Introduction
Varicose veins (VVs) are a common pathology
affecting the lower extremities and are manifested by a range of
conditions, including pain, ankle edema, itch, eczema,
lipodermatosclerosis and ulceration, affect approximately one third
of the adult western population and ~20% of the adult Chinese
population (1). Despite several
hypotheses, the mechanism involved in VVs remains to be elucidated.
Increasing lines of evidence indicates that venous wall alterations
and dilation are the primary events responsible for the formation
of VVs and suggests that chronic venous insufficiency,
characterized by symptoms or signs produced by venous hypertension,
may be the result of venous wall changes. Structural and
biochemical changes, which have been reported in the walls of VVs
include intimal hyperplasia, dysregulated apoptosis, smooth muscle
cell proliferation, abnormal glucose metabolism, altered
angiogenesis, changes in collagen and elastin content, and
imbalances of matrix metalloproteases and their tissue inhibitors
(1,2).
Venous hypoxia secondary to blood stasis in chronic
venous disease has been hypothesized to contribute to changes in
the walls of VVs (3).
Hypoxia-inducible factors (HIFs) are nuclear transcription factors.
HIF-α dimerizes with HIF-β in the nucleus and binds the
hypoxia-responsive element of target genes, which regulate their
transcription responses to altered oxygenation (4,5).
In vitro studies have clearly demonstrated that hypoxia
upregulates the formation of new vessels through activation of the
HIF pathway, leading to the release of pro-angiogenic factors,
including vascular endothelial growth factor (VEGF) and basic
fibroblast growth factor (6,7).
Together with nitric oxide, VEGF, one of the target genes of HIF-1,
is central in maintaining vascular integrity and reactivity by
mediating vaso-permeability and dilatory responses (8,9).
In mammals, several behavioral and physiological
processes exhibit circadian (~24 h) rhythms, which are controlled
by a clock system. This system includes a central circadian clock
residing in the hypothalamic suprachiasmatic nucleus (SCN) and a
peripheral clock located in a number of peripheral tissues
(10). Previous studies have
demonstrated that peripheral tissues and cells contain a similar
clock system to that in the SCN (11,12).
It has been reported that the circadian rhythmicity of human
plaque-derived vascular smooth muscle cells (VSMCs) differs from
that of normal carotid VSMCs (13). Rhythmic changes in the expression
of circadian clock genes, including circadian locomotor output
cycles kaput (CLOCK), in plaque-derived VSMCs may be involved in
the process of vessel disease. This hypothesis is supported by
increasing evidence that cross-talk between mediators of hypoxic
and circadian pathways can regulate target genes (14). To further understand the pathology
of VVs, the present study compared the expression of the CLOCK gene
in initial and advanced varicose lesions, and examined the
correlation between the CLOCK gene, HIF-1α and its target gene,
VEGF, in VVs.
Patients and methods
Patient recruitment
The procedures performed in the present study were
performed following the principles outlined in the Declaration of
Helsinki and approved by the Ethics Committee of Zhongshan
Hospital, Fudan University (Shanghai, China). All human tissues
were collected following the provision of informed consent by the
patients. Following removal of adherent connective tissue, all
venous specimens were snap-frozen in liquid nitrogen (Shanghai
Pujiang Special Gas Co., Ltd., Shanghai, China) and then stored at
−80°C or preserved in 4% paraformaldehyde (Sangon Biotech Co.,
Ltd., Shanghai, China) until use.
The samples of VVs were obtained from symptomatic
patients undergoing saphenofemoral ligation and stripping of the
great saphenous vein (GSV) for primary VVs due to incompetence of
the GSV. Prior to surgery, a duplex ultrasound scan was performed
in a vascular laboratory by a qualified independent vascular
surgeon. Prior to removal of the varicose lesions, physical
examination and hemodynamic findings were used to classify the
stage of disease, according to the
clinical-etiology-anatomy-pathophysiology (CEAP) criteria of the
American Venous Forum for chronic lower-extremity venous disease
(15). As venous ulcers in C5
patients (a healed ulcer) or C6 patients (an active ulcer) can
significantly reduce health-associated quality of life, the
patients with VV were divided into those with initial varicose
lesions (C3 and C4) and advanced varicose lesions (C5 and C6).
