Introduction
Acute lymphoblastic leukemia (ALL) is the most
common childhood malignant disease, and accounts for the greatest
percent of malignancies in children newly diagnosed with cancer in
the USA (1,2). Following chemotherapy and
hematopoietic stem cell transplantation, novel therapeutic
strategies have been developed to improve the complete remission
(CR) rate and overall survival of ALL patients (3,4).
However, significant toxicity, relapse due to a state of minimal
residual disease (MRD) and transplant-associated mortality limit
the efficacy of allogeneic stem cell transplantation (5). Therefore, the development of
additional immunotherapeutic strategies that selectively recognize
and destroy leukaemia cells is required, with the aim of reducing
relapse rates. In previous years, dendritic cell (DC)-based
immunotherapy has presented as a promising strategy for the
elimination of MRD in patients with ALL (6–8).
DCs are professional antigen-presenting cells (APC),
and are critical in the induction of cellular and humoral immunity
(9). Various studies have reported
that the injection of tumor antigen-loaded DCs induces
tumor-specific cytotoxic T lymphocyte (CTL) responses and leukemia
resistance (6,10–12).
However, the primary obstacles to the introduction of this
therapeutic strategy in clinical practice include insufficient
numbers of DCs and insufficient production of cytokines. Therefore,
DC vaccine therapy relies on either the generation of sufficient
numbers of DCs, to prime CTLs, or administration of
immunomodulatory agents to overcome deficiencies in DC and CTL
function (5). Previous studies
that focused on an in vitro methodology have revealed that
it is possible to derive DCs from peripheral blood mononuclear
cells (PBMCs) using cytokines, which can be used to harvest
sufficient numbers of DCs for use in vaccine therapy (13,14).
In addition, specific immunomodulatory agents that induce DC
maturation may improve DC vaccine therapy for the treatment of
leukaemia (12).
Thymosin α1 (Tα1) is a naturally
occurring thymic peptide, consisting of 28 amino acid residues,
that is widely distributed in numerous tissues and cells (15,16).
Tα1 is administered worldwide for the treatment of certain
immunodeficiencies, malignancies and infections (17). For example, Tα1 was shown to induce
apoptosis and inhibit proliferation in human leukemia cell lines,
suggesting its potential therapeutic effects in leukemia (18). Additionally, Tα1 exerts
immunomodulatory effects on T cells, natural killer cells and
macrophages, and has an important role in the modulation of
differentiation, maturation and the function of DCs. However, it
remains unclear as to whether Tα1 affects the functional maturation
of DCs that are derived from PBMCs of ALL patients.
In the present study, the influence of Tα1 on
functional maturation of PBMC-derived DCs was investigated by
analysis of the morphology, phenotype and CTL cytotoxicity in HL-60
cells. It was hypothesized that the immunomodulatory agent, Tα1 may
be an effective adjuvant to DC vaccine therapy for the treatment of
hematological malignancies.
Materials and methods
Patients
A total of 10 consecutive patients (males, n=5;
females, n=5) with ALL (mean age, 5.5 years) were enrolled at the
Department of Pediatric Hematology at The Affiliated Hospital of
Qingdao University (Qingdao, China). The patients had achieved CR
for ≥6 months and had not been administered with immunomodulators
for ≥4 weeks. Informed consent to participate in the current study
was obtained from their guardians and the study was approved by the
Ethical Committee of The Affiliated Hospital of Qingdao
University.
HL-60 cell culture and antigen
preparation
The acute promyelocytic leukemia cell line HL-60
cells were obtained from Shanghai Institutes for Biological
Sciences of Chinese Academy of Sciences (Shanghai, China). The
cells were cultured in RPMI-1640 medium (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS; Gibco-BRL) and 1% penicillin-streptomycin solution (100
units/ml; Gibco-BRL) under a humidified 5% CO2
atmosphere at 37°C, as previously described (19). A half-medium exchange was performed
every 2 days with fresh RPMI-1640 medium and the cells were
subcultured under the same conditions.
Approximately 1.0×107 cells/ml were
collected by centrifugation (at 3,000 ×g for 15 min) during the
logarithmic growth phase and the cells were subjected to five rapid
repetitive freeze-thaw cycles (−140°C to 37°C) to obtain the
whole-cell (HL-60) tumor lysates. Removal of cell debris was
conducted by centrifugation, leaving the tumor lysate-containing
supernatant, which was stored at −20°C for the subsequent loading
of DCs with antigens.
