Introduction
Stem cell transplantation has been investigated
extensively as a therapeutic approach to regenerate tissues
following injury (1). Mesenchymal
stem cells (MSCs), which can be readily isolated and expanded, have
neurogenic, chondrogenic, adipogenic, osteogenic and myogenic
properties under specific differentiating conditions (2). Furthermore, MSCs are genetically
stable with have a low risk of immune rejection and, therefore, are
often used as seeding cells in tissue engineering and in stem cell
therapy (3).
The ability to accurately regulate of cell fate
determination is a prerequisite for the future therapeutic use of
MSCs. MicroRNAs (miRNAs), a class of small non-coding RNAs,
negatively regulate the expression of a variety of genes (4). miRNAs affect diverse cellular
pathways by inducing RNA interference-based mechanisms at a
post-transcriptional level (5).
Based on the complicated association between miRNAs and the mRNA
3′-untranslated region (UTR), several online tools have been
developed, including TargetScan, PicTar and TargetRank, which
provide miRNA target predictions based on sequence complementarity
in the optimal base-pairing of miRNA to the seed region and
sequence conservation (6–8).
Roundabout 1 (Robo1) is expressed in multiple cell
types, including embryonic stem cells, cadiocytes, vascular
endothelial cells and MSCs (9,10).
In addition, Robo1 is crucial in the regulation of cell
proliferation, migration and differentiation (11,12).
Inhibition of the expression of Robo1 has a suppressive effect on
cell proliferation (11,13), however, the role of Robo1 in MSCs
remains to be fully elucidated.
In order to identify novel miRNAs, which target
Robo1, the present study used bioinformatic predications and
confirmed the association between miR-29a and Robo1 by performing
luciferase reporter assays.
Materials and methods
Cell transfection
Human MSCs were purchased from the American Type
Culture Collection (Manassas, VA, USA) and were cultured in
RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL, Carlsbad, CA,
USA) at 37°C in a humidified atmosphere with 5% CO2. To
achieve overexpression of miR-29a, the cells were transfected with
an miR-29a lentivirus (Genepharma Co., Ltd., Shanghai, China) using
Lipofectamine 2000 (Invitrogen Life Technologies). The
overexpression of Robo1 was achieved using a Robo1 open reading
frame-expressing clone (GeneCopoecia, Guangzhou, China). The cells
were plated in 6-well plates or 96-well plates, transfected and
incubated at 37°C for 24 or 48 h prior to their use in subsequent
assays or for RNA and protein extraction.
Construction of lentiviral vectors
overexpressing miR-29a and Robo1
To produce a lentivirus expressing mature miR-29a,
the pre-miRNA sequence was synthesized and a control scrambled
construct (control RNAi) with no homology to the human genome was
designed (5′-AAT GTA CTG CGC GTG GAG A-3′). The sequences were
cloned into the HpaI and XhoI sites of pGCSIL-green
fluorescent protein (GFP) (GeneChem, Shanghai, China) to produce
the pGCSIL-GFP-miR-29a or pGCSIL-GFP-control, respectively. Small
interfering (si)RNA targeting Robo1 was purchased from Auragene
Bioscience, Inc. (Changsha, China).
Lentivirus production, titration and
infection
To produce the miR-29a and the control lentivirus,
the plasmids encoding either miR-29a or the control scrambled
sequence were cotransfected into 293T cells with the pHelper1.0 and
pHelper 2.0 plasmids (GeneChem), which contained elements required
for virus packaging, using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer's instructions. The
culture supernatants containing the lentivirus were harvested,
ultra-centrifuged at 70,000 x g for 90 min, and the viral titers
were determined using plaque-based assays. For lentiviral
infection, the target cells were plated at 40–50% confluency and
incubated overnight for 16 h at 37°C. The culture medium,
consisting of Dulbecco's modified Eagle's medium (Cellgro,
Manassas, VA, USA) containing 10% fetal bovine serum (Gibco-BRL),
was then replaced with viral supernatant (1.5 ml/well) and
incubated at 37°C for 10 h, prior to replacement of the viral
supernatant with fresh media. After 48 h incubation, the infected
cells were selected using puromycin (2 mg/ml) (InvivoGen, Toulouse,
France) for 5 days.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
The MSCs were seeded (~1.0×106) into
6-well culture plates for 72 h prior to harvesting. Small RNAs
(~200 nt) were isolated using a mirVana™ PARIS™ kit (Ambion® Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. For the RT reactions, 1 mg small RNAs were reverse
transcribed using an miScript RT kit (Qiagen, Hilden, Germany) at
37°C for 60 min followed by a final incubation at 95°C for 5 min.
RT-qPCR was performed using an miScript SYBR Green PCR kit (Qiagen)
on a CFX96 real-time PCR machine (Bio-rad Laboratories, Inc.,
Hercules, CA, USA). The qPCR was performed at 95°C for 15 min,
followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C
for 30 sec. The expression level of each miRNA was normalized to U6
short non-coding RNA.