Samples of a normal control vein were obtained from
patients undergoing cardiac bypass procedures, who had no symptoms
or clinically evident signs of varicose disease in either limb. The
veins were confirmed with a duplex ultrasound scan using a Philips
iU22 (Philips, Bothell, WA, USA) ultrasound system, which was
performed in the vascular laboratory at the Qingpu Branch of
Zhongshan Hospital by a qualified independent vascular surgeon. The
veins were confirmed to be normal with a preoperative duplex scan,
which indicated that the vessel was disease-free and without
valvular incompetence in any segment. Patients with a history of
recent infection, rheumatoid arthritis, steroid use, cancer or
connective tissue disease, those <18 years of age or those who
did not sign a consent form, were excluded from the
investigation.
RNA isolation and reverse-transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA from each vein was extracted using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) in an
RNase-free environment, according to the manufacturer's standard
instructions. First-strand cDNA was synthesized and amplified from
1 µg of the total RNA using the ReverTra Ace qPCR RT kit
(Toyobo Co., Ltd., Osaka, Japan). Messenger RNA (mRNA) levels of
the target genes were determined using an ABI 7900HT machine
(Applied Biosystems Life Technologies, Foster City, CA, USA) in
triplicate, in 10 µl reaction mixtures containing SYBR Green
Real-time PCR master mix (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The relative quantification of gene expression was analyzed
from the measured threshold cycles (Ct) using the 2−ΔΔCt
method, as described previously (16). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as an internal standard to normalize
the expression level of other genes. Primers were designed by
PRIMER 5.0 (Premier Biosoft International, Palo Alto, CA, USA) and
synthesized by Sangon Biotech Co., Ltd., as follows: CLOCK, forward
5′-GGCATGTCCCAGTTTCAGTTT-3′ and reverse
5′-ATGCGTGTCCGTTGTTCCAAT-3′; HIF-1α, forward
5′-CACCACAGGACAGTACAGGAT-3′ and reverse
5′-CGTGCTGAATAATACCACTCACA-3′; VEGF, forward
5′-AGGGCAGAATCATCACGAAGT-3′ and reverse 5′-AGGGTCTCGATTGGATGGCA-3′
and GAPDH, forward 5′-TGTTGCCATCAATGACCCCTT-3′ and reverse
5′-CTCCACGACGTACTCAGCG-3′.
Immunohistochemistry (IHC)
For histological analysis of the venous tissue, 4
µm sections of the paraffin-embedded veins were used.
Hematoxylin and eosin staining was used for general histological
evaluation, and calcification was observed using von Kossa staining
(cat. no. ab150687l; Abcam, Cambridge, MA, USA). Formalin-fixed
paraffin embedded sections (4 µm thickness) of varicose
veins were used for CLOCK immunostaining, according to the
manufacturer's standard instructions, as described previously
(17). In brief, the slides were
deparaffinized in xylene and rehydrated with alcohol prior to being
placed in a 3% H2O2/methanol blocking
solution to quench endogenous peroxidase activity. This was
followed by subsequent antigen retrieval. The non-specific binding
was blocked with normal horse or goat serum. The slides were
incubated with rabbit anti-human polyclonal antibody for 10 minutes
at 95°C in the specified antigen retrieval solution (pH 6.0, Abcam)
at a 1:50 dilution overnight in a humidified chamber at 4°C.
Following washing with phosphate-buffered saline, the slides were
incubated with goat anti-rabbit IgG conjugated to a horseradish
peroxidase-labeled polymer (Envision System; Dako, Carpinteria, CA,
USA) for 30 min at room temperature. Reactions were performed using
3,3′-diaminobenzidine chromogen and coun-terstained with
hematoxylin. Negative controls comprised of tissue sections
incubated with non-specific IgG in place of the primary
antibody.
All sections were quantitatively analyzed and scored
by two experienced pathologists under a light microscope. The
scoring of the IHC was based on two independent criteria: The
proportion of immunopositive cells and their intensity of
immunoreactivity. The percentage of immunopositive cells was
categorized as follows: 0, <10%; 1, ≥10 to <25%; 2, 25 to
<50%; 3, ≥50 to <75% and 4, ≥75%. The intensity of staining
was categorized by the relative intensity as follows: 0, no
positivity; 1, weak positivity; 2, moderate positivity and 3,
marked positivity. A final immunoreactivity score of each section
was assigned by multiplying the two individual scores.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). The results are
expressed as the mean ± standard deviation. The values for mRNA
levels are presented as relative values in all experiments.