Generation of PBMC-derived DCs and Tα1
treatment
PB samples (20 ml) from the ALL patients were
obtained by venipuncture and maintained in sterile heparinized
tubes; the blood samples were subjected to Ficoll-Hypaque density
gradient centrifugation at 400 ×g for 30 min (Ficoll-Hypaque, GE
Healthcare, Little Chalfont, UK) (20) at room temperature. The PBMCs were
collected from the interface and washed three times using Hank's
buffer (Gibco-BRL). The DCs were prepared according to a previously
described method (21). Briefly,
the cell density was adjusted to 2.0×106/ml with
RPMI-1640 medium and incubated at 37°C in 24-well culture plates
(Sino-American Biotechnology, Shanghai, China) for 3 h. Nonadherent
cells were gently removed by washing twice with RPMI-1640 medium
and the adherent monocytes were cultured in the RPMI-1640 medium
supplemented with 100 ng/ml recombinant human
granulocyte-macrophage colony-stimulating factor (GM-CSF)
(Peprotech, Rocky Hill, NJ, USA) and 75 ng/ml interleukin (IL)-4
(Peprotech) at 37°C to differentiate into immature DCs. On the
third day of culture, the medium was aspirated and the cells were
divided into three groups, in triplicate (n=3 wells in each group):
i) Control; ii) AN (antigen + no Tα1); and iii) AT (antigen + Tα1;
Patheon Italia S.P.A, Italy). The groups were incubated with fresh
medium supplemented with 100 ng/ml GM-CSF and 75 ng/ml IL-4.
Additionally, 100 µl freeze-thaw antigens were added to the
AN and AT groups. Following 5 days of incubation, the new medium
containing recombinant human tumor necrosis factor-α (TNF-α; 50
ng/ml) was added to each well, in addition to the GM-CSF and IL-4,
to induce DC maturation. The AT group was then treated with 100
ng/ml Tα1: A Tα1 dose-dependent curve had previously been generated
(data not shown), and optimal changes could be observed at a
concentration of 100 ng/ml (21).
After an additional 3 days, the DCs were harvested and used for
in vitro analysis.
Morphological analysis
Morphological characteristics of PBMCs cultured for
3, 5, and 7 days in the presence of GM-CSF, IL-4 and TNF-α were
analyzed on an inverted light microscope (magnification, ×1,000;
SZH-ILLB; Olympus, Tokyo, Japan). On day 8 of the culture, the
morphology of mature DCs in each group was analyzed.
Phenotypic analysis of DCs by flow
cytometry
Cultured DCs were harvested and washed twice with
phosphate-buffered saline (PBS; Shanghai Threebio Technology Co.,
Ltd, Shanghai, China). Subsequently, the cells were incubated for 1
h at 4°C with 1 µl of the following DC marker-specific
antibodies: Mouse anti-human CD1a monoclonal antibody (cat no.
300102; BioLegend), mouse anti-human CD83 monoclonal antibody (cat
no. 305305, BioLegend) and mouse anti-human HLA-DR monoclonal
antibody (cat no. 307602; BioLegend). After washing with PBS, the
stained DCs were incubated with fluorescein isothiocyanate
(FITC)-conjugated Alexa Fluor 488F secondary antibody (mouse
anti-human monoclonal antibody; cat no. A-10631; Invitrogen Life
Technologies) for 30 min at room temperature. Isotype controls
comprising mouse anti-human immunoglobulin G1 FITC-conjugated
antibodies were included. After washing twice with PBS, 10,000
scatter-gated cells in each sample were analyzed with a FACScan
flow cytometer using CellQuest software (BD Biosciences, Franklin
Lakes, NJ, USA).
Cytotoxicity assays
T lymphocytes isolated from PB were purified using a
nylon wool column filtration method, as previously described
(22). The autologous cells
(1.0×106/ml) were cultured in the 6-well culture plate
(2 ml/well) with the RPMI-1640-containing medium, 10% FBS and 100
µl IL-2 (20 ng/ml), at 37°C in a 5% CO2
atmosphere for 7 days. Half of the medium was exchanged for fresh
culture medium supplemented with IL-2 (100 IU/ml) every other day.
On day 8, the T lymphocytes were co-cultured with DCs from the
different groups at a ratio of 10:1 in 24-well culture plates,
which contained the medium of RPMI-1640 with 10% FBS, IL-2 and 25
µg/ml mitomycin C (Roche Diagnostics, Basel, Switzerland)
for an additional 4 days to obtain the CTLs.