The mRNA expression level of Robo1 was detected by
RT-qPCR using a SYBR Green RT-PCR kit (Bio-Rad Laboratories, Inc.)
according to the manufacturer's instructions. Briefly, the total
RNA was extracted from the cells using TRIzol reagent (Invitrogen
Life Technologies) and the cDNA was synthesized using a RevertAid
First-Strand cDNA Synthesis kit (Fermentas, Pittsburgh, PA, USA),
according to the manufacturer's instructions. Each cDNA sample was
used as a template for the qPCR in triplicate using iQTM SYBR Green
Supermix (Bio-Rad Laboratories, Inc.) by denaturation at 94°C for 1
min, 30 cycles at 94°C for 40 sec and 60°C for 40 sec, followed by
extension at 72°C for 6 min. The specific primer pairs were: Robo1
(107 bp), sense 5′-GGC GGT GAA GGA GAT GAA C-3′ and antisense
5′-TGA TGA GGA AAT CCA CGA TAG AG-3′ and β-actin (202 bp), sense
5′-GGC GGC ACC ACC ATG TAC CCT-3′ and antisense 5′-AGG GGC CGG ACT
CGT CAT ACT-3′. The relative mRNA expression levels of Robo1 were
normalized to the internal control, β-actin. The relative gene
expression was quantified using CFX Manager version 1.6 software
(Bio-Rad Laboratories, Inc.) and data were expressed as a
percentage compared with the control cells.
Western blotting
The cells were cultured in 35 mm dishes prior to
lysis in 0.2 ml lysis buffer containing 0.1% SDS, 1% NP-40, 50 mM
HEPES (pH 7.4), 2 mM EDTA, 100 mM NaCl, 5 mM sodium orthovanadate,
40 µM p-nitrophenyl phosphate and 1% protease inhibitor
mixture set I (Calbiochem, La Jolla, CA, USA). The lysates were
centrifuged at 12,000 rpm for 15 min and the supernatants were
collected, denatured and separated using 10% SDS-PAGE gels and
blotted onto polyvinylidene difluoride membranes. The membranes
were then blocked in 5% bovine serum albumin for 1.5 h at room
temperature, followed by incubation overnight at 4°C with the
following antibodies: Mouse anti-human monoclonal anti-BrdU (B2531;
dilution 1:1,000; Sigma-Aldrich, St. Louis, MO, USA) and polyclonal
mouse anti-β-actin (sc-47778; dilution 1:2,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The membranes were rinsed
and incubated for 1 h with goat anti-mouse HRP-conjugated secondary
antibodies (sc-2005; dilution 1:5,000; Santa Cruz Biotechnology,
Inc.). Chemiluminescent detection was performed using an Enhanced
Chemiluminescence Detection kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Luciferase reporter assay
A luciferase reporter assay was performed in human
embryonic kidney 293a (HEK293a) cells (Huiying Biological
Technology Co., Ltd., Xian, China). Vectors, based on either
pMIR-REPORT, containing the wild-type (WT) 350 bp fragment of the
Robo1 3′-UTR or the same fragment with mutation (MUT) of the
miR-29a binding site (199-194 bp), were inserted downstream of the
luciferase reporter gene stop codon in pMIR-REPORT using
HindIII and SpeI sites. The cells were cotransfected
with either the miR-29a lentivirus, the miR-67-negative-control
lentivirus (50 nM), the pMIR-REPORT vectors containing the WT or
MUT miR-29a binding sites (400 ng) or pRL-SV40 (Promega
Corporation, Madison, WI, USA) expressing Renilla luciferase (400
ng) for normalization of the transfection efficiency. The cells
were grown in high-glucose Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum and the luciferase
activities were measured 48 h after transfection using a
Dual-Luciferase Reporter assay system (Promega Corporation).
MTT assay
The cell viability was evaluated using a modified
MTT assay. The MSCs were seeded (2×103) into 96-well
plates and the viability of the cells transfected with either the
miR-29a or control were assessed at five time points (days 1–5).
Briefly, the quantification of mitochondrial dehydrogenase activity
was determined via the enzymatic conversion of MTT (Sigma-Aldrich)
to a colored formazan product. MTT (10 µl; 10 mg/ml) was
added to the cells and incubated for 4 h prior to termination of
the reaction by removing the supernatant and additing 100 µl
dimethylsulfoxide to dissolve the formazan product. Following
incubation for 30 min, the optical density of each well was
measured at 570 nm using a plate reader (EL×808; BioTek
Instruments, Inc., Winooski, VT, USA).
