Introduction
Glaucoma, characterized by progressive optic
neuropathy, is the second most commonly occurring disease causing
blindness worldwide. Glaucoma filtration surgery (GFS) provides the
gold standard for the management of intraocular pressure (IOP),
after medication and laser surgery have failed to control IOP
adequately. However, scar tissue, which can form under the
conjunctiva, obstructs aqueous flow and causes the filter to fail.
Previous studies have demonstrated that human Tenon's capsule
fibroblasts (HTFs) located in the incision area exert a major role
in scar formation by promoting the proliferation, migration and
synthesis of the extracellular matrix (1). Several antimetabolites, including
mitomycin C and 5-fluorouracil, have been used to prevent
post-operative ocular scar tissue formation. However, a
disadvantage is that these antimetabolites are associated with
marked side-effects, including hypotony, endophthalmitis, bleb
leakage and loss of vision (2,3).
Several alternative methods have been demonstrated to reduce scar
formation through the inhibition of the proliferation of the HTFs
(4,5).
Protein kinase C (PKC) comprises a family of protein
isoforms, which occupy a central role in cellular processes,
including proliferation, differentiation, mitosis and inflammation.
To date, at least 12 isoforms of PKC have been cloned, and these
are divided into three major groups: Classical PKCs
(PKCα, PKCβI, PKCβII and
PKCγ), novel PKCs (PKCδ, PKCε,
PKCη, PKCθ and PKCµ) and
atypical PKCs (PKCζ, PKCλ and
PKCι) (6). The
differences in functionality among the specific PKC isoforms are
predominantly due to their subcellular localization, activation or
inhibition by different stimuli, and transcriptional regulation. It
was reported that tranilast inhibits the proliferation and
migration of HTFs in vitro, at least in part, by
downregulating the expression of PKC (7,8).
Alkylphosphocholines were identified as effective inhibitors of HTF
proliferation and migration, and cell-mediated contraction of
collagen gels at non-toxic concentrations. A previous study
indicated that the mechanism of action appeared to involve the
inhibition of the PKC pathway (9).
It was therefore feasible that several of the PKC isoforms may
exert a role in HTF proliferation, and the present study was
focused on analyzing the expression of the 12 PKC isoforms in
cultured HTFs with a view towards elucidating their role(s) in HTF
proliferation.
Materials and methods
Culture of HTFs
Fresh human Tenon's capsule tissues (48 h
post-mortem) from donors, were obtained from the Eye Bank of
Zhongshan Ophthalmic Center (Guangzhou, China). The HTFs were
cultured in 6-well plates (BD Biosciences, Lincoln Park, NJ, USA)
with explants in Dulbecco's modified Eagle's medium/F12 nutrient
mixture (Invitrogen Life Technologies, Grand Island, NY, USA),
containing 5% FBS (Hyclone, Logan, UT, USA), 100 U/ml penicillin G
(Gibco Life Technologies, Carlsbad, CA, USA), 100 mg/ml
streptomycin sulfate (Gibco Life Technologies) and L-glutamate
(Invitrogen Life Technologies), as previously described (10). HTFs from cell passage 3 to 5 were
subsequently used in the experiments. The cells were lysed for
total RNA extraction to assess the mRNA expression levels, or to
extract proteins and assess the protein expression levels.
Immunofluorescence analysis
HTFs (1×106/liter) grown on coverslips
were fixed in 4% paraformaldehyde for 15 min at room temperature,
prior to rinsing three times in phosphate buffered saline (PBS).
The cell cultures were permeabilized with 0.2% Triton X-100
(Sigma-Aldrich, St. Louis, MO, USA) in PBS at room temperature for
10 min. Indirect immunostaining was performed, as described
previously (11). The primary
antibodies, rabbit anti-PKCα polyclonal antibody (1:200; cat. no.
sc-208), rabbit anti-PKCγ polyclonal antibody (1:100; cat. no.
sc-211) and mouse anti-PKCη monoclonal antibody (1:100; cat. no.
sc-136036) were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA); mouse anti-PKCδ monoclonal antibody (1:100; cat.
no. 610397), mouse anti-PKCε monoclonal antibody (1:200; cat. no.
610086), mouse anti-PKCθ monoclonal antibody (1:50; cat. no.
612734), mouse anti-PKCι monoclonal antibody (1:50; cat. no.
610175) and mouse anti-PKCλ monoclonal antibody (1:50; cat. no.