The CTLs, which served as effector cells (E),
continued to co-culture with the wild-type HL-60 cells, which were
regarded as target cells (T), at a ratio of 20:1 (E:T) for 4 h at
37°C. The cytotoxicity was measured using the lactate dehydrogenase
(LDH) Cytotoxicity Assay kit (Sigma-Aldrich, St. Louis, MO, USA)
according to the manufacturer's instructions. The optical density
(OD) was measured at 490 nm using a microplate reader (model MR
5000, Dynatech, Paris, France) and the cytotoxicity of CTL cells
was calculated according to the following formula: Cytotoxicity (%)
= (experimental OD value - natural release OD value)/(target
maximum OD value - natural release OD value) × 100.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Statistical analysis was performed using SPSS
13.0 (SPSS Inc., Chicago, IL, USA). The different groups were
compared by analysis of variance and P<0.05 was considered to
indicate a statistically significant difference.
Results
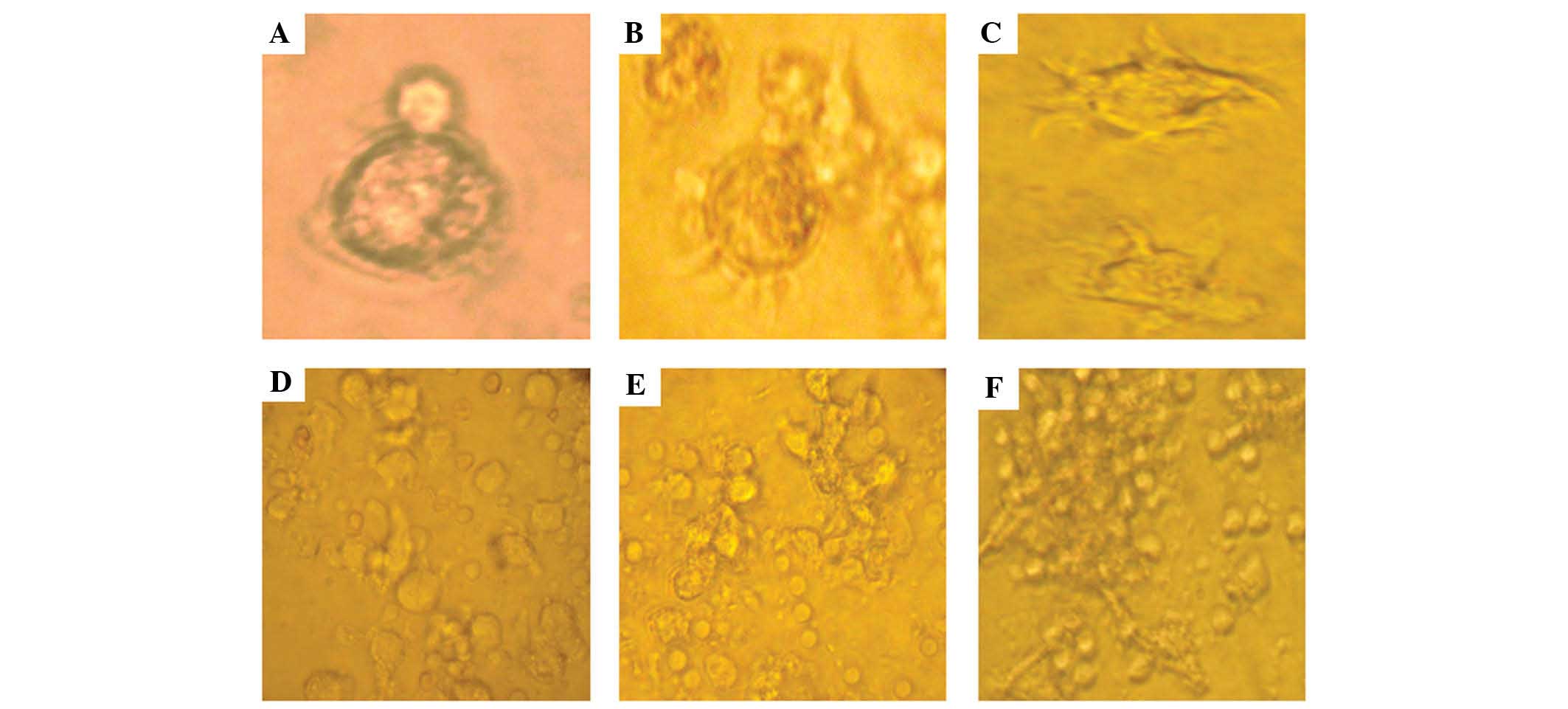
Alterations in DC morphology
DC morphology was observed under an inverted light
microscope every other day (Fig.
