Introduction
Giant cell tumor of bone (GCTB) is a relatively
uncommon, aggressive, non-cancerous type of tumor, characterized by
the presence of multinucleated giant (osteoclast-like) cells. GCTB
generally occurs in adults between the ages of 20 and 40 years, and
occurs in males and females with equal frequency (1,2). The
majority of GCTBs arise in the metaphyseal-epiphyseal areas of the
distal femur, proximal tibia and distal radius (3,4).
Despite being classified as benign, GCTBs are aggressive and up to
50% of cases are associated with local recurrence. Furthermore, up
to 5% of GCTBs metastasize to the lungs and GCTB undergoes
spontaneous transformation into high-grade malignancy in 1–3% of
patients (2,5). However, the pathogenesis and
histogenesis of GCTBs have remained elusive, without predictable
histological values for evaluating the clinical outcome.
Hypoxia has been confirmed to be associated with a
variety of types of cancer, and has become one of the key issues in
the study of tumor physiology (6,7). A
group of transcription factors has been reported to be involved in
the regulation of genes responsible for the metabolic changes
induced under hypoxic conditions (8,9). A
pivotal component of this group is hypoxia-inducible factor 1
(HIF-1), which exists as a heterologous dimer, comprised of an
oxygen sensitive HIF-1α subunit and a constitutively expressed
HIF-1β subunit (10). HIF-1 binds
to a conserved DNA consensus sequence on the promoters of its
target genes, known as hypoxia-responsive elements (11–13).
Upregulation of HIF promotes the expression of gene products
required for hypoxic adaptation (14), and regulates vascular endothelial
growth factor (VEGF) and additional angiogenic factors (15,16),
which have key roles in the growth and progression of solid tumors
(17–20). As a result of the generally
insidious onset of symptoms in patients with GCTB, the tumors
frequently grow to a large size prior to diagnosis, and therefore
potentially undergo hypoxia as the overgrowth of tumor cells
distances them from the local microvessels (21). Few studies have previously
indicated the significant role of HIF in hypoxia adaptation in
GCTBs; however, Knowles et al (21) revealed that HIF is promoted in
GCTBs and mediates paracrine effects on monocyte-osteoclast
differentiation via the induction of VEGF.
The neoplastic 'driver' role of the stromal cell was
indicated by Knowles et al (21) and the results of additional studies
(22). The in vitro
experiments by Knowles et al (21) indicated that HIF was induced in
primary GCTB stromal cells following hypoxia. It was also
demonstrated that the accumulation of giant cells is due, in part,
to the high levels of receptor activator of nuclear factor-κB
ligand expression by the neoplastic stromal cells (23–25),
suggesting a significant role for the stromal cell. Treatment of
GCTB stromal cells with parathyroid hormone-related protein
significantly increased the number of multinucleated cells formed
from RAW 264.7 cells in co-culture experiments (26). Interleukin-17A has also been shown
to be overexpressed in GCTB stromal cells, and to stimulate the
progression of GCTBs (27).
Additionally, it was demonstrated that stromal cells in GCTB
expressed matrix metalloproteinase-9 (28,29)
and several types of bone morphogenetic protein (BMP-2, 3, 4, 5 and
6) (30).
MicroRNAs (miRNAs) are 18–22 nucleotide non-coding
RNA molecules that regulate gene expression in a variety of
organisms, ranging from nematodes to humans (31), and are involved in a broad array of
mammalian cellular processes (32–34).
The suppression of target genes by miRNAs induce diverse biological
outcomes during normal development and pathological responses. In
particular, alterations in miRNA expression have marked effects on
the progression of tumorigenesis (35–37).
Studies have indicated the oncogenic or tumor suppressive roles of
certain deregulated miRNAs in bone tumors, particularly in
osteosarcoma (38–43). miR-210 is a key regulator of the
hypoxic response, and its upregulation has been observed in all
cell types evaluated under hypoxic conditions so far (44). In addition, miR-210 was found to be
a positive regulator of osteoblastic differentiation via inhibition
of activin A receptor type 1B (45). However, to the best of our
knowledge there has been no previous report regarding miR-210, the
characteristic miRNA deregulated under hypoxia, in GCTBs.
In the present study, the expression of miR-210 and
HIF-1α was quantitatively determined in 42 GCTB specimens by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analyses. The induction of miR-210 and
HIF-1α expression in primary stromal cells isolated from GCTB
tissues under hypoxic conditions was also further confirmed.
Subsequently, the regulatory role of HIF-1α on miR-210 expression
in primary stromal cells was evaluated. The present study aimed to
provide novel information regarding the mechanisms underlying GCTB
tumorigenesis.
Materials and methods
Tissue specimens and ethical
approval
The utilization of 42 sacral GCTB specimens was
approved by the Internal Review Board of the Second Affiliated
Hospital of Inner Mongolia Medical University (Hohhot, China). All
42 specimens were obtained from surgical resections from sacral
GCTB patients registered at the aforementioned hospital, between
January 2008 and December 2011. A total of 11 osteochondroma
tissues were obtained from patients matched for age, gender, and
tumor location for use as control specimens. All tissue samples for
miR-210 and HIF-1α expression analysis were frozen at −80°C
immediately following surgical resection. The GCTB tissue for
primary stromal cell isolation was obtained from a GCT located on
the distal femur and permission was given by the patient who was
registered at the Second Affiliated Hospital of Inner Mongolia
Medical University in September 2013. The GCT tissue was put on ice
and treated for cell isolation immediately following surgical
resection. The GCTB patients selected for this study were all
treated with identical programs. Prior to the operation, patients
granted consent for the use of the excised cancer tissue in medical
or scientific research.
Isolation, culture and treatment of
primary GCTB stromal cells or U2 osteosarcoma (OS) cells
Primary GCTB stromal cells were isolated from the
GCT tissue from the distal femur according to a previously
published protocol (46,47). Briefly, the fresh tissue specimens
were placed in ice-cold isolation solution (Miltenyi Biotec, Inc.,
San Diego, CA, USA), and homogenized. The stromal cells were
isolated and basal CD146 expression was enriched with anti-CD146
monoclonal antibody-coupled magnetic beads, following the removal
of CD14- and CD45-positive cells (46). The stromal cells were cultured with
RPMI-1640 (Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS) (Invitrogen Life Technologies,