Student's t-test was performed to examine the differences between
the groups. A one-way analysis of variance, followed by Fisher's
test, was used when appropriate. Pearson's correlation analysis was
performed to examine the correlation between CLOCK and HIF-1α or
the levels of VEGF in the human VVs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics and clinical
characteristics
The demographics and characteristics of the patients
in the clinical investigations are summarized in Table I. The patients with VVs exhibited
incompetence of the saphenofemoral junction, combined with
superficial and perforator reflux (CEAP classification, C3–6). The
mean age of the control group was 67.4 years (standard deviation,
6.9) and the ratio of males to females was 9:2. The patients with
either initial VVs or advanced VVs were significantly younger than
the control patients (58.6±9.2 and 57.0±7.1 years, vs. 67.4±6.9
years, respectively; P<0.05). Fewer patients in the VV group had
hypertension or diabetes mellitus, compared with the control group
(P=0.040 and P<0.001, respectively). No other differences in the
demographics were identified among the three groups.
 | Table IPatient demographics and clinical risk
factors. |
Table I
Patient demographics and clinical risk
factors.
| Characteristic | Varicose vein group
| Control group
(n=11) | P-value |
|---|
| Initial (n=44) | Advanced (n=26) |
|---|
| CEAP
classification | C3 and C4 | C5 and C6 | Normal | – |
| Age (years) | 58.6±9.2 | 57.0±7.1 | 67.4±6.9a | P<0.05 |
| Male:female | 25:19 | 19:7 | 9:2 | P=0.181 |
| Hypertension (n) | 14 | 9 | 8b | P=0.040 |
| Diabetes mellitus
(n) | 4 | 2 | 7c | P<0.001 |
| Dyslipidemia (n) | 9 | 10 | 5 | P=0.131 |
| COPD (n) | 0 | 0 | 1 | P=0.136 |
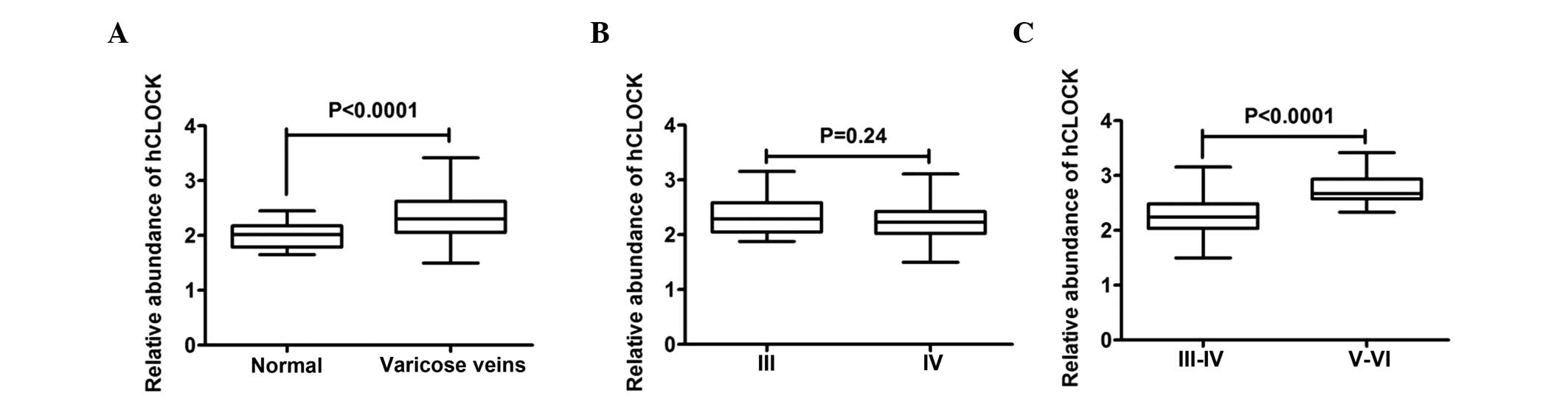
Quantification of the gene expression of
CLOCK in VVs and normal vein tissues
To analyze the gene expression of CLOCK in the
veins, RT-qPCR analysis of the veins from VVs and normal control
veins was performed. As shown in Fig.
1A, a high mRNA expression level of CLOCK was detected in the
VVs. Densitometric analysis revealed that the mRNA levels of CLOCK
in the VVs were higher than those in the normal veins (control
group). Although variations between individuals were observed, the
expression pattern was similar in all the vein samples examined.