1). On the third day of culture, the cells displayed smooth
round contours without apparent dendrites, which is the
characteristic morphology of immature DCs (Fig. 1A). On day 5, the DCs had increased
in size, and most of the nonadherent cells had acquired a typical
dendritic morphology (Fig. 1B).
After 7 days, the DCs revealed a typical mature dendritic cell
morphology (Fig. 1C). Furthermore,
following 8 days of culture, numerous clusters of cells with long
dendrites were apparent in the control, AN and AT groups. DCs in
the AT group exhibited looser adherence and more dendritic-like
cytoplasmic projections when compared with the other groups
(Fig. 1D–F).
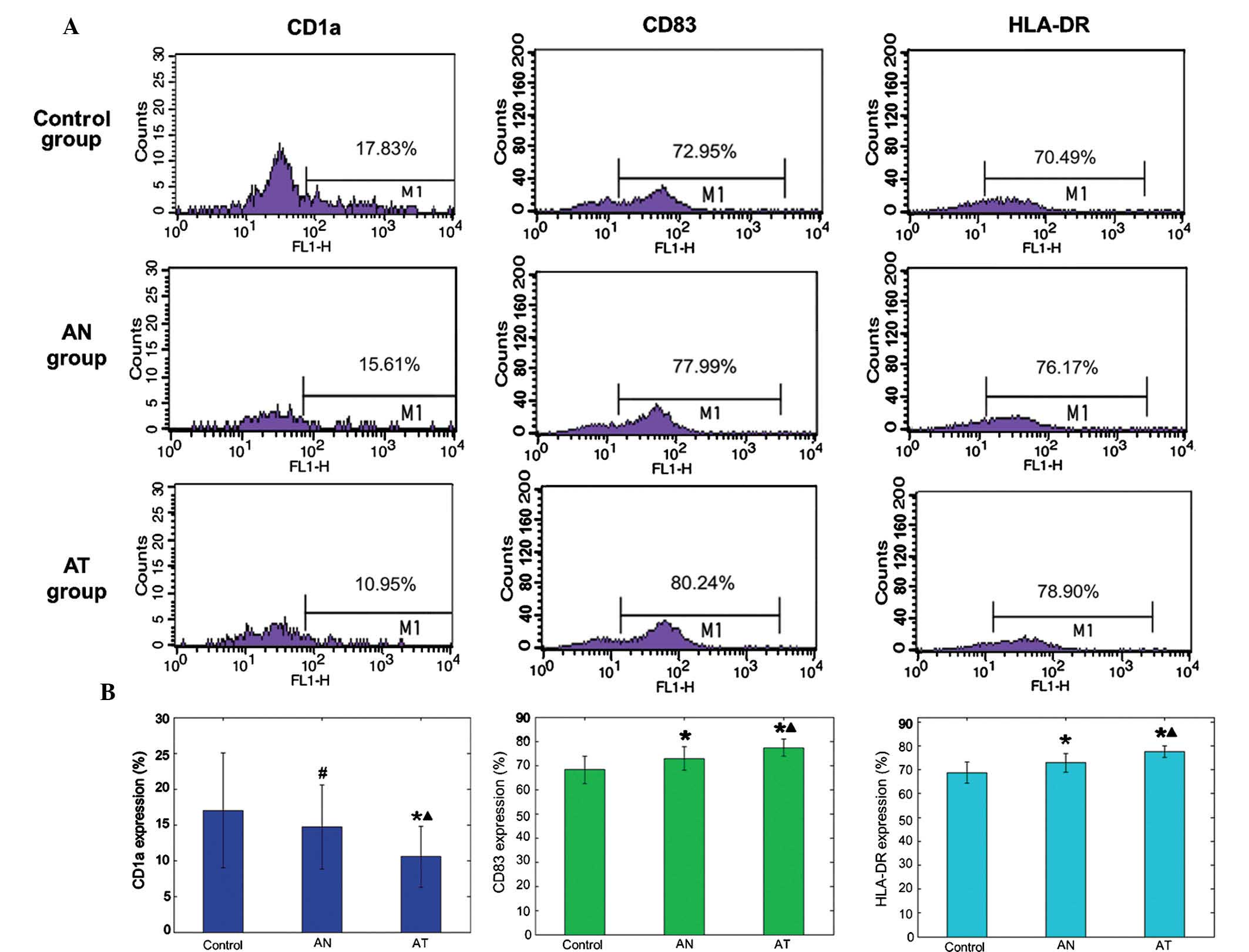
Immunophenotypic characteristics of
DCs
No significant difference was noted in CD1a
expression between the AN and control group (14.68±5.86 vs.