610208) were purchased from BD Biosciences; rabbit anti-PKCβI
polyclonal antibody (1:200; cat. no. p3078), rabbit anti-PKCβII
polyclonal antibody (1:100; cat. no. p8371), rabbit anti-PKCζ
polyclonal antibody (1:30; cat. no. SAB2104776) and rabbit
anti-PKCµ polyclonal antibody (1:50; cat. no. SAB1306354)
were from Sigma-Aldrich. The cells were incubated with the
antibodies overnight in a solution of PBS at a temperature of 4°C.
Following washing with PBS, Alexa Fluor 488-conjugated secondary
antibodies [1:200 (cat. nos. A28175 and A27034); Invitrogen Life
Technologies] were applied for 1 h and 1 µg/ml Hoechst 33342
(Sigma-Aldrich) was used for nuclear counter-staining. Either
secondary antibody alone, without primary antibodies, or isoform
immunoglobulin G (BD Biosciences) served as the negative control.
The staining was imaged using a laser-scanning confocal microscope
(LSCM510META; Zeiss, Thornwood, NY, USA). Each antibody was used in
a minimum of three separate experiments.
Total RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
The total RNA was isolated from the cells using an
RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the
manufacturer's instructions. The RNA concentration was quantified
spectrophotometry, using an ND-1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA) prior to storage at −80°C. The
RNA (5 µg) was reverse-transcribed using the SuperScript™
first-strand synthesis system, according to the manufacturer's
instructions (Invitrogen Life Technologies). cDNAs (2 µg)
encoding the PKC isoform genes were amplified by PCR as follows:
Denaturation for 30 sec, followed by annealing (56°C for
PKCα, PKCβII, PKCγ,
PKCε, PKCη, PKCθ, PKCι,
PKCζ and PKCµ; 60°C for
PKCδ; 52°C for PKCβI) for 30 sec, and
elongation at 72°C for 60 sec, for 30 cycles. The resulting PCR
products were analyzed by 2% gel electrophoresis. Primer sequences
for 11 PKC isoforms and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) were published previously (12), and are listed in Table 1 (PKCλ cannot be detected due to
the absence of its human PKC cDNA in GeneBank; http://www.ncbi.nlm.nih.gov/genbank/).
Each PCR experiment was performed a minimum of three times with
each set of primers.
 | Table IPrimers and reverse
transcription-polymerase chain reaction conditions. |
Table I
Primers and reverse
transcription-polymerase chain reaction conditions.
| Primer | Sequence (5′→3′) | Amplicon (bp) |
|---|
| PKCα | Forward:
ATCCGCAGTGGAATGAGTCCTTTACAT
Reverse: TTGGAAGGTTGTTTCCTGTCTTCAGAG | 327 |
| PKCβI | Forward:
CTGTGGAACTGACTCCCACTG
Reverse: ATACTGAAGCATTTTGGTATC | 404 |
| PKCβII | Forward:
GACCGTTTTTCACCCGCCA
Reverse: CCATCTCATAGAGATGCTCC | 309 |
| PKCγ | Forward:
CACGAAGTCAAGAGCCACAA
Reverse: TAGCTATGCAGGCGGAACTT | 233 |
| PKCδ | Forward:
CAACTACATGAGCCCCACCT
Reverse: GAGGCTCTCTGGGTGACTTG | 189 |
| PKCε | Forward:
GATGCAGAAGGTCACTGCAA
Reverse: GTCGTCATGGAGGATGGACT | 249 |
| PKCζ | Forward:
GTTATCGATGGGATGGATGG
Reverse: GCACCAGCTCTTTCTTCACC | 166 |
| PKCη | Forward:
GAACAGAGGTTCGGGATCAA
Reverse: ATATTTCCGGGTTGGAGACC | 239 |
| PKCθ | Forward:
ACAAACAGGGCTACCAGTGC
Reverse: ATGCCACATGCATCACACTT | 250 |
| PKCι | Forward:
TACGGCCAGGAGATACAACC
Reverse: TCGGAGCTCCCAACAATATC | 169 |
| PKCµ | Forward:
ACGGCACTATTGGAGATTGG
Reverse: TGACCACATTTTCTCCCACA | 206 |
| GAPDH | Forward:
ACCCAGAAGACTGTGGATGG
Reverse: TGCTGTAGCCAAATTCGTTG | 415 |
Western blot analysis
The culture medium was removed and washed twice with
ice-cold PBS. The HTFs were lysed using sample buffer [60 mM
Tris/HCl (pH 6.8), 2% (w/v) sodium dodecyl sulfate (SDS), 100 mM
2-mercaptoethanol and 0.01% (w/v) Bromophenol Blue] (13). The lysates were incubated on ice
for 30 min and the extracts were harvested using a cell scraper,
and boiled for 5 min prior to storage at −20°C.