The present study subsequently investigated whether the
deterioration in the VVs accounted for the elevated gene expression
of CLOCK in the VVs. Subgroup comparison of the C3 and C4 lesions
revealed no significant differences in the gene expression of CLOCK
in these initial VVs (Fig. 1B).
However, comparison of the mRNA expression level of CLOCK in the
advanced varicose lesions (C5 and C6), with those in the initial
varicose lesions (C3 and C4) revealed significantly higher levels
in the C5 and C6 lesions (Fig.
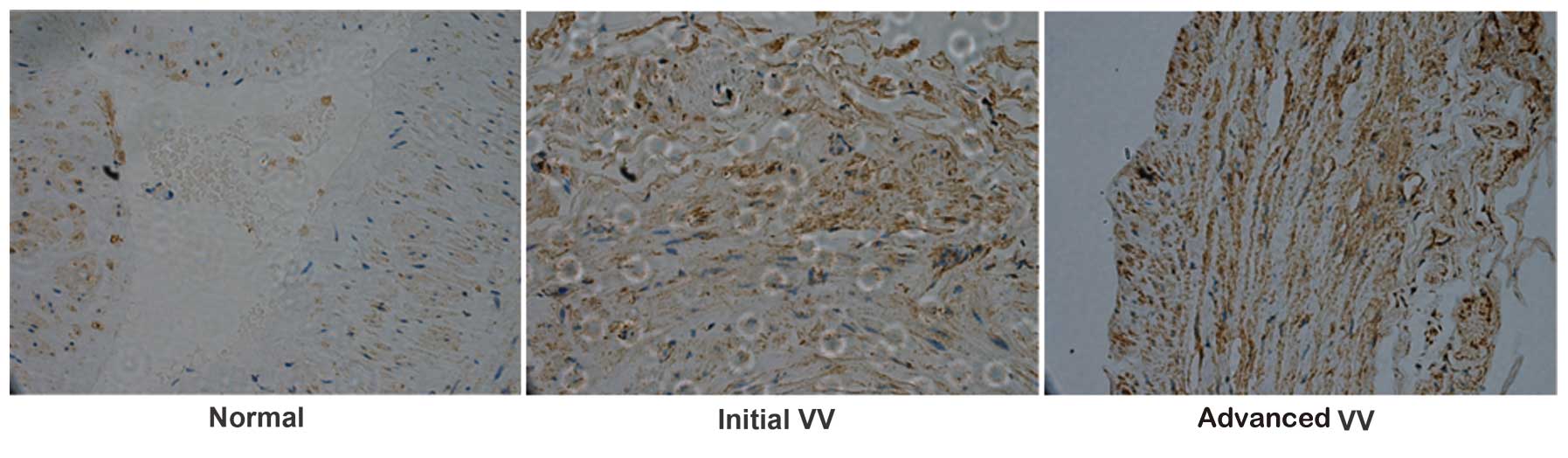
1C). To confirm these results, IHC analysis was performed to
investigate the expression of CLOCK in the VVs and normal control
veins. The IHC analysis revealed that the expression of CLOCK was
predominantly located in the cytoplasm (Fig. 2). Normal control veins exhibited
negative or weak staining, while staining in the VVs was often more
marked (Fig. 2). Taken together,
these data revealed that upregulation in the mRNA expression of
CLOCK is a characteristic of VVs.