17.01±7.99; P>0.05), while the AN group demonstrated higher
levels of CD83 (72.85±4.79 vs. 68.23±5.65 and HLA-DR (72.91±3.92
vs. 68.81±4.4) compared with the control group (Fig. 2; P<0.05). Compared with the
control and AN groups, the AT group expressed significantly lower
CD1a levels, and significantly higher levels of CD83 and HLA-DR as
a result of treatment with freeze-thaw antigens and Tα1:
10.55±4.25, 77.31±3.51 and 77.51±2.40%, respectively (Fig. 2; P<0.05).
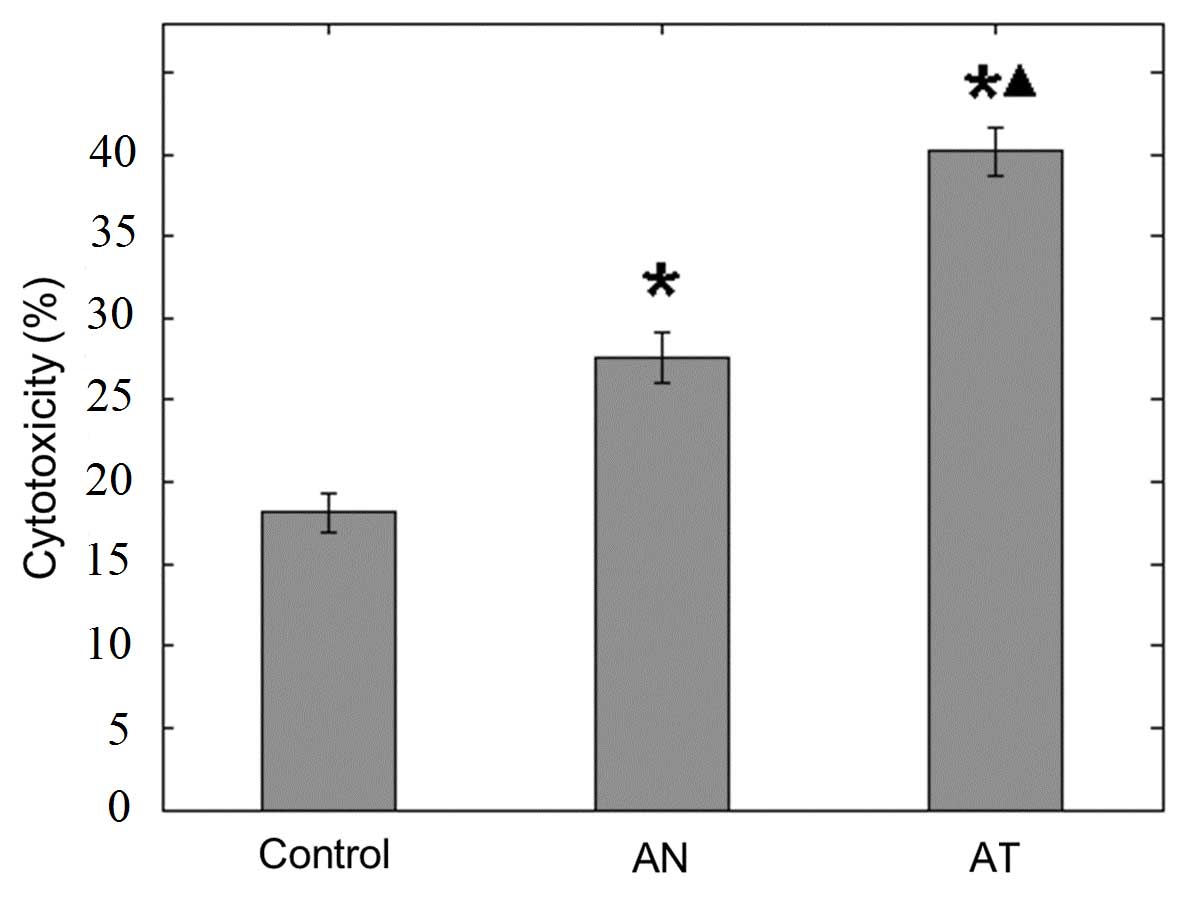
CTL cytotoxicity
The CTL cytotoxicity assay response to the wild-type
HL-60 cells was measured using the LDH release method according to
the manufacturer's instructions. The lowest cytotoxicity
(18.15±1.20%) was observed in the control group, while the AT group
exhibited the highest cytotoxicity (40.20±1.48%) among the three
groups (Fig. 3; P<0.01).