Subsequently, western blotting was performed, as
previously described (14).
Briefly, 40 µg protein per well, measured using a
bicinchoninic acid protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), was loaded onto a 12% SDS-polyacrylamide gel
for SDS-polyacrylamide gel electrophoresis. The proteins were
separated and electro-transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Bedford, MA, USA) for 1 h at 350 mA. The
membranes were blocked with 5% non-fat milk disolved in TTBS
buffer, containing, 50 mM Tris/HCl (pH 7.5), 0.9% NaCl and 0.1%
Tween-20, for 1 h at room temperature and incubated with primary
antibodies overnight at 4°C, prior to subsequent incubation with
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature. The signals were detected using an enhanced
chemiluminescence kit (GE Healthcare, Inc., Piscataway, NJ, USA),
according to the manufacturer's instructions. Each PKC isoform was
detected in a minimum of three independent experiments. Rat brain
lysate (BD Biosciences) served as a positive control, as
recommended by the manufacturer's instructions for the primary
antibody.
Results
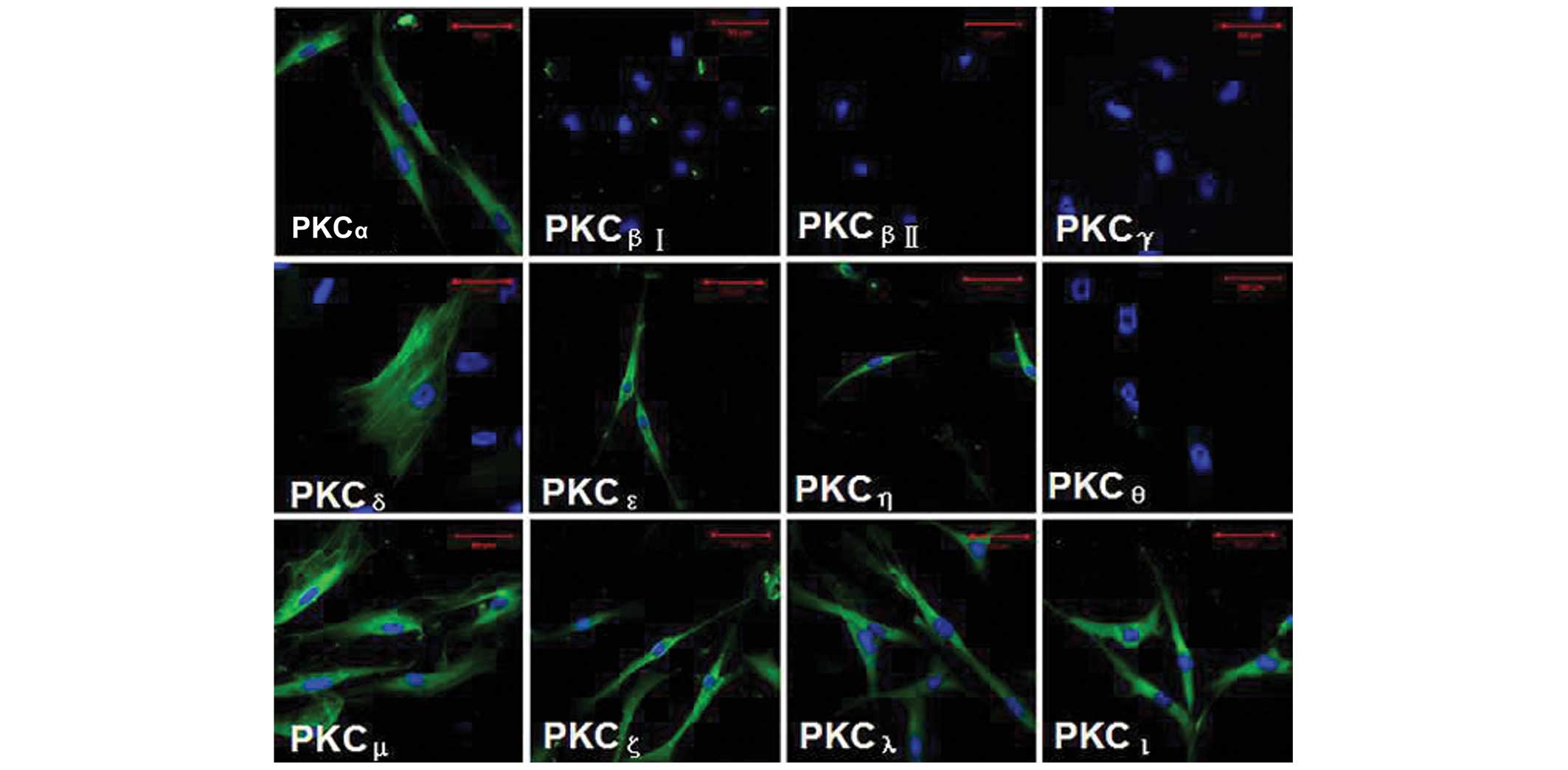