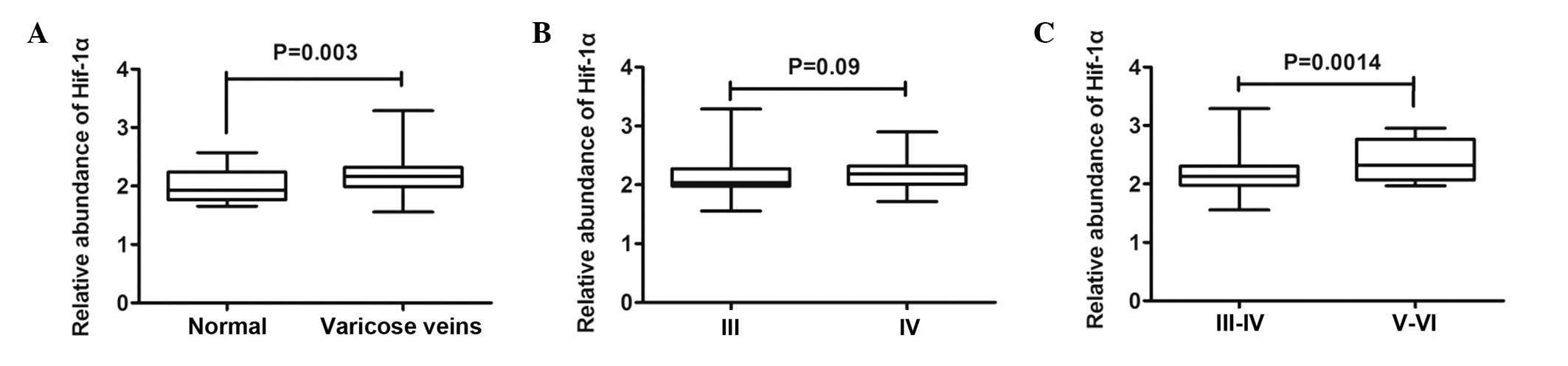
Expression levels of HIF-1α and VEGF are
elevated in VVs
Previous studies have demonstrated that hypoxia
increases the protein expression levles of CLOCK in mice, and the
co-operative effect of HIF-1α and CLOCK lead to the transcriptional
activity of vasopressin (14,18).
In the present study, the expression levels of HIF-1α and VEGF were
assessed. Compared with the control veins, RT-qPCR revealed a
significant increase in the mRNA expression levels of HIF-1α in the
VVs (Fig. 3A), however, no
significant differences were observed between the C3 and C4
subgroups (Fig. 3B). Furthermore,
it was also observed that the mRNA expression levels of HIF-1α were
significantly increased in the advanced varicose lesions (C5 and
C6), compared with those in the initial varicose lesions (C3 and
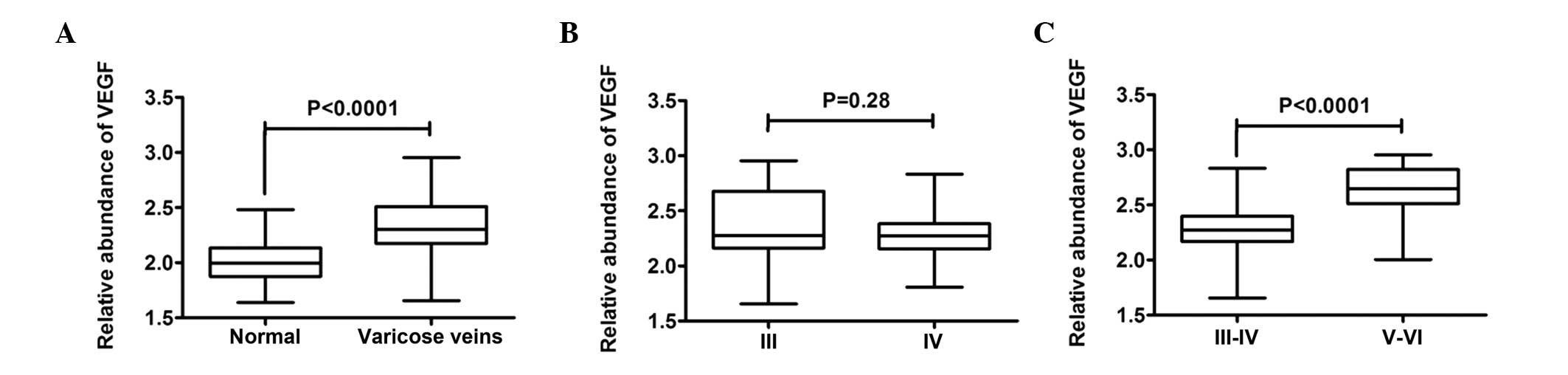
C4; Fig. 3C). Similarly, RT-qPCR
revealed a significant increase in the mRNA expression levels of
VEGF in the VVs, compared with the normal control veins (Fig. 4A), however, no significant
differences were observed between the C3 subgroup and C4 subgroup
(Fig. 4B). In addition, the mRNA
expression levels of VEGF were significantly increased in the
advanced varicose lesions (C5 and C6), compared with those in the
initial varicose lesions (C3 and C4; Fig. 4C). Despite individual variations,
the same expression pattern was observed in all the specimens
assessed. Together, these results revealed marked expression levels
of HIF-1α and VEGF associated with venous disease, the expression
pattern of which were distinct, compared with those observed in the
control veins.