Discussion
Immunotherapy of malignant diseases that is mediated
by a tumor antigen-loaded DC vaccine is considered to be a
promising novel therapeutic strategy for the treatment of
malignancies. (11,23–25).
Previous studies have revealed that Tα1 exerts immunomodulatory
effects on the maturation, differentiation and function of DCs from
murine bone marrow (18,26) and healthy human PB (21). However, there are few published
studies on the effects of Tα1 on DC-based vaccines for ALL. In the
present study, via analysis of morphology, phenotype and CTL
cytotoxicity in HL-60 cells, Tα1 was shown to induce the phenotypic
and functional maturation of DCs with the capacity to induce
antitumor immunity toward leukemic cells.
It is generally accepted that the degree of maturity
of DCs correlates with the level of cytotoxicity and it is
considered to be a critical factor that requires investigation to
allow the DC vaccination to be improved (27,28).
Furthermore, the ability of therapeutic DCs to migrate to the lymph
nodes is influenced by the DC maturation stage and is considered to
be important (5,29). A previous study regarding melanoma
demonstrated that mature DCs loaded with melanoma antigens were
superior to immature DCs loaded with melanoma antigens in the
induction of immunological and clinical responses (30). The maturation of DCs is
characterized by the altered expression of the DC markers, CD1a,
CD80, CD86, DC-specific intercellular adhesion molecule-3-grabbing
non-integrin and HLA-DR (31,32).
CD1 molecules efficiently present antigens in immature DCs,
therefore, a high CD1a level indicates an immature DC status
(33,34). The surface expression of CD1a is
downregulated during DC maturation (35). CD83, one of the primary markers of
mature DCs, is upregulated concurrently with DC maturation
(36,37). In addition, the major
histocompatibility complex class II receptor, HLA-DR is also an
important maturation marker for DCs, and the upregulated expression
of it is apparent during DC maturation (38). In the current study, the DCs
treated with lysates obtained by freeze-thaw cycling exhibited
apparent dendritic morphology and markedly increased expression
levels of CD83 and HLA-DR, when compared with the control group.
However, the combined treatment, with lysates and Tα1, induced a
reduced expression level of CD1a and increased levels of CD83 and
HLA-DR expression in the DC surface phenotype, when compared with
the lysate-alone treatment. Thus, Tα1 appears to promote the
phenotypic maturation of leukaemia cell-derived antigen-loaded
DCs.
In addition, an LDH release assay was used to
measure the killing activity of CTLs (39). In the present study, the killing
rate of CTL in the group of leukemic cell lysates + Tα1 (the AT
group) was higher than that of the lysate-alone (the AN group) and
the control, indicating that Tα1 significantly improves the antigen
presentation capacity of DCs and ultimately enhances the killing
activity of CTL on leukemia cells.
In conclusion, the present study demonstrates that
Tα1 promotes the phenotypic and functional maturation of DCs, thus
inducing enhanced CTL killing activity on leukemia cells. These
findings may provide a basis to further evaluate Tα1 as a potential
immunomodulator for DC-directed vaccines and therapeutic strategies
for the treatment of children with ALL.
References
|
1
|
Essig S, Li Q, Chen Y, Hitzler J,
Leisenring W, Greenberg M, Sklar C, Hudson MM, Armstrong GT, Krull
KR, et al: Risk of late effects of treatment in children newly
diagnosed with standard-risk acute lymphoblastic leukaemia: A
report from the Childhood Cancer Survivor Study cohort. Lancet
Oncol. 15:841–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turcotte LM and Neglia JP: Survivors of
childhood cancer: Risk of new primary neoplasms of the CNS. Tumors
of the Central Nervous System. Hayat MA: ISSN: 2215-096X12.