Immunofluorescence analysis of the PKC
isoforms in HTFs
Using laser scanning confocal microscopy (LSCM),
eight PKC isoforms (PKCα, PKCδ,
PKCε, PKCζ, PKCη, PKCι,
PKCλ and PKCµ) were identified in the
cultured HTFs, predominantly localized in the cytoplasm of the
cells, as revealed by immunofluorescence staining. In particular,
PKCδ was expressed in the cytoskeleton. However, no
staining was identified for the PKCβI,
PKCβII, PKCγ or PKCθ isoforms in
the HTFs (Fig. 1). Note that the
primary antibody replaced with β-actin, and the primary antibody
replaced with IgG isotype (positive and negative controls,
respectively), are not shown in Fig.
1.
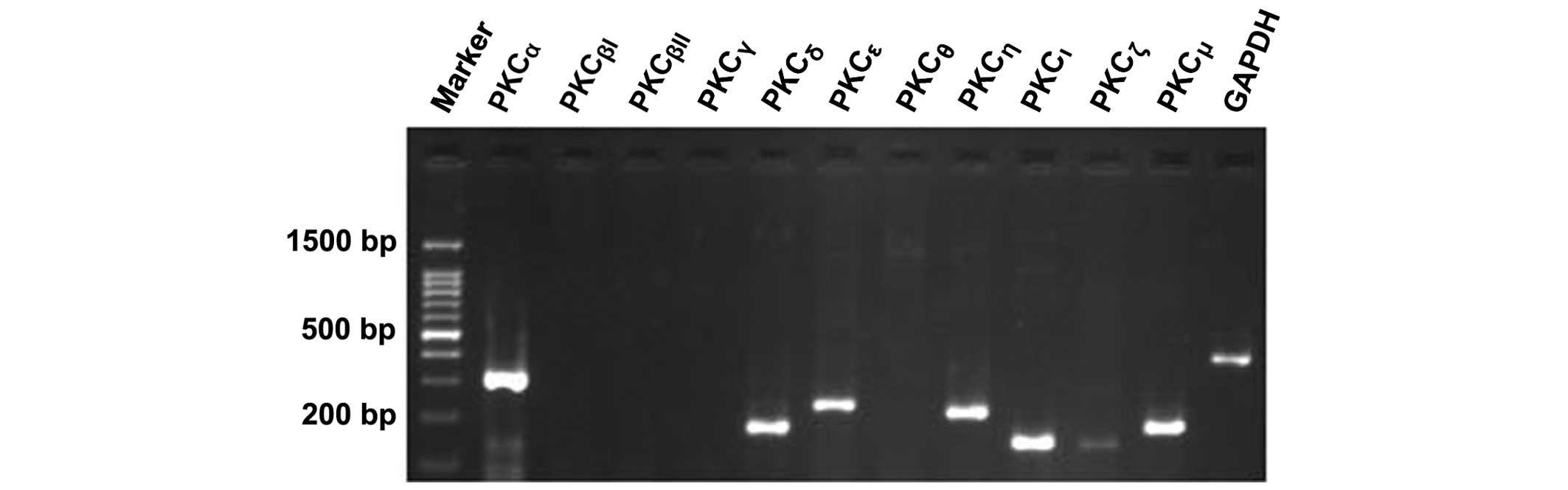
mRNA expression levels of the PKC
isoforms in HTFs
The results from the 2% agarose gel electrophoresis
experiment revealed the presence of mRNAs coding for seven of the
PKC isoforms. PKCα (327 bp), PKCδ (189 bp),
PKCε (249 bp), PKCη (239 bp), PKCι
(169 bp) and PKCµ (206 bp) were present at a
higher level compared with PKCζ (166 bp), which only
produced a weak signal. PKCβI, PKCβII,
PKCγ and PKCθ were not detected at the mRNA
level (Fig. 2). GAPDH was used as
a positive control.