5-Bromo-2-deoxyuridine (BrdU)
incorporation assay
The DNA synthesis in the proliferating cells was
determined by measuring BrdU incorporation. BrdU assays were
performed at 24 and 48 h after the transfection of the MSCs with
the miR-29a or control vectors. The transfected cells were seeded
(2×103) into 96-well plates, cultured for 24 or 48 h and
incubated with a final concentration of 10 µM BrdU (BD
Pharmingen, San Diego, CA, USA) for 2–24 h at 37°C. Following
incubation, the medium was removed and the cells were fixed with 4%
paraformaldehyde (Amsbio, Beijing, China) for 30 min at room
temperature, prior to incubation with a peroxidase-coupled
monoclonal mouse anti-human BrdU-antibody (1:1,000; Sigma-Aldrich)
for 60 min at room temperature. The cells were then washed three
times with phosphate-buffered saline, incubated with
tetramethylbenzidine for 30 min and the absorbance values were
measured at 490 nm using the EL×808 microplate reader. The
background BrdU immunofluorescence was determined using
BrdU-negative cells stained with the BrdU antibody.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent experiments and processed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The expression of miR-29a
in the MSC tissues and the paired adjacent normal colonic tissues
were compared using Wilcoxon's paired test. The differences between
the groups in the migration and invasion assays were evaluated by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
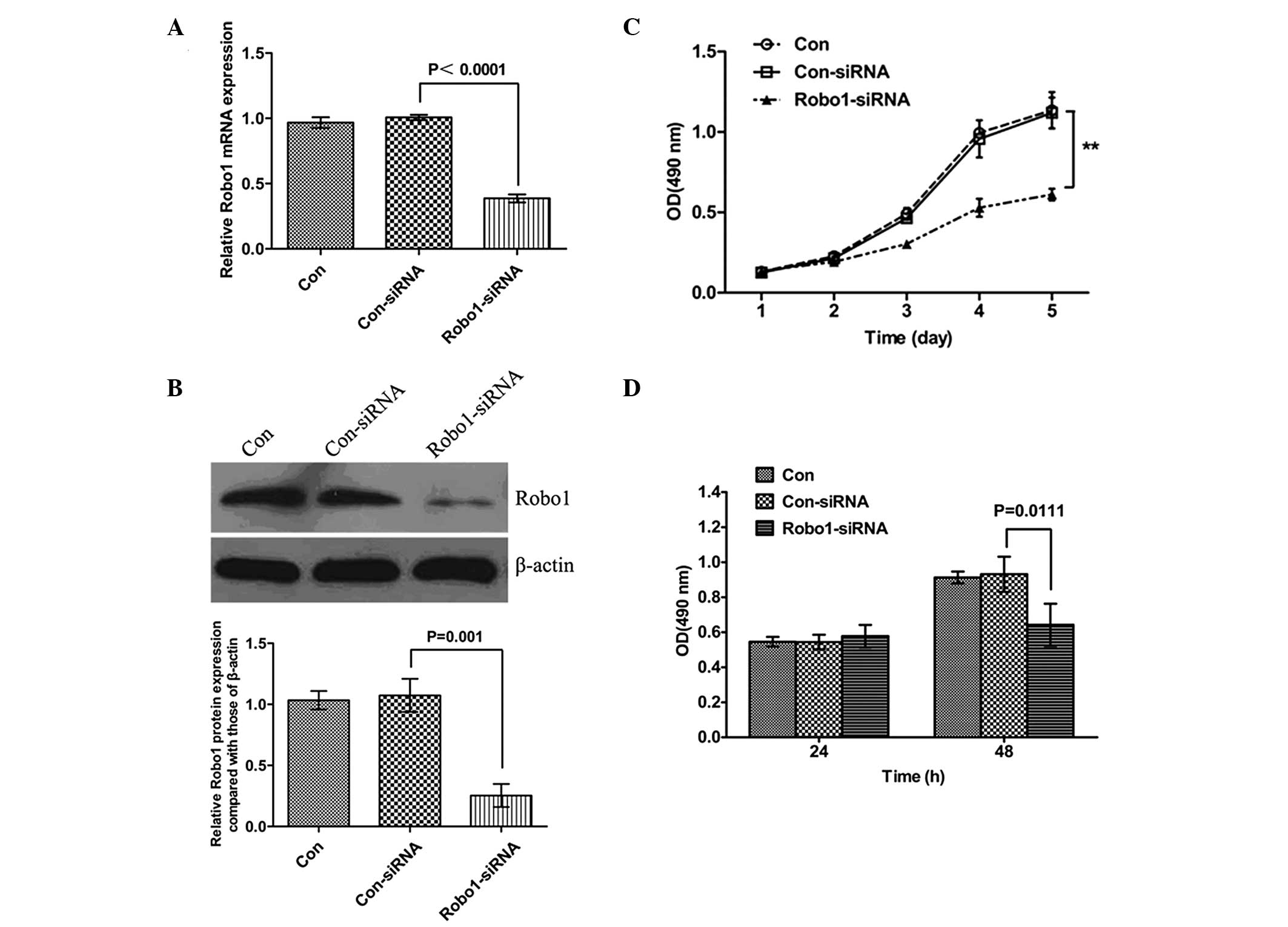
Downregulation of Robo1 using siRNA
inhibits the viability and proliferation of MSCs
To determine the potential role for Robo1 in MSCs,
Robo1-specific siRNA was transfected into MSCs to suppress the
expression of Robo1. Following transfection, the mRNA and protein
levels of Robo1 in the MSCs were determined. The mRNA and protein
levels were notably downregulated following transfection with
Robo1-siRNA (Fig. 1A and B),
indicating that the transfection efficiency was satisfactory. The
MTT and BrdU incorporation assays were subsequently used to
determine the effects of Robo1 downregulation on MSC viability and
proliferations. As shown in Fig. 1C
and D, the viability and proliferation of the
Robo1-siRNA-transfected MSCs were reduced compared with the
non-transfected cells or those transfected with the control siRNA,
indicating that the inhibition of Robo1 suppressed the viability
and proliferation of the MSCs.