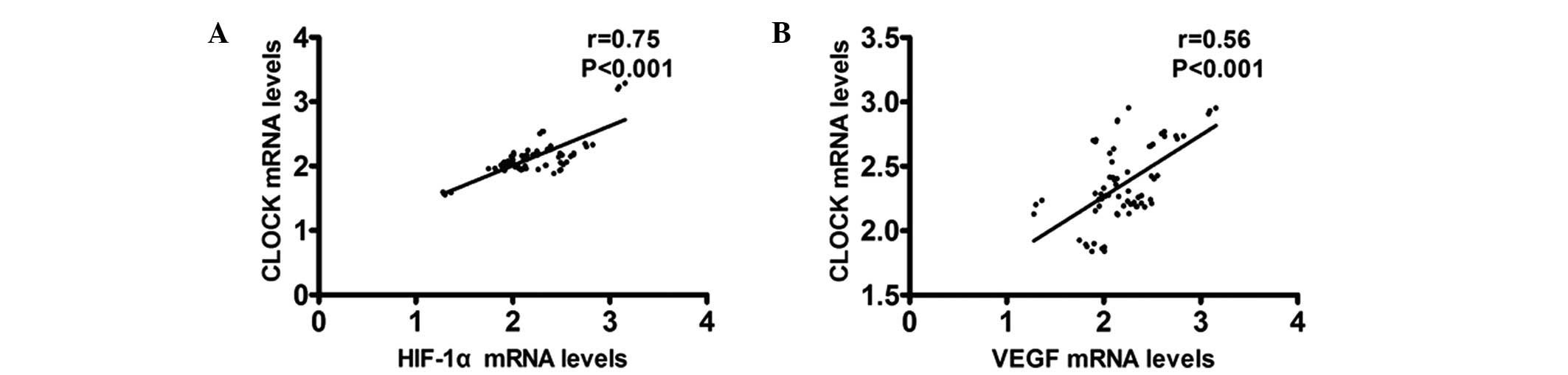
Upregulation of CLOCK is associated with
the expression of HIF-1α and VEGF in VVs
To better understand the clinical significance of
CLOCK in VVs, the association between the expression of CLOCK,
HIF-1α and VEGF in VVs was investigated. A relatively higher
expression level of CLOCK was detected in the early and advanced VV
tissues, with high expression levels of HIF-1α. The expression
levels of CLOCK and HIF-1α were positively correlated in the early
and advanced VV tissues (r=0.75; P<0.001; Fig. 5A). The same trends were observed in
the expression levels of CLOCK and VEGF in the advanced VVs
(r=0.56, P<0.001; Fig. 5B).
Discussion
At present, the pathogenesis of VVs remains to be
fully elucidated. Understanding the pathophysiology of chronic
venous disease is important to establish pharmacological approaches
to complement current treatment strategies. For the first time, to
the best of our knowledge, the present study demonstrated that high
mRNA expression of CLOCK is a hallmark of VVs, and that
upregulation in the expression of the CLOCK gene is correlated with
HIF-1α following deterioration in VVs. These findings indicate a
potential link between the expression of HIF-1α and the circadian
clock in the pathogenesis of this disease.
Venous hypoxia has been hypothesized to contribute
to VV wall changes and it is well known that activation of the HIF
pathway is increased in cells and tissues associated with hypoxia
(19). In the present study, the
expression of HIF-1α in the VVs was elevated, compared with that of
the control group containing normal veins, the results of which are
in agreement with previous in vitro studies (20,21).
The increased expression of HIF in the VVs is a significant
finding, as HIFs regulate ~1% of human genes (5). Several HIF target genes are involved
in functions, which are known to cause pathophysiological changes
in the walls of veins. However, what stage of VV formation the HIF
pathway is activated remains to be elucidated. The present study
demonstrated for the first time, to the best of our knowledge, that
the pathway was activated following the deterioration of VVs (in C5
and C6 lesions). Analysis of the subgroups revealed that the
expression of HIF-1α in the advanced varicose lesions was
significantly higher, compared with that of the initial varicose
lesions.
Several groups have demonstrated that hypoxia can
stimulate the production of VEGF (8,22).