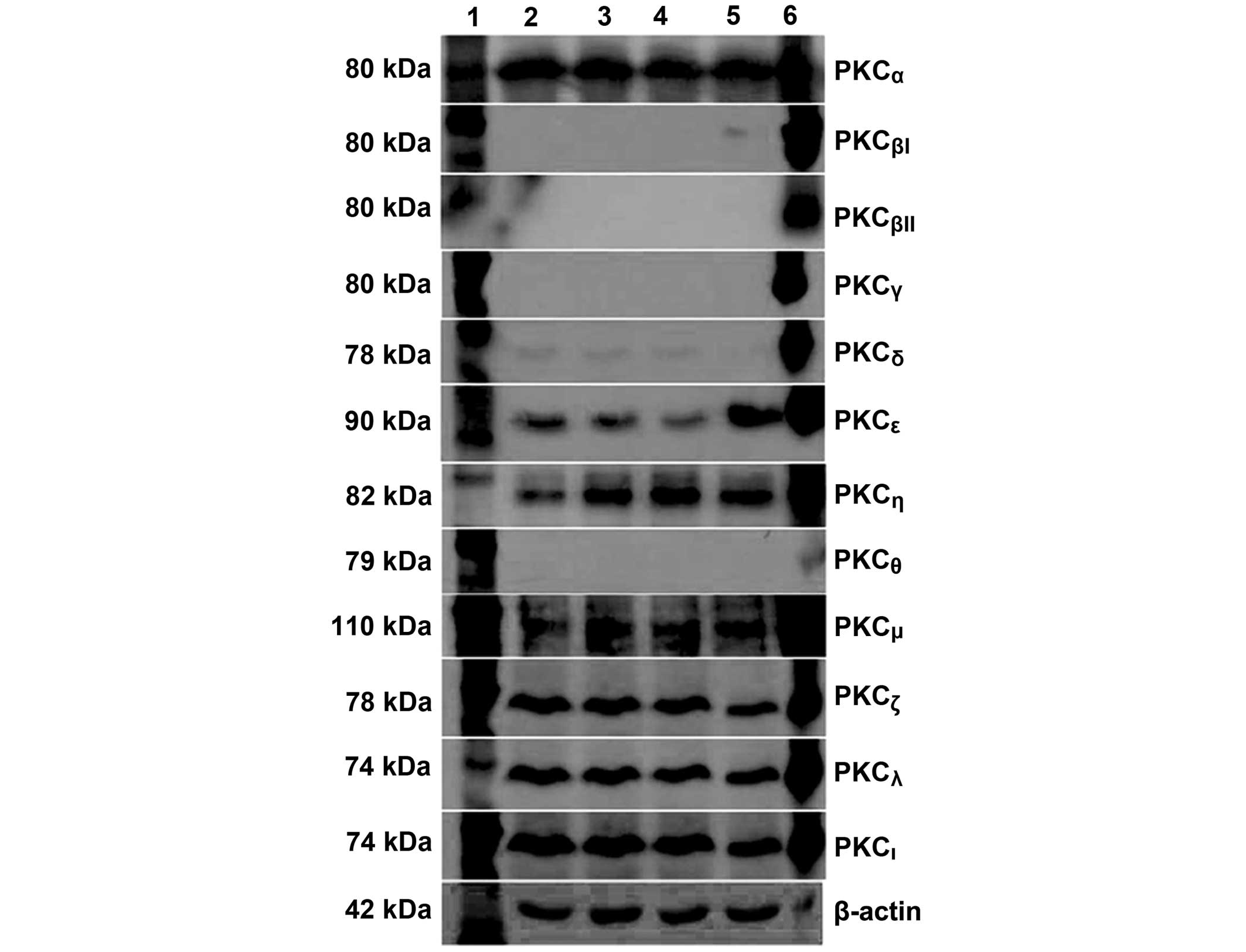
Protein expression levels of the PKC
isoforms in HTFs
An analysis of the protein expression levels of the
PKC isoforms in HTFs was also performed. Using western blotting,
seven PKC isoforms [PKCα (80 kDa), PKCδ (78
kDa), PKCε (90 kDa), PKCζ (78 kDa),
PKCη (82 kDa), PKCι (74 kDa) and PKCµ
(110 kDa)] were observed to be expressed in the HTFs, corroborating
the results of the mRNA expression level analysis. In addition,
PKCλ (74 kDa) was also expressed in the HTFs. However,
the other four isoforms, PKCβI, PKCβII,
PKCγ and PKCη, were not detected (Fig. 3). Lanes 2–5 feature HTF protein
lysates derived from independent cell lines. Rat brain lysate was
used as a positive control (lane 6) and β-actin (42 kDa) was used
for protein normalization.
