data were analyzed by one-way analysis of variance
followed by post hoc least significant difference test using SPSS
software package Version 19.0 for Windows (SPSS, Inc., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of WRC3 on the proliferation of
B16 melanoma cells
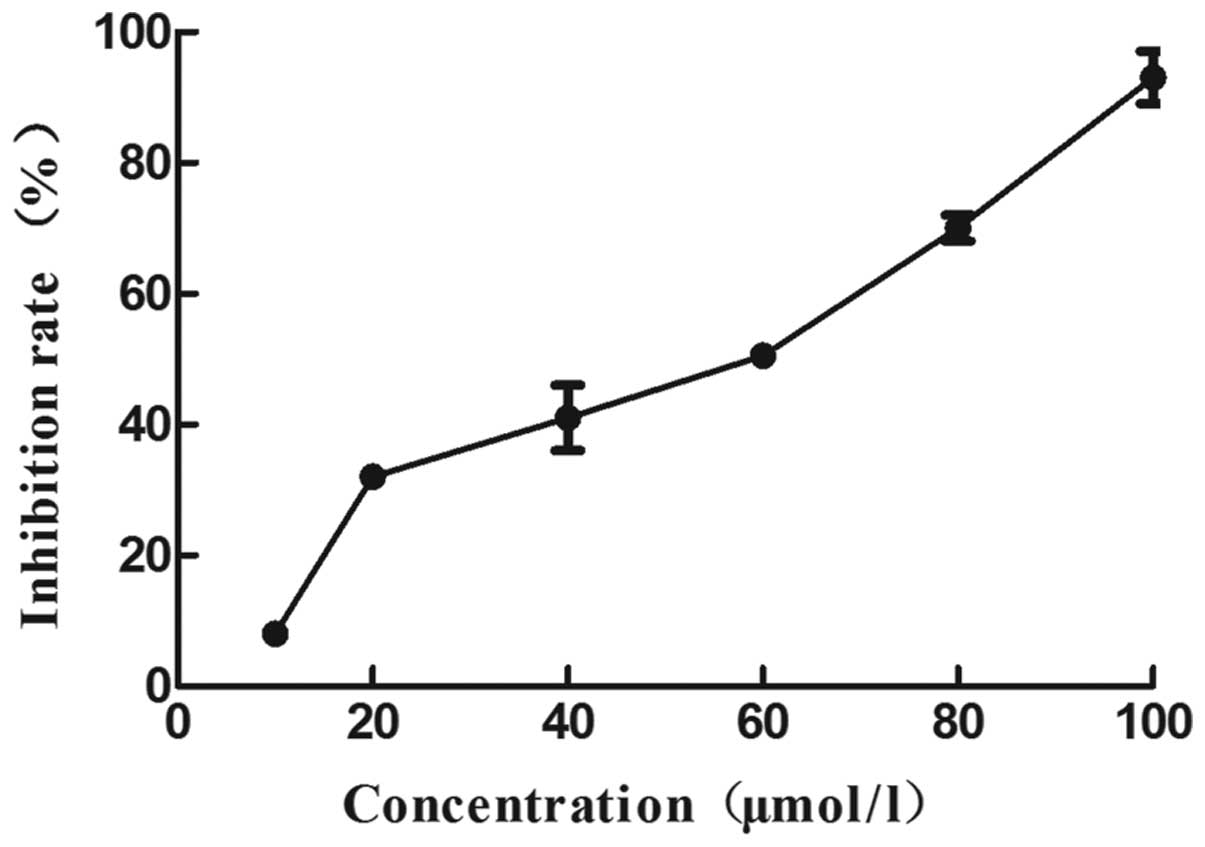
The survival rate of B16 melanoma cells treated with
WRC3 was determined using an MTT assay. As shown in Figs. 2 and 3, the effect of WRC3 on the survival rate
of B16 melanoma cells was examined. Significant WRC3 cytotoxic