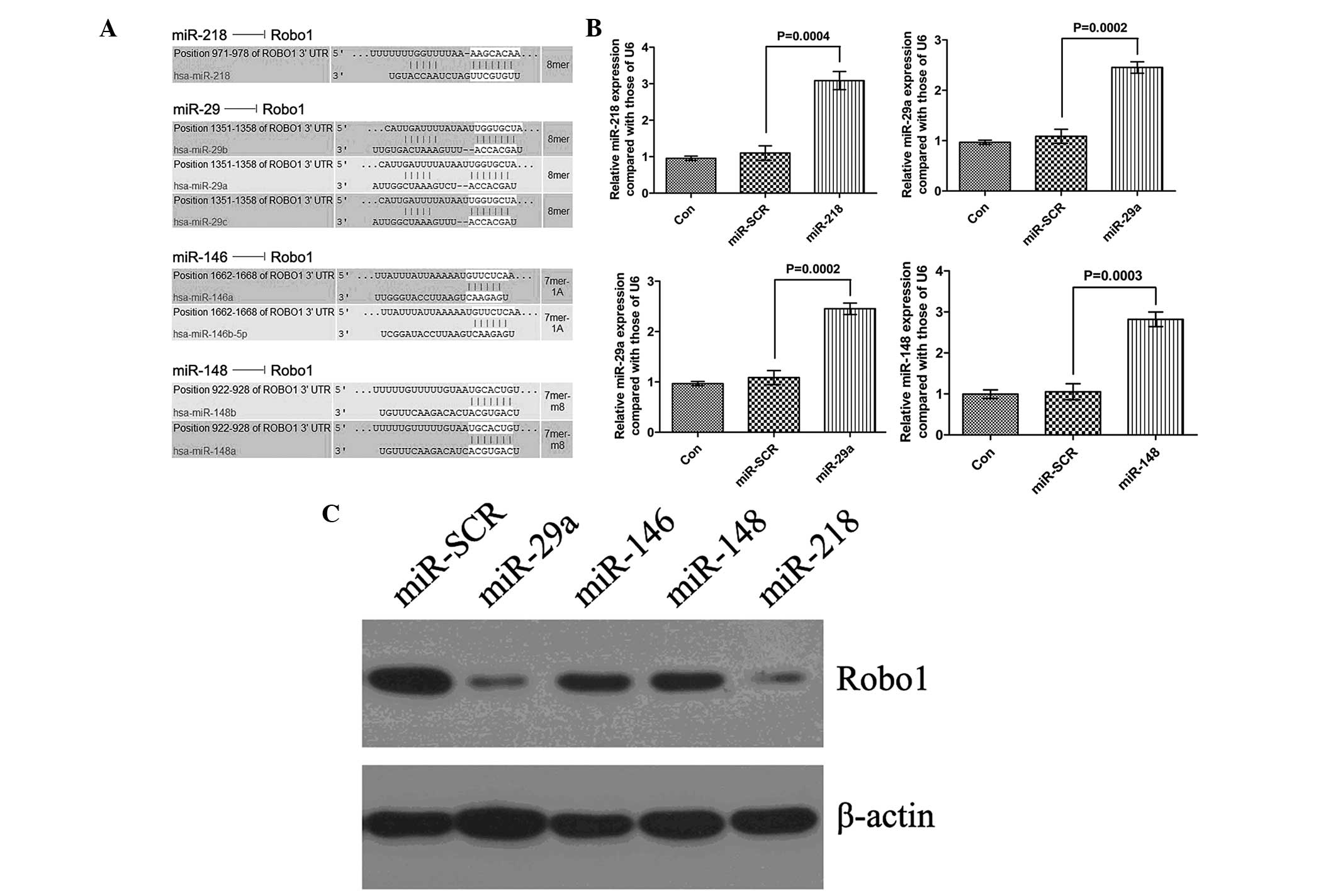
miR-29a markedly inhibits the protein
expression of Robo1 in MSCs
To examine the regulatory effects of miRNAs on the
expression of Robo1 in the MSCs, bioinformatical predication was
performed. Several putative miRNAs were identified, including
miR-218, miR-29a, miR-146 and miR-148 (Fig. 2A). The MSCs were then transfected
with the identified miRNA mimics. Following transfection, the
expression levels of the four miRNAs were determined using RT-qPCR,
all of which were significantly increased (Fig. 2B), indicating a satisfactory
transfection efficiency. Subsequently, western blotting was
performed to examine the protein expression of Robo1. All four
miRNAs inhibited the protein expression of Robo1, however, miR-29a
had the most marked effect (Fig.