Discussion
Scarring is the predominant reason for the failure
of GFS. Successful filtration surgery depends directly on an
individual's wound-healing response. HTFs are crucially important
in this process. Previous studies have revealed that
subconjunctival scarring of the filtering bleb site is
predominantly mediated by HTF proliferation, migration and
contraction (15,16). In order to assess the role of PKCs
in the biological function of HTFs, the specific expression of the
12 PKC isoforms were characterized. The present study assessed for
the first time, to the best of our knowledge, the expression levels
of the 12 PKC isoforms in HTFs at the cellular, mRNA and protein
level, using LSCM, RT-PCR and western blotting, respectively.
A similar expression pattern of the PKC isoforms was
identified by each of the three methods. Eight of the PKC isoforms,
PKCα, PKCδ, PKCε, PKCζ,
PKCη, PKCι, PKCλ and
PKCµ, were expressed in cultured HTFs; however,
no expression was observed for the four other isoforms,
PKCβI, PKCβII, PKCγ and
PKCθ. With the exception of PKCλ, the protein
expression levels of the other 11 isoforms were consistent with
their gene expression levels. A previous report revealed that 8 of
10 of the PKC isoforms were expressed in rat subconjunctival
fibroblasts, as determined using western blot analysis, including
PKCα, PKCβ, PKCγ, PKCδ,
PKCε, PKCη, PKCι and
PKCλ. PKCζ was not examined (17). The differences identified between
the two studies are likely to be species-specific.
It has been suggested that isoform-specific
functions may be conferred by the subcellular localization of the
PKCs. Using immunofluorescence staining, the present study revealed
that the subcellular localization of the PKC isoforms occurs
predominantly in the cytoplasm of the cells. In addition,
PKCδ was localized specifically to the cytoskeleton,
which may be associated with its function in HTFs.
Although the PKC isoforms exhibit very few
differences in terms of their structures, substrate preferences,
expression and localization, individual PKC isoforms still appear
to be tissue- and cell-specific. Epidermal growth factor activates
the PKCα phosphorylation pathway to stimulate goblet
cell proliferation (18).
Hepatocyte growth factor induces the migration of retinal pigment
epithelial cells by enhancing the activation of PKCδ and
the phosphorylation of ERK (19).
However, the majority of studies to date have focused on the
activation of PKC in general (17). Therefore it is necessary to
elucidate which PKC isoforms exert specific roles in HTFs
proliferation.
In conclusion, the present study demonstrated that 8
of the 12 PKC isoforms were expressed in HTFs at both the protein
and the mRNA level. This represents an important step in
understanding their precise physiological role, and how they are
regulated during the process of proliferation. To elucidate whether
one or several of the isoforms are involved in HTF proliferation,
due to their role in cellular signal transduction of scarring
post-GFS, remains to be elucidated by further experiments.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81100643).
References
|
1
|
Occleston NL, Daniels JT, Tarnuzzer RW,
Sethi KK, Alexander RA, Bhattacharya SS, Schultz GS and Khaw PT:
Single exposures to antiproliferatives: Long-term effects on ocular
fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci.
38:1998–2007. 1997.PubMed/NCBI
|
|
2
|
Anand N, Arora S and Clowes M: Mitomycin C
augmented glaucoma surgery: Evolution of filtering bleb
avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol.
90:175–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beckers HJ, Kinders KC and Webers CA:
Five-year results of trabeculectomy with mitomycin C. Graefes Arch
Clin Exp Ophthalmol. 241:106–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer-Ter-Vehn T, Katzenberger B, Han H,
Grehn F and Schlunck G: Lovastatin inhibits TGF-β-induced
myofibroblast transdifferentiation in human tenon fibroblasts.