effects were observed and the viability of the B16 melanoma cells
decreased to 37% at 20 mM, and 71% at 80 µM (Fig. 4). Notably, WRC3 significantly
inhibited B16 cell survival in a dose-dependent manner, starting a
12.09 µM of IC50. In addition, the survival of
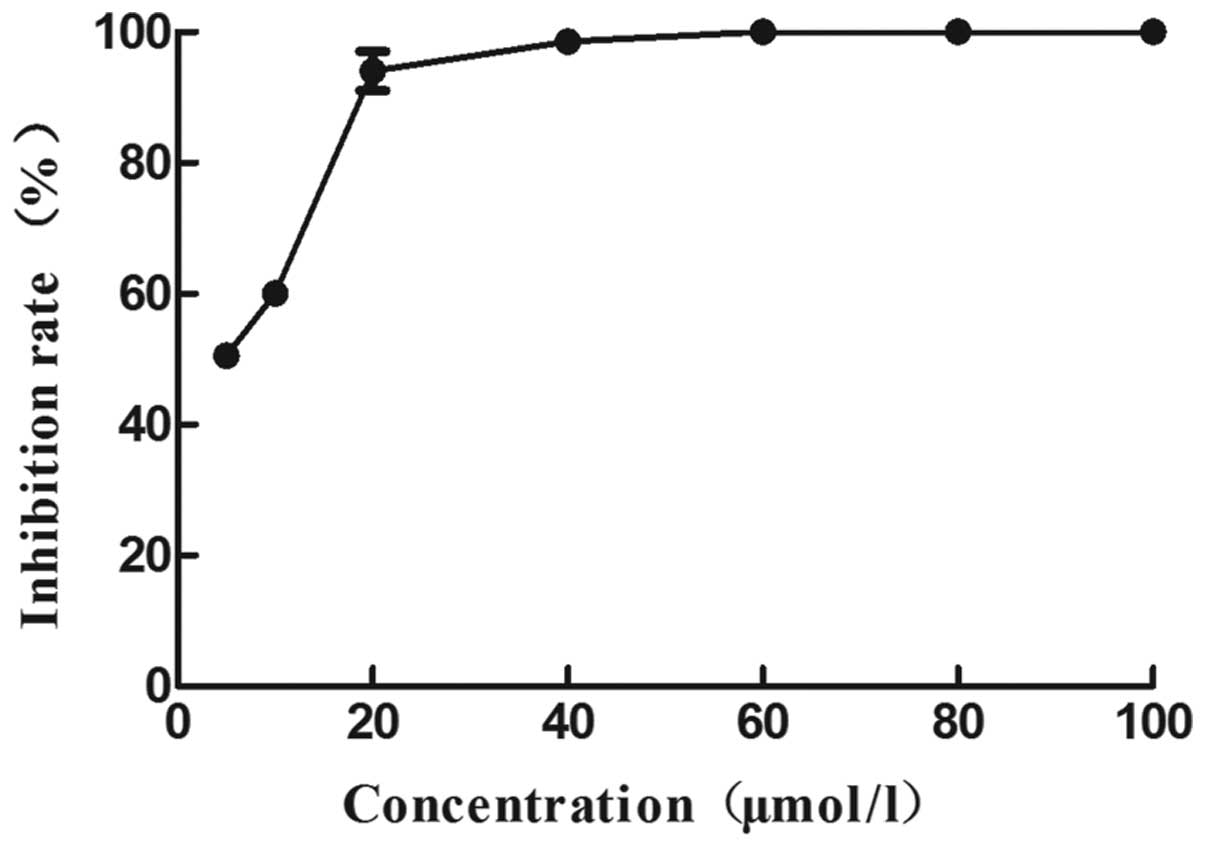
the cells treated with 20 µM WRC3 for 48 h was significantly
decreased (<10%), compared with that at 24 h (Fig. 3). These results suggest that the
inhibitory effect of WRC3 on B16 cells increases in a
time-dependent manner. Therefore, a 24 h treatment time was
selected for the subsequent experiments.