Springer; Dordrecht: pp. 137–pp145. 2014, View Article : Google Scholar
|
|
3
|
McCarthy PL, Owzar K and Hahn T:
Autologous hematopoietic stem cell transplantation and maintenance
therapy for multiple myeloma. Int J Hematol Oncol. 2:71–83. 2013.
View Article : Google Scholar
|
|
4
|
Rein LA, Sung AD and Rizzieri DA: New
approaches to manipulate minimal residual disease after allogeneic
stem cell transplantation. Int J Hematol Oncol. 2:39–48. 2013.
View Article : Google Scholar
|
|
5
|
Duncan C and Roddie H: Dendritic cell
vaccines in acute leukaemia. Best Pract Res Clin Haematol.
21:521–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conrad DP, Tsang J, Maclean M, Diallo JS,
Le Boeuf F, Lemay CG, Falls TJ and Parato KA: Leukemia
cell-rhabdovirus vaccine: Personalized immunotherapy for acute
lymphoblastic leukemia. Clin Cancer Res. 19:3832–3843. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pospísilová D, Borovicková J, Poloucková
A, Spísek R, Sedivá A, Hrusák O, Starý J and Bartůnková J:
Generation of functional dendritic cells for potential use in the
treatment of acute lympho-blastic leukemia. Cancer Immunol
Immunother. 51:72–78. 2002. View Article : Google Scholar
|
|
8
|
Maggio R, Peragine N, Calabrese E, De
Propris MS, Intoppa S, Della Starza I, Ariola C, Vitale A, Foà R
and Guarini A: Generation of functional dendritic cells (DC) in
adult acute lymphoblastic leukemia: Rationale for a DC-based
vaccination program for patients in complete hematological
remission. Leuk Lymphoma. 48:302–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malissen B, Tamoutounour S and Henri S:
The origins and functions of dendritic cells and macrophages in the
skin. Nat Rev Immunol. 14:417–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Y, Aymeric L, Locher C, Kroemer G and
Zitvogel L: The dendritic cell-tumor cross-talk in cancer. Curr
Opin Immunol. 23:146–152. 2011. View Article : Google Scholar
|
|
11
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Subklewe M, Geiger C, Lichtenegger FS,
Javorovic M, Kvalheim G, Schendel DJ and Bigalke I: New generation
dendritic cell vaccine for immunotherapy of acute myeloid leukemia.
Cancer Immunol Immunother. 63:1093–1103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anton D, Dabadghao S, Palucka K, Holm G
and Yi Q: Generation of dendritic cells from peripheral blood
adherent cells in medium with human serum. Scand J Immunol.
47:116–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeras M, Bergant M and Repnik U: In vitro
preparation and functional assessment of human monocyte-derived
dendritic cells-potential antigen-specific modulators of in vivo
immune responses. Transpl Immunol. 14:231–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bach JF: Thymic hormones. J
Immunopharmacol. 1:277–310. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein AL, Guha A, Zatz MM, Hardy MA
and White A: Purification and biological activity of thymosin, a
hormone of the thymus gland. Proc Natl Acad Sci USA. 69:1800–1803.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Romani L, Bistoni F, Montagnoli C, Gaziano
R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E, et
al: Thymosin alpha1: An endogenous regulator of inflammation,
immunity, and tolerance. Ann N Y Acad Sci. 1112:326–338. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan Y, Chang H, Yu Y, Liu J and Wang R:
Thymosin α 1 suppresses proliferation and induces apoptosis in
human leukemia cell lines. Peptides. 27:2165–2173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin JJ, Hsu HY, Yang JS, Lu KW, Wu RS, Wu
KC, Lai TY, Chen PY, Ma CY, Wood WG, et al: Molecular evidence of
anti-leukemia activity of gypenosides on human myeloid leukemia
HL-60 cells in vitro and in vivo using a HL-60 cells murine
xenograft model. Phytomedicine. 18:1075–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fidan I, Yesilyurt E, Kalkanci A, Aslan
SO, Sahin N, Ogan MC and Dizbay M: Immunomodulatory effects of
voriconazole and caspofungin on human peripheral blood mononuclear
cells stimulated by Candida albicans and Candida krusei. Am J Med
Sci. 348:219–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao Q, Doan LX, Zhang R, Bharadwaj U, Li M
and Chen C: Thymosin-alpha1 modulates dendritic cell
differentiation and functional maturation from human peripheral
blood CD14+ monocytes. Immunol Lett. 110:110–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisen S, Wedner H and Parker C: Isolation
of pure human pexipherac blood T-lymphocytes using nylon wool
columns. Immunol Invest. 1:571–577. 1972. View Article : Google Scholar
|
|
23
|
Timmerman JM and Levy R: Dendritic cell
vaccines for cancer immunotherapy. Annu Rev Med. 50:507–529. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banchereau J and Palucka AK: Dendritic
cells as therapeutic vaccines against cancer. Nat Rev Immunol.