2C).
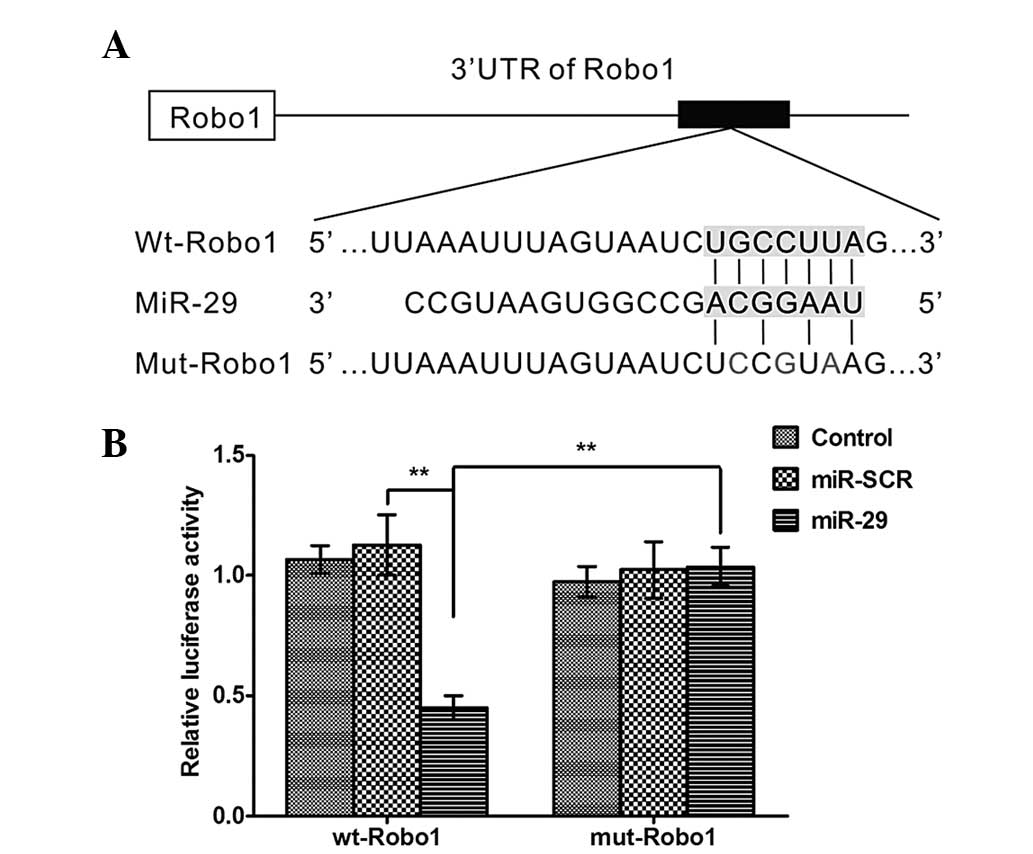
Robo1 mRNA is a direct target of
miR-29a
To confirm the hypothesis that miR-29a binds to the
3′-UTR of Robo1 mRNA (Fig. 3A), a
WT Robo1 3′-UTR luciferase reporter vector (WT-Robo1) and a mutated
version of the Robo1 3′-UTR luciferase reporter vector (MUT-Robo1)
were produced by sequentially mutating the predicted 8 bp miR-29a
binding site of the Robo1 3′-UTR (Fig.
3A). The WT-Robo1 vector was cotransfected with either the
miR-29a lentivirus or the scrambled control into the HEK293 cells.
The luciferase activity of the Robo1 3′-UTR luciferase reporter
vector was significantly reduced in the miR-29a-transfected cells
compared with the scrambled control cells (Fig. 3B). In addition, the
miR-29a-mediated repression of Robo1 3′-UTR luciferase reporter
activity was eliminated by mutation of the putative miR-29a binding
site in the Robo1 3′-UTR (Fig.
3B).
Overexpression of miR-29a inhibits the
expression of Slit2 and Robo1 in MSCs
To investigate the role of miR-29a in MSCs, the MSCs
were infected with an miR-29a or miR-scramble lentivirus. RT-qPCR
revealed that miR-29a was significantly upregulated in the MSCs
infected with the miR-29a lentivirus compared with those infected
with the scrambled control lentivirus (Fig. 4A). In addition, overexpression of
miR-29a resulted in a significant decrease in the mRNA and protein
expression levels of Slit2 and Robo1 in the MSCs (Fig. 4B and C). These findings indicated
that miR-29a inhibits the regulation of the Slit2 and Robo1
expression in the MSCs.