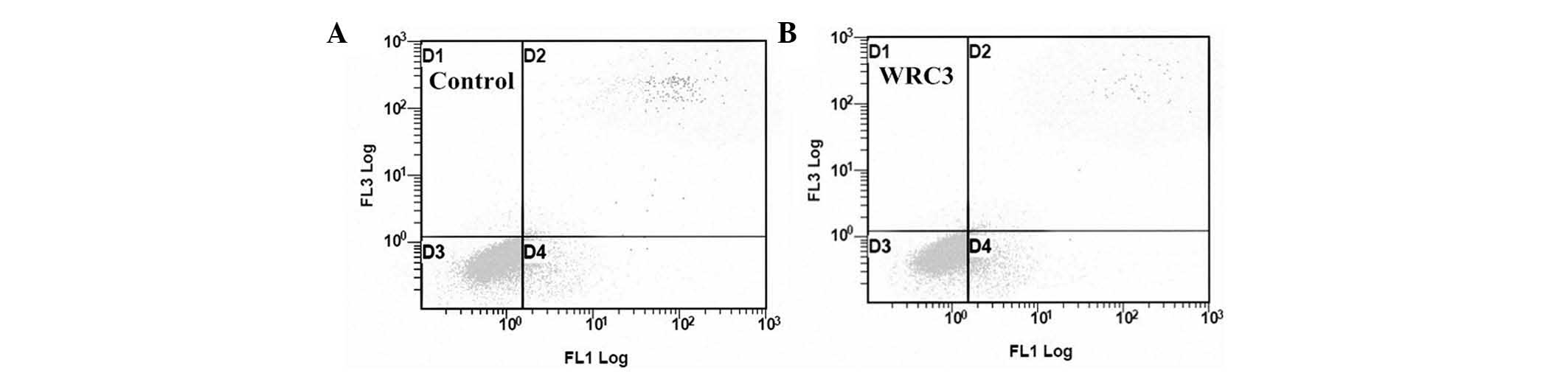
Analysis of apoptosis by flow cytometry
in B16 cells
Flow cytometric analysis using an Annexin V-FITC
apoptosis kit identified apoptosis of B16 cells induced by WRC3. As
shown in Fig. 5, the B16 cells
were treated with WRC3 (2.5 µM) for 24 h, which caused the
percentage of early apoptotic cells to increase to 10.64% and the
percentage of late apoptotic cells to decrease to 1.15%. In the
control group, the early and late apoptotic cells were observed to
be 7.17 and 2.42%, respectively. These results suggest that WRC3
predominantly induces early apoptosis in B16 cells.
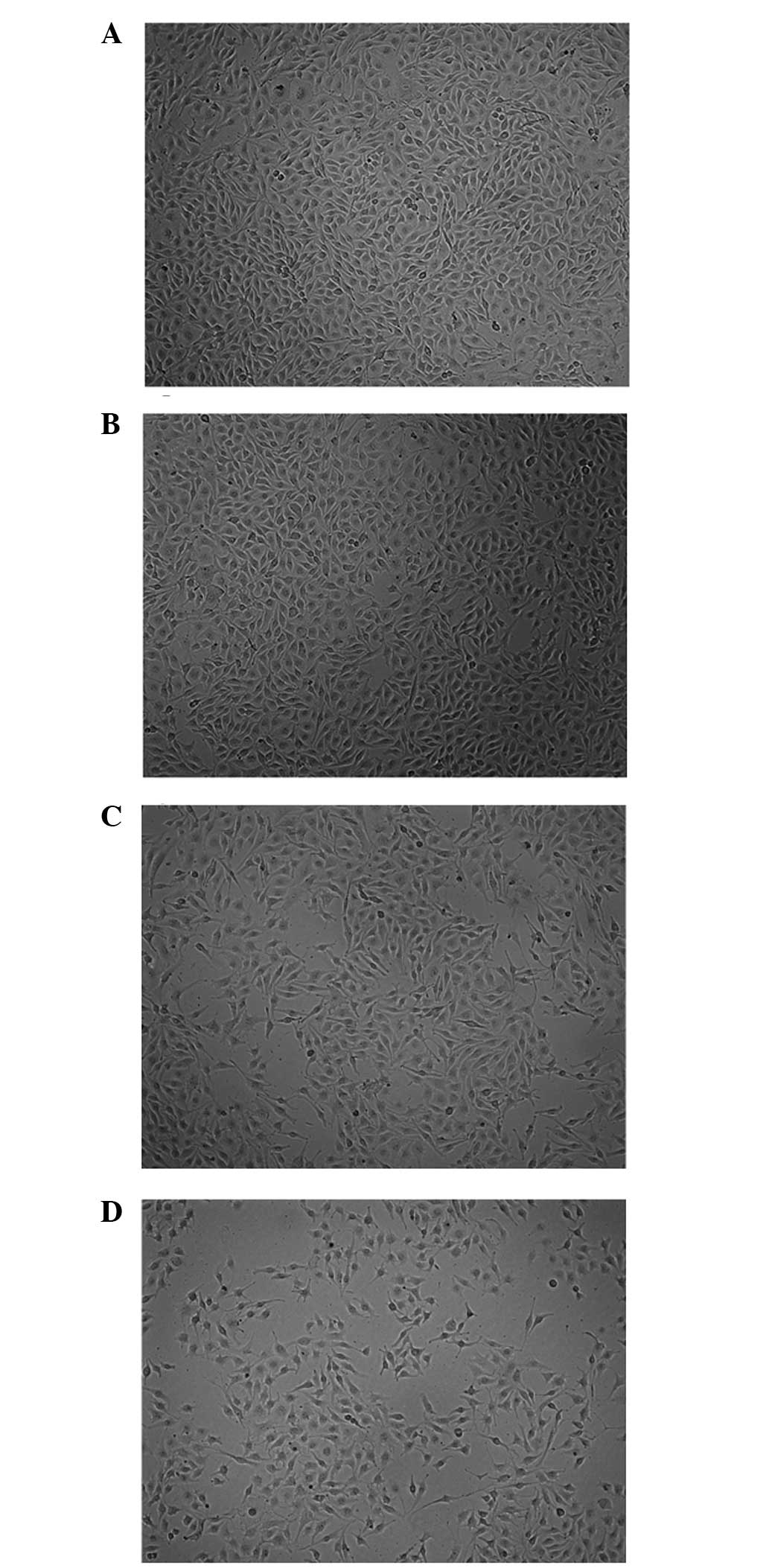
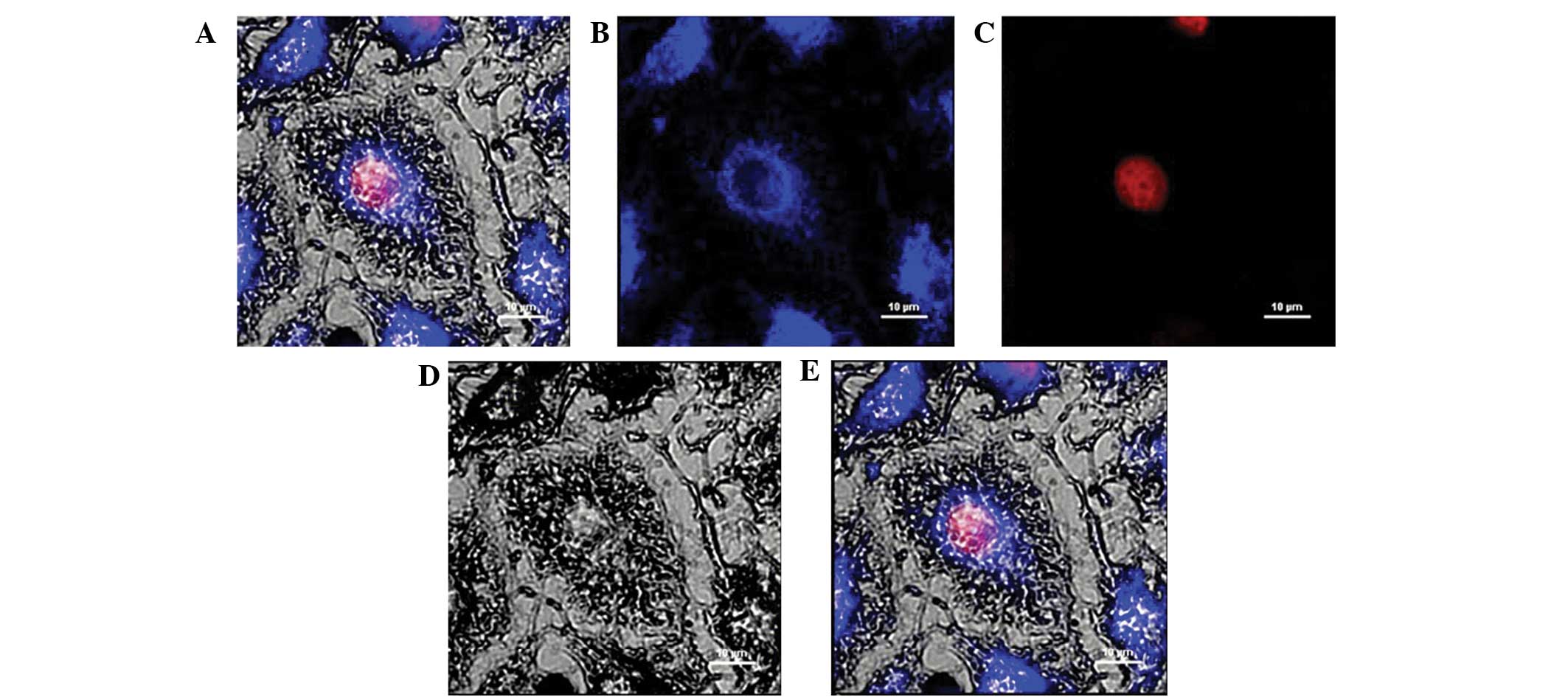
Visualization of WRC3 in melanoma B16
cells
Fluorescence analysis was used to investigate the
distribution of the drug compounds, which may provide information
for future drug development. The use of a murine B16 cell line
verified that WRC4 was not located in the nucleus, but was
distributed in the cytoplasm. In addition, the specific
sequestration of WRC4 in the cell was monitored by confocal
microscopy, to produce high resolution images. Notably, WRC4 was
not located in the nucleus, but was distributed in the cytoplasm
(Fig. 6), suggesting that WRC3 may
enter the cell and reach the cytoplasm where the primary targets of
WRC3 are located, and interfere with cell growth without causing
genotoxicity.
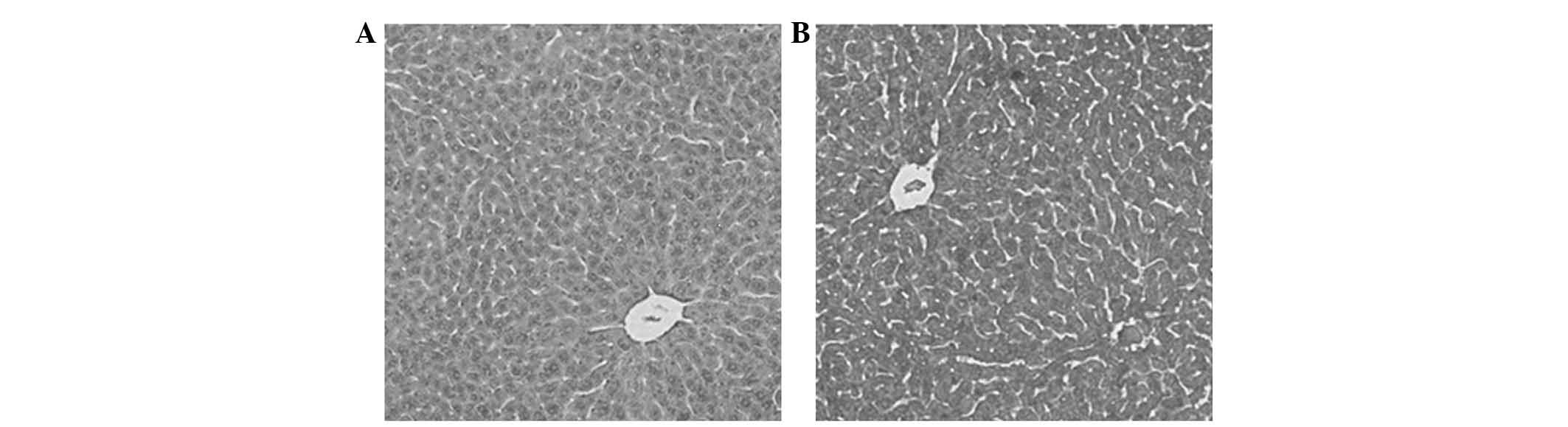
Acute toxicity studies in mice
In the acute toxicity study (Table I), no cell death was recorded in
any of the animals that received WRC3 (2.5, 5.0 and 7.5 g/kg body
weight). In addition, no obvious alterations in organ color were
observed in the treated animals, as compared with the control. The
levels of ALT, AST, CREA and UREA in the serum of mice treated with
various concentrations of WRC3 (2.5, 5.0 and 7.5 g/kg body weight)
were not observed to be significantly different (Table I). Histopathological examination of
the mice treated with or without WRC3 was evaluated by HE staining
to determine the toxicity of WRC3 on organ tissue samples. Fig. 7 revealed that the liver tissue
samples of the mice fed with WRC3 did not exhibit significant
histological changes, and the structure of the mouse liver lobules
and sinusoids were clearly defined, as compared with the control
group, suggesting that WRC3 exhibits no hepatotoxicity.
 | Table IEffects of the WRC3 on AST, ALT, CREA
and UREA levels in the serum of rats. |
Table I
Effects of the WRC3 on AST, ALT, CREA
and UREA levels in the serum of rats.
| Group | AST (U/l) | ALT (U/l) | CREA
(µmol/l) | UREA
(µmol/l) |
|---|
| Control | 104.67±0.81 | 35.73±0.93 | 49.87±0.81 | 4.20±0.14 |
| 2.5 g/kg of WRC3 | 171.33±6.58 | 50.13±2.89 | 58.13±0.35 | 5.45±0.35 |
| 5.0 g/kg of WRC3 | 135.60±7.43 | 43.33±0.74 | 50.67±0.71 | 5.77±0.74 |
| 7.5 g/kg of WRC3 | 128.80±1.44 | 37.73±0.93 | 62.13±0.35 | 6.37±0.71 |
Discussion
Steroidal saponins have diversity of structure, and
are reported to have various biological activities, including anti
hyperlipidemic, antibacterial, anti-inflammatory, immunomodulatory,
anti-human immunodeficiency virus and anticarcinogenic activities
(18). However, the mechanism and
site of action underlying the effects of the compounds remains to
be elucidated. In the present study, the effects of WRC3, a saponin
derivative, was investigated on the proliferation of B16 cells. The
IC50 of diosgenin was >20 µM in the B16 cells
(data not shown), whereas that of WRC3 was 12.09 µM, which
is lower than that of diosgenin. The results of the present study
indicated that WRC3 is able to enter melanoma cells and inhibit the
growth of cancer cells in a time and concentration-dependent
manner. In addition, the liver of mice treated with WRC3 did not
exhibit significant histological changes. Simultaneously, the
target of WRC3 in B16 cells was investigated by fluorescence
analysis.
A greater understanding of the mechanisms of action
associated with anticancer activity will facilitate the use of drug
intervention as an important strategy to prevent cancer development
(19). A previous study reported
that the pathogenesis of numerous diseases, including cancer, are
closely associated with aberrantly regulated apoptotic cell death
(20). In the present study, the
saponin derivative WRC3 was able to suppress the proliferation of
B16 cells in a dose-dependent manner, and the results from the
Annexin V-FITC/PI staining assay also demonstrated that WRC3
induced early apoptosis in B16 cells, as compared with the control
group. These results indicated that WRC3 may prove beneficial in
cancer treatment by mediating apoptotic cell death.