VEGF is central in the maintenance of vascular integrity and
reactivity by mediating vaso-permeability and dilatory responses
(9). VEGF, which is produced in
vascular smooth muscle, can stimulate the production of nitric
oxide from the vascular endothelium, and continued and elevated
expression of VEGF then leads to elevated production of nitric
oxide, resulting in the generation of reactive oxygen species,
contributing to the further development of the pathology (8). Although it has been previously
demonstrated that patients with primary VV exhibit a loss of VEGF
release following mild, experimentally-induced venous stasis that
has been observed in control individuals (23), the present study clearly
demonstrated a significant rise in the expression of VEGF in the
VVs, as well as altered expression of VEGF following deterioration
in the veins. The ability to maintain or regulate the levels of
VEGF may be reduced in the VVs.
Notably, our previously study demonstrated that
VEGFα is one of a few genes expressed in a circadian
rhythm-dependent manner in the hearts of C57BL/6J and
ApoE−/− mice (24). In
mammals, several behavioral and physiological processes exhibit
circadian (~24 h) rhythms, which are controlled by a clock system.
The circadian rhythmicity of peripheral tissues is considered to be
uniquely controlled by the SCN via neural and humoral signals.
Previous studies have demonstrated that peripheral tissues and
cells contain a similar clock system to that in the SCN (11,12).
Our previous study revealed that the levels and rhythms of the
expression of core circadian genes were altered in human carotid
plaque-derived VSMCs (13).
Therefore, in the present study, focus was placed on the potential
role of the CLOCK gene in the pathogenesis of varicose lesions. The
present study revealed, for the first time, to the best of our
knowledge, that the expression of the CLOCK gene was significantly
upregulated in the advanced VVs, compared with normal control veins
or initial VVs. This finding further supports the hypothesis that
changes in the expression of circadian clock genes may be involved
in the progression of vascular diseases.
In common with several features of VVs, the cause
and effect association between the CLOCK gene and VVs may be
difficult to ascertain, particularly whether the association
contributes to a vicious cycle of disease progression. The control
of gene transcription and the maintenance of vascular reactivity
may be altered by a causative mechanism or simply by the
pathological process. As noted previously, increasing evidence has
demonstrated that cross-talk between mediators of the hypoxic and
circadian pathways regulate target genes. At the molecular level,
transient transfection experiments investigating the effect of
hypoxia on the circadian clock have revealed that the CLOCK gene
interacts with HIF-1α and drives transcription (14). HIF-1α does not form a DNA binding
complex with the CLOCK gene at E-box A, the control element of
several circadian clock genes and the core sequence of the
hypoxia-responsive element. However, the brain and muscle ARNT-like
protein 1 complex at E-box A is regulated by co-transfected
HIF-1α/CLOCK. Therefore, the E-box may be a key element in the
interaction between hypoxia signaling and the circadian clock
(14). A previous study revealed a
bidirectional link between the hypoxic and circadian pathway
(25). Further examination of such
genes may assist in elucidating the mechanism underlying the
development of VVs.
In conclusion, the present study demonstrated a
significant increase in the gene expression of CLOCK, as well as
HIF-1α and its target gene, VEGF, in advanced varicose lesions. The
upregulation of the circadian clock gene in venous vessels may be
involved in the pathogenesis of VVs and promote the progression of
venous disease. However, further investigations are required to
ascertain the correlations among the CLOCK gene, hypoxia and
HIF-1α, and the bidirectional link between the hypoxic and
circadian pathways in the pathogenesis of VVs. Investigations are
also required to detect the protein expression of these circadian
clock genes and identify other downstream genes that they mediate.
Pharmacological therapy aimed at hypoxia signaling and the
circadian clock may have a role in the management of venous
diseases in the future.
Acknowledgments
The present study was supported a by grant from the
Zhongshan Hospital Youth Talent Training Program (grant. no.
201514).
References
|
1
|
Lim CS and Davies AH: Pathogenesis of
primary varicose veins. Br J Surg. 96:1231–1242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raffetto JD and Khalil RA: Mechanisms of
varicose vein formation: Valve dysfunction and wall dilation.
Phlebology. 23:85–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim CS, Gohel MS, Shepherd AC, Paleolog E
and Davies AH: Venous hypoxia: A poorly studied etiological factor
of varicose veins. J Vasc Res. 48:185–194. 2011. View Article : Google Scholar
|
|
4
|
Semenza GL: Regulation of oxygen
homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda).