Overexpression of miR-29a inhibits MSC
viability and proliferation by targeting Robo1
To investigate whether miR-29a regulates MSC
viability and proliferation by targeting Robo1, the MSCs were
infected with either the miR-29a lentivirus, the control or the
Robo1 lentivirus or were co-transfected with the miR-29a and Robo1
lentiviruses. Prior to performing cell viability and proliferation
assays, the efficiency of the overexpression of Robo1 was
determined. As shown in Fig. 5A and
B, following infection with Robo1 lentivirus, the mRNA and
protein levels of Robo1 were markedly upregulated in the MSCs. MTT
and BrdU incorporation assays were subsequently performed to
determine the cell viability and proliferation of the cells in each
group. As shown in Fig. 5C and D,
the forced upregulation of miR-29a significantly inhibited MSC
viability and proliferation, while overexpression of Robo1 notably
promoted the viability and proliferation in the MSCs. In addition,
the inhibitory effects of miR-29a upregulation on the viability and
proliferation of the MSCs were restored by the overexpression of
Robo1, indicating that miR-29a inhibited MSC viability and
proliferation, at least partially, by directly targeting Robo1
(Fig. 5C and D).
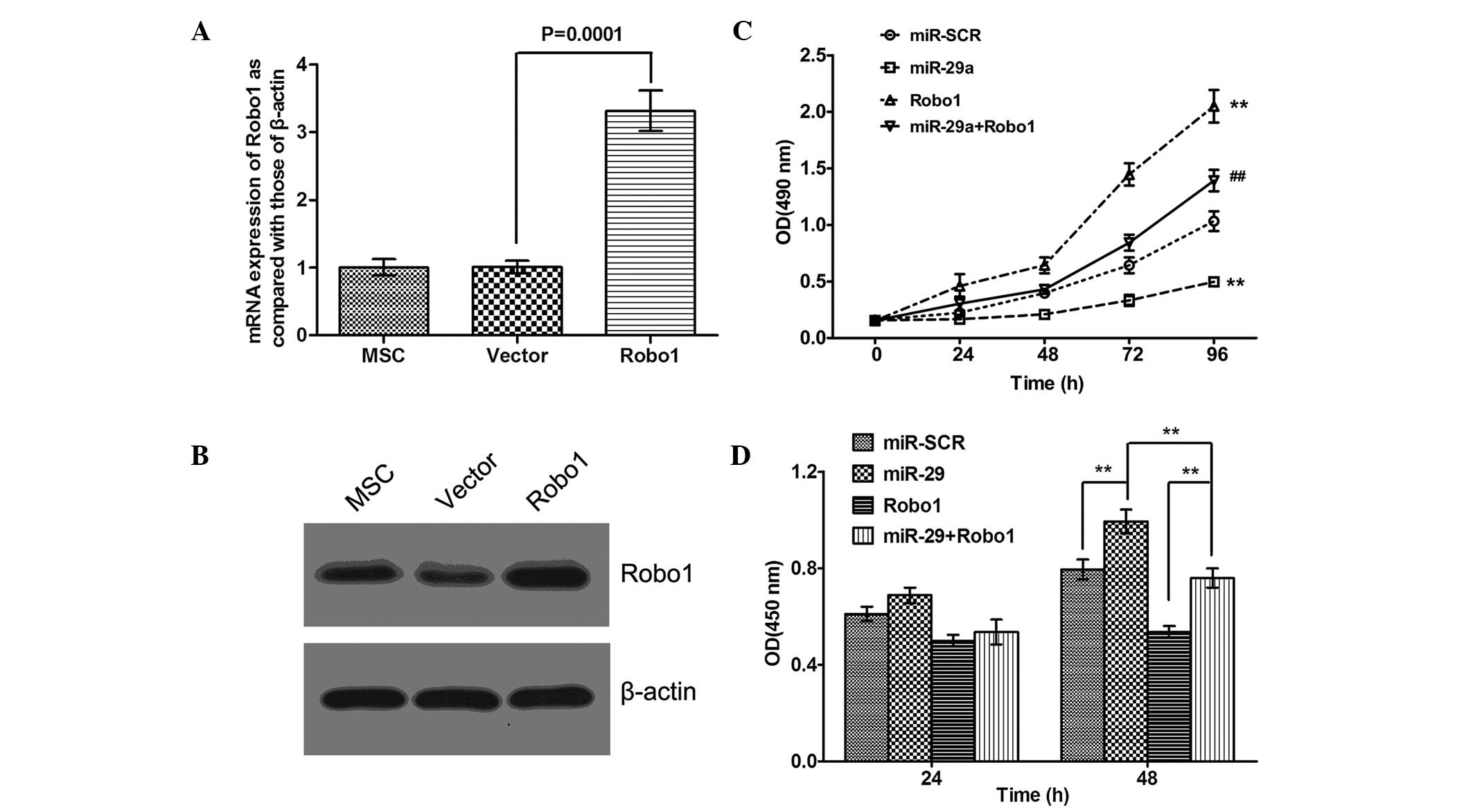 | Figure 5Overexpression of miR-29a inhibits MSC
viability and proliferation by targeting Robo1. Following MSC
infection with the Robo1 overexpressing lentivirus or blank vector
lentivirus, the (A) mRNA level of Robo1 was determined using
reverse transcription quantitative polymerase chain reaction. Data
are expressed as the mean ± standard deviation. (B) protein level
of Robo1 was determined using western blotting. (C) Following MSC
infection with miR-29a lentivirus and/or Robo1 overexpressing
lentivirus, an MTT assay was performed to determine the MSC
viability in each group. MSCs infected with miR-SCR were used as a
control (##P<0.05, vs. miR-SCR and
**P<0.01, vs. miR-SCR). Data are expressed as the
mean ± standard deviation. (D) Following MSC infection with miR-29a
lentivirus and/or Robo1 overexpressing lentivirus, a BrdU assay was
performed to determine the MSC proliferation in each group. MSCs
infected with miR-SCR were used as a control
(**P<0.01). Data are expressed as the mean ± standard
deviation. MSC, mesenchymal stem cell; Robo1, Roundabout 1; miR,
microRNA; BrdU, 5-bromo-2-deoxyuridine; OD, optical density. |
Discussion
The present study, was the first, to the best of our
knowledge, to reveal the crucial role of the miR-29a/Robo1 axis in
the regulation of MSC viability and proliferation. In addition, the
results identified that Robo1 was a direct target of miR-29a and
that miR-29a had an inhibitory effect on MSC viability and
proliferation, at least partly through direct inhibition of
Robo1.
Robo1 has been found to be fundamental in the
regulation of multiple biological processes, including cellular
proliferation, differentiation, migration, embryonic development,
angiogenesis and types of cancer (10,11,14).
Several studies have revealed that Slit-Robo signaling is involved
in the regulation of MSC biological processes (15,16).
Sun et al demonstrated that the mRNAs of Slit2 and Robo1 are
expressed during the osteoblastic differentiation of MSC-derived