Invest Ophthalmol Vis Sci. 49:3955–3960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao YQ, Liu K, Shen JF, Xu GT and Ye W:
SB-431542 inhibition of scar formation after filtration surgery and
its potential mechanism. Invest Ophthalmol Vis Sci. 50:1698–1706.
2009. View Article : Google Scholar
|
|
6
|
Johannes FJ, Prestle J, Eis S,
Oberhagemann P and Pfizenmaier K: PKCu is a novel, atypical member
of the protein kinase C family. J Biol Chem. 269:6140–6148.
1994.PubMed/NCBI
|
|
7
|
Ji CN, Hu YZ, Ding ZP and Li GG: The
investigation of tranilast on the proliferation and migration of
human Tenon's capsule fibroblasts. Zhonghua Yan Ke Za Zhi.
40:165–169. 2004.In Chinese. PubMed/NCBI
|
|
8
|
Chihara E, Dong J, Ochiai H and Hamada S:
Effects of tranilast on filtering blebs: A pilot study. J Glaucoma.
11:127–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eibl KH, Banas B, Kook D, Ohlmann AV,
Priglinger S, Kampik A and Welge-Luessen UC: Alkylphosphocholines:
A new therapeutic option in glaucoma filtration surgery. Invest
Ophthalmol Vis Sci. 45:2619–2624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang SS, Ge J, Wang LN, Yin XB, Wei YT
and Ma P: Preliminary study of inhibition of human Tenon's capsule
fibroblasts in vitro by RNA interference targeting IKK-beta.
Zhonghua Yan Ke Za Zhi. 141:1076–1081. 2005.In Chinese.
|
|
11
|
Ma P, Wang Z, Pflugfelder SC and Li DQ:
Toll-like receptors mediate induction of peptidoglycan recognition
proteins in human corneal epithelial cells. Exp Eye Res.
90:130–136. 2010. View Article : Google Scholar :
|
|
12
|
Yu K, Ma P, Ge J, Willey CD, Yang P, Wang
Z and Gao Q: Expression of protein kinase C isoforms in cultured
human retinal pigment epithelial cells. Graefes Arch Clin Exp
Ophthalmol. 245:993–999. 2007. View Article : Google Scholar
|
|
13
|
Campa MJ, Wang MZ, Howard B, Fitzgerald MC
and Patz EF Jr: Protein expression profiling identifies macrophage
migration inhibitory factor and cyclophilin a as potential
molecular targets in non-small cell lung cancer. Cancer Res.
63:1652–1656. 2003.PubMed/NCBI
|
|
14
|
Ma P, Bian F, Wang Z, Zheng X,
Chotikavanich S, Pflugfelder SC and Li DQ: Human corneal
epithelium-derived thymic stromal lymphopoietin links the innate
and adaptive immune responses via TLRs and Th2 cytokines. Invest
Ophthalmol Vis Sci. 50:2702–2709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Bergen T, Vandewalle E, Van de Veire
S, Dewerchin M, Stassen JM, Moons L and Stalmans I: The role of
different VEGF isoforms in scar formation after glaucoma filtration
surgery. Exp Eye Res. 93:689–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye H, Qian Y, Lin M, Duan Y, Sun X, Zhuo Y
and Ge J: Cationic nano-copolymers mediated IKKβ targeting siRNA to
modulate wound healing in a monkey model of glaucoma filtration
surgery. Mol Vis. 16:2502–2510. 2010.PubMed/NCBI
|
|
17
|
Nomura N, Nomura M, Takahira M and
Sugiyama K: Phorbol 12-myristate 13-acetate-activated protein
kinase C increased migratory activity of subconjunctival
fibroblasts via stress-activated protein kinase pathways. Mol Vis.
13:2320–2327. 2007.
|
|
18
|
Li D, Shatos MA, Hodges RR and Dartt DA:
Role of PKCα activation of Src, PI-3K/AKT, and ERK in
EGF-stimulated proliferation of rat and human conjunctival goblet
cells. Invest Ophthalmol Vis Sci. 54:5661–5674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YJ, Tsai RK, Wu WC, He MS, Kao YH and
Wu WS: Enhanced PKCδ and ERK signaling mediate cell migration of
retinal pigment epithelial cells synergistically induced by HGF and
EGF. PLoS One. 7:e449372012. View Article : Google Scholar
|