24:97–106. 2009. View Article : Google Scholar
|
|
5
|
Muz B, Khan MN, Kiriakidis S and Paleolog
EM: Hypoxia. The role of hypoxia and HIF-dependent signalling
events in rheumatoid arthritis. Arthritis Res Ther. 11:2012009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an
HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in
human endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar
|
|
7
|
Zhu XY, Daghini E, Chade AR, Lavi R,
Napoli C, Lerman A and Lerman LO: Disparate effects of simvastatin
on angiogenesis during hypoxia and inflammation. Life Sci.
83:801–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Servos S, Zachary I and Martin JF: VEGF
modulates NO production: The basis of a cytoprotective effect?
Cardiovasc Res. 41:509–510. 1999.PubMed/NCBI
|
|
9
|
Tomasian D, Keaney JF and Vita JA:
Antioxidants and the bioactivity of endothelium-derived nitric
oxide. Cardiovasc Res. 47:426–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore RY: Circadian rhythms: Basic
neurobiology and clinical applications. Annu Rev Med. 48:253–266.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balsalobre A: Clock genes in mammalian
peripheral tissues. Cell Tissue Res. 309:193–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh D, Rani S and Kumar V: Daily
expression of six clock genes in central and peripheral tissues of
a night-migratory songbird: Evidence for tissue-specific circadian
timing. Chronobiol Int. 30:1208–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin C, Tang X, Zhu Z, Liao X, Zhao R, Fu
W, Chen B, Jiang J, Qian R and Guo D: The rhythmic expression of
clock genes attenuated in human plaque-derived vascular smooth
muscle cells. Lipids Health Dis. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghorbel MT, Coulson JM and Murphy D:
Cross-talk between hypoxic and circadian pathways: Cooperative
roles for hypoxia-inducible factor 1alpha and CLOCK in
transcriptional activation of the vasopressin gene. Mol Cell
Neurosci. 22:396–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Classification and grading of chronic
venous disease in the lower limbs. A consensus statement. Ad Hoc
Committee, American Venous Forum. J Cardiovasc Surg (Torino).
38:437–441. 1997.
|
|
16
|
Zhu XC, Dong QZ, Zhang XF, et al:
microRNA-29a suppresses cell proliferation by targeting SPARC in
hepatocellular carcinoma. Int J Mol Med. 30:1321–1326.
2012.PubMed/NCBI
|
|
17
|
Hashimoto T, Yanaihara N, Okamoto A,
Nikaido T, Saito M, Takakura S, Yasuda M, Sasaki H, Ochiai K and
Tanaka T: Cyclin D1 predicts the prognosis of advanced serous
ovarian cancer. Exp Ther Med. 2:213–219. 2011.PubMed/NCBI
|
|
18
|
Chilov D, Hofer T, Bauer C, Wenger RH and
Gassmann M: Hypoxia affects expression of circadian genes PER1 and
CLOCK in mouse brain. FASEB J. 15:2613–2622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
20
|
Lim CS, Kiriakidis S, Paleolog EM and
Davies AH: Increased activation of the hypoxia-inducible factor
pathway in varicose veins. J Vasc Surg. 55:1427–1439. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JD, Yang WK and Lee TH: Increased
expression of hypoxia-inducible factor-1alpha and Bcl-2 in
varicocele and varicose veins. Ann Vasc Surg. 26:1100–1105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas KA: Vascular endothelial growth
factor, a potent and selective angiogenic agent. J Biol Chem.
271:603–606. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hollingsworth SJ, Tang CB, Dialynas M and
Barker SG: Varicose veins: Loss of release of vascular endothelial
growth factor and reduced plasma nitric oxide. Eur J Vasc Endovasc
Surg. 22:551–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu C, Lu C, Hua L, Jin H, Yin L, Chen S
and Qian R: Rhythm changes of clock genes, apoptosis-related genes
and atherosclerosis-related genes in apolipoprotein E knockout
mice. Can J Cardiol. 25:473–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egg M, Köblitz L, Hirayama J, Schwerte T,
Folterbauer C, Kurz A, Fiechtner B, Möst M, Salvenmoser W,
Sassone-Corsi P, et al: Linking oxygen to time: The bidirectional
interaction between the hypoxic signaling pathway and the circadian
clock. Chronobiol Int. 30:510–529. 2013. View Article : Google Scholar : PubMed/NCBI
|