cell lines (15). Another previous
study revealed that Slit2 cooperated with Robo1 to maintain
Robo-expressing cells, including MSCs in bone marrow niches at
steady state and following radiation (16). However, no direct evidence has been
found to confirm that Robo1 is involved in the regulation of MSC
viability and proliferation. The present study demonstrated that
silencing of Robo1 by siRNA suppressed the viability and
proliferation of MSCs.
miRNAs, as small molecular regulators of gene
expression, are important in stem cell function (17). Their ability to fine-tune protein
levels is often exploited against key proteins involved in
self-renewal or differentiation, including the targeting of
Slit/Robo signaling by miR-218 in angiogenesis (18). Additionally, miR-29 has multiple
distinct functions and the role of miR-29a in the differentiation
of MSC-derived cells has been previously reported. miR-29b
initiates the osteogenic signaling by suppressing anti-osteogenic
factors, including activin receptor type-2A, catenin, β-interacting
protein 1, histone deacetylase 4, transforming growth factor-β3 and
dual specificity protein phosphatase 2 (19). Guerit et al demonstrated
that miRNA-29a is involved in the forkhead box O3 controlled
chondrogenic differentiation of MSCs and cartilage formation
(20). miR-29a also acts as a
mediator in the miR-335-5p/sex determining region Y-box 9
controlled chondrogenesis in mouse MSCs (21) and Yan et al reported that
miR-29a is involved in the polyhydroxyalkanoate-induced
chondrogenic differentiation of MSCs by directly targeting col2a1,
which encodes type II collagen (22). Therefore, miR-29a is important in
regulating the osteogenic and chondrogenic differentiation of MSCs.
However, no other functions on the effects of miR-29a on the
biological processes in MSCs have been reported. The present study,
was the first, to the best of our knowledge, to demonstrate that
miR-29a has an inhibitory effect in the regulation of MSC viability
and proliferation.
As Robo1 was identified as a novel target of miR-29a
and had reverse effects on MSC viability and proliferation, the
present study hypothesized that miR-29a inhibited MSCs viability
and proliferation by directly targeting Robo1. To confirm this
hypothesis, the present study upregulated the expression levels of
miR-29a and/or Robo1 in MSCs. Upregulation of miR-29a inhibited the
protein expression of Robo1 and inhibited MSC viability and
proliferation. The restoration of Robo1 partially rescued the
inhibitory effects of the miR-29a upregulation on the viability and
proliferation of the MSCs, which suggested that miR-29a had a
suppressive effect in the regulation of MSC viability and
proliferation, at least partly via the direct downregulation of
Robo1. MSCs have also been demonstrated to be regulated by other
miRNAs. Chen et al demonstrated that miR-125b suppresses the
proliferation and osteogenic differentiation of human bone
marrow-derived MSCs (23) and Mao
et al demonstrated that miR-23a is involved in tumor
necrosis factor-α induced apoptosis in MSCs (24). These findings and those of the
present study provide evidence supporting a role for miRNAs in the
regulation of MSC biological processes.