5:296–306. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabado RL, Miller E, Spadaccia M, Vengco
I, Hasan F and Bhardwaj N: Preparation of Tumor Antigen-loaded
Mature Dendritic Cells for Immunotherapy. J Vis Exp. View Article : Google Scholar : 2013.PubMed/NCBI
|
|
26
|
Huang Y, Chen Z, Zhou C, Yao H, Li M and
Xu C: The modulation of thymosin alpha 1 in the maturation,
differentiation and function of murine bone marrow-derived
dendritic cells in the absence or presence of tumor necrosis
factor-alpha. Int Immunopharmacol. 4:539–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ragde H, Cavanagh WA and Tjoa BA:
Dendritic cell based vaccines: Progress in immunotherapy studies
for prostate cancer. J Urol. 172:2532–2538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Yu Y, Zhang M, Wang W and Cao X:
The involvement of TNF-alpha-related apoptosis-inducing ligand in
the enhanced cytotoxicity of IFN-beta-stimulated human dendritic
cells to tumor cells. J Immunol. 166:5407–5415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MartIn-Fontecha A, Sebastiani S, Höpken
UE, Uguccioni M, Lipp M, Lanzavecchia A and Sallusto F: Regulation
of dendritic cell migration to the draining lymph node: Impact on T
lymphocyte traffic and priming. J Exp Med. 198:615–621. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Vries IJ, Lesterhuis WJ, Scharenborg
NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM,
Torensma R, Adema GJ, et al: Maturation of dendritic cells is a
prerequisite for inducing immune responses in advanced melanoma
patients. Clin Cancer Res. 9:5091–5100. 2003.PubMed/NCBI
|
|
31
|
León B and Ardavín C: Monocyte-derived
dendritic cells in innate and adaptive immunity. Immunol Cell Biol.
86:320–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slukvin II, Thomson JA, Vodyanyk MA and
Gumenyuk ME: Method of forming dendritic cells from embryonic stem
cells. US Patent no 8,785,189. Washington, DC: U.S. Patent and
Trademark Office; 2014, Filed Feb 1, 2012; issued May 7, 2013.
|
|
33
|
Cao X, Sugita M, van der Wel N, Lai J,
Rogers RA, Peters PJ and Brenner MB: CD1 molecules efficiently
present antigen in immature dendritic cells and traffic
independently of MHC class II during dendritic cell maturation. J
Immunol. 169:4770–4777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan SM, Kapp M, Flechsig C, Kapp K, Rachor
JE, Eyrich M, Loeffler J, Einsele H and Grigoleit GU: Stimulating
surface molecules, Th1-polarizing cytokines, proven trafficking-a
new protocol for the generation of clinical-grade dendritic cells.
Cytotherapy. 15:492–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Wel NN, Sugita M, Fluitsma DM, Cao
X, Schreibelt G, Brenner MB and Peters PJ: CD1 and major
histocompatibility complex II molecules follow a different course
during dendritic cell maturation. Mol Biol Cell. 14:3378–3388.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lechmann M, Berchtold S, Steinkasserer A
and Hauber J: CD83 on dendritic cells: More than just a marker for
maturation. Trends Immunol. 23:273–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prechtel AT, Turza NM, Theodoridis AA and
Steinkasserer A: CD83 knockdown in monocyte-derived dendritic cells
by small interfering RNA leads to a diminished T cell stimulation.
J Immunol. 178:5454–5464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Drénou B, Amiot L, Setterblad N, Taque S,
Guilloux V, Charron D, Fauchet R and Mooney N: MHC class II
signaling function is regulated during maturation of plasmacytoid
dendritic cells. J Leukoc Biol. 77:560–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li XL, Zhao YX, Sun LR, Yang J and Xu HJ:
The preparation of HL-60 cells vaccine expressing BCG heat shock
protein 70 and detection of its immunogenicity in vitro. Hum Vaccin
Immunother. 8:1376–1381. 2012. View
Article : Google Scholar : PubMed/NCBI
|