The ability of drugs to enter cells to effect the
growth of cancer cells is important. Knowledge regarding the
cellular distribution of drugs is important for understanding and
predicting drug action and toxicity. Therefore, identifying the
targets of a drug is important for drug development (21). The present study investigated the
distribution of WRC3 using a fluorescence tracker technique, and
demonstrated that WRC4 was not located in the nucleus, but was
instead distributed in the cytoplasm. The results of the present
study also demonstrated that the fluorescence tracker technique is
an important tool for the detection of drug distribution. WRC3
contains a hydroxyl group (chemical handle), which is important for
the fluorescence tracker technique. The chemical handle is able to
react with terminal alkynyl compounds to obtain alkynyl-WRC3.
Alkynyl-WRC3 and 3-azido-7-hydroxy-coumarin reacted in the cells
catalyzed by CuSO4 and sodium ascorbate, which may be
detected in the cells by fluorescence microscopy. In our previous
studies, a WRC3 analogue with alkynes was designed to investigate
WRC3 distribution in cells (22,23).
WRC3 was observed in the cytoplasm of B16 cells by confocal
microscopy (Fig. 6) suggesting
that WRC3 can enter into B16 cells to induce the apoptotic cell
death of cancer cells without causing genetic toxicity. ALT, AST,
CREA and UREA levels in the serum together with tissue H&E
staining, indicate that WRC3 was not toxic to mice organs.
In conclusion, it was demonstrated that a diosgenyl
analogue WRC3 inhibits cancer cells in vitro and is not
toxic to the liver and kidneys. In addition, the present study also
provided a method for locating the action site of WRC3 using
click-chemistry, which is important for the determination of the
drug target in the cells by fluorescence labeling. The results
further provided evidence that the sugar moiety of steroidal
saponins may be crucial for their antitumor activity. These
findings were concordant with those of a previous study
demonstrating that the anti-cancer effects of steroidal saponins
with sugar moieties are markedly higher compared with those of
parent nucleus diosgenin (7).
Saponins have the potential to be developed as anticancer agents,
and thus require further investigation.
Acknowledgments
This study was supported by the China National
'12.5' Foundation (grant no. 2011BAJ07B04) and National Natural
Science Foundation of China (grant no. 20972105).
References
|
1
|
Li H, Huang W, Wen Y, Gong G, Zhao Q and
Yu G: Anti-thrombotic activity and chemical characterization of
steroidal saponins from Dioscorea zingiberensis C.H Wright.
Fitoterapia. 81:1147–1156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu W, Huang W, Sun W, Zhu Y and Ni J:
Production of diosgenin from yellow ginger (Dioscorea zingiberensis
C.H. Wright) saponins by commercial cellulase. World J Microbiol
Biotechnol. 26:1171–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sparg SG, Light ME and van Staden J:
Biological activities and distribution of plant saponins. J
Ethnopharmacol. 94:219–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung DH, Park HJ, Byun HE, Park YM, Kim
TW, Kim BO, Um SH and Pyo S: Diosgenin inhibits macrophage-derived
inflammatory mediators through downregulation of CK2, JNK,
NF-kappaB and AP-1 activation. Int Immunopharmacol. 10:1047–1054.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong G, Qin Y and Huang W: Anti-thrombosis
effect of diosgenin extract from Dioscorea zingiberensis C.H.
Wright in vitro and in vivo. Phytomedicine. 18:458–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CH, Liu DZ and Jan TR: Diosgenin, a
plant-derived sapogenin, enhances regulatory T-cell immunity in the
intestine of mice with food allergy. J Nat Prod. 73:1033–1037.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong QY, He Y, Zhao QB, Qing Y, Huang W
and Wu XH: Cytotoxicity and apoptosis-inducing effect of steroidal
saponins from Dioscorea zingiberensis Wright against cancer cells.
Steroids. 77:1219–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rivera DG, Concepción O, Perez-Labrada K
and Coll F: Synthesis of diamino-furostan sapogenins and their use
as scaffolds for positioning peptides in a preorganized form.
Tetrahedron. 64:5298–5305. 2008. View Article : Google Scholar
|
|
9
|
Kvasnica M, Budesinsky M, Swaczynova J,
Pouzar V and Kohout L: Platinum (II) complexes with steroidal
esters of L-methionine and L-histidine: Synthesis, characterization
and cytotoxic activity. Bioorg Med Chem. 16:3704–3713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang B, Du D, Zhang R, Wu X, Xing Z, He Y
and Huang W: Synthesis, characterization and biological studies of
diosgenyl analogues. Bioorg Med Chem Lett. 22:7330–7334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin Y, Wu X, Huang W, Gong G, Li D, He Y
and Zhao Y: Acute toxicity and sub-chronic toxicity of steroidal
saponins from Dioscorea zingiberensis C.H. Wright in rodents. J
Ethnopharmacol. 126:543–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang R, Huang B, Du D, Guo X, Xin G, Xing
Z, Liang Y, Chen Y, Chen Q, He Y, et al: Anti-thrombosis effect of
diosgenyl saponins in vitro and in vivo. Steroids. 78:1064–1070.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu TL, Hanson SR, Kishikawa K, Wang SK,
Sawa M and Wong CH: Alkynyl sugar analogs for the labeling and
visualization of glycoconjugates in cells. Proc Natl Acad Sci USA.
104:2614–2619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson
SR, Vogt PK and Wong CH: Glycoproteomic probes for fluorescent
imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA.
103:12371–12376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KS, Kim JA, Eom SY, Lee SH, Min KR and
Kim Y: Inhibitory effect of piperlonguminine on melanin production
in melanoma B16 cell line by downregulation of tyrosinase
expression. Pigment Cell Res. 19:90–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jadeja RN, Thounaojam MC, Devkar RV and
Ramachandran AV: Clerodendron glandulosum. Coleb extract prevents
in vitro human LDL oxidation and oxidized LDL induced apoptosis in
human monocyte derived macrophages. Food Chem Toxicol.
49:1195–1202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong G, Qin Y, Huang W, Zhou S, Yang X and
Li D: Rutin inhibits hydrogen peroxide-induced apoptosis through
regulating reactive oxygen species mediated mitochondrial
dysfunction pathway in human umbilical vein endothelial cells. Eur
J Pharmacol. 628:27–35. 2010. View Article : Google Scholar
|
|
18
|
Tuntiwechapikul W, Taka T, Songsomboon C,
Kaewtunjai N, Imsumran A, Makonkawkeyoon L, Pompimon W and Lee TR:
Ginger extract inhibits human telomerase reverse transcriptase and
c-Myc expression in A549 lung cancer cells. J Med Food.
13:1347–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong G, Qin Y, Huang W, Zhou S, Wu X, Yang
X, Zhao Y and Li D: Protective effects of diosgenin in the
hyperlipidemic rat model and in human vascular endothelial cells
against hydrogen peroxide-induced apoptosis. Chem Biol Interact.
184:366–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu M, Xu L, Yin L, Qi Y, Li H, Xu Y, Han
X, Peng J and Wan X: Cytotoxicity of dioscin in human gastric
carcinoma cells through death receptor and mitochondrial pathways.
J Appl Toxicol. 33:712–722. 2013. View
Article : Google Scholar
|
|
21
|
Hanson S, Best M, Bryan MC and Wong CH:
Chemoenzymatic synthesis of oligosaccharides and glycoproteins.
Trends Biochem Sci. 29:656–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rostovtsev VV, Green LG, Fokin VV and
Sharpless KB: A stepwise huisgen cycloaddition process: copper
(I)-catalyzed regioselective 'ligation' of azides and terminal
alkynes. Angew Chem Int Ed Engl. 41:2596–2599. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Q, Chan TR, Hilgraf R, Fokin VV,
Sharpless KB and Finn MG: Bioconjugation by copper(I)-catalyzed
azide-alkyne [3+2] cycloaddition. J Am Chem Soc. 125:3192–3193.
2003. View Article : Google Scholar : PubMed/NCBI
|