In conclusion, the present study demonstrated that
miR-29a inhibited MSC viability and proliferation via the targeting
of Robo1. These results suggested that the miR-29a/Robo1 axis is
important in MSCs, raising the possibility that administering
miR-29a inhibitors may be used as a therapeutic technique for
diseases for the expansion of MSCs prior to transplantation.
References
|
1
|
Bayraktar UD and Ciurea SO: Strategies in
haploidentical stem cell transplantation in adults. Turk J
Haematol. 30:342–350. 2013. View Article : Google Scholar
|
|
2
|
Tanabe S: Role of mesenchymal stem cells
in cell life and their signaling. World J Stem Cells. 6:24–32.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das M, Sundell IB and Koka PS: Adult
mesenchymal stem cells and their potency in the cell-based therapy.
J Stem Cells. 8:1–16. 2013.
|
|
4
|
Moss EG: MicroRNAs: Hidden in the genome.
Curr Biol. 12:R138–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarkar D, Leung EY, Baguley BC, Finlay GJ
and Askarian-Amiri ME: Epigenetic regulation in human melanoma:
Past and future. Epigenetics. Jan 14–2015.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5:232014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krek A, Grun D, Poy MN, et al:
Combinatorial microRNA target predictions. Nat Genet. 37:495–500.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrews W, Barber M, Hernadez-Miranda LR,
et al: The role of Slit-Robo signaling in the generation, migration
and morphological differentiation of cortical interneurons. Dev
Biol. 313:648–658. 2008. View Article : Google Scholar
|
|
10
|
Yuen DA and Robinson LA: Slit2-Robo
signaling: A novel regulator of vascular injury. Curr Opin Nephrol
Hypertens. 22:445–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Li Y, Wang XY, et al: Slit/Robo1
signaling regulates neural tube development by balancing
neuroepithelial cell proliferation and differentiation. Exp Cell
Res. 319:1083–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dontula R, Dinasarapu A, Chetty C, et al:
MicroRNA 203 modulates glioma cell migration via Robo1/ERK/MMP-9
signaling. Genes Cancer. 4:285–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang L, Xu Y, Yu W, et al: Effect of
Robo1 on retinal pigment epithelial cells and experimental
proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci.
51:3193–3204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Je EM, Gwak M, Oh H, et al: Frameshift
mutations of axon guidance genes ROBO1 and ROBO2 in gastric and
colorectal cancers with microsatellite instability. Pathology.
45:645–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Dai K, Tang T and Zhang X:
Regulation of osteoblast differentiation by slit2 in osteoblastic
cells. Cells Tissues Organs. 190:69–80. 2009. View Article : Google Scholar
|
|
16
|
Smith-Berdan sec, Schepers K, Ly A,
Passegue E and Forsberg EC: Dynamic expression of the Robo ligand
Slit2 in bone marrow cell populations. Cell Cycle. 11:675–682.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KS, Kim JS, Lee MR, Jeong HS and Kim
J: A study of microRNAs in silico and in vivo: Emerging regulators
of embryonic stem cells. FEBS J. 276:2140–2149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fish JE, Wythe JD, Xiao T, et al: A
Slit/miR-218/Robo regulatory loop is required during heart tube
formation in zebrafish. Development. 138:1409–1419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi E and Hwang KC: MicroRNAs as novel
regulators of stem cell fate. World J Stem Cells. 5:172–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerit D, Brondello JM, Chuchana P, et al:
FoxO3A regulation by miRNA-29a controls chondrogenic
differentiation of mesenchymal stem cells and cartilage formation.
Stem Cells Dev. 23:1195–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Wu L, Zhang Z, et al: MiR-335-5p
promotes chondrogenesis in mouse mesenchymal stem cells and is
regulated through two positive feedback loops. J Bone Miner Res.
29:1575–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan C, Wang Y, Shen XY, et al: MicroRNA
regulation ass°Ciated chondrogenesis of mouse MSCs grown on
polyhydroxyalkanoates. Biomaterials. 32:6435–6444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Yang L, Jie Q, et al: MicroRNA125b
suppresses the proliferation and osteogenic differentiation of
human bone marrowderived mesenchymal stem cells. Mol Med Rep.
9:1820–1826. 2014.PubMed/NCBI
|
|
24
|
Mao J, Lv Z and Zhuang Y: MicroRNA-23a is
involved in tumor necrosis factor-alpha induced apoptosis in
mesenchymal stem cells and myocardial infarction. Exp Mol Pathol.
2013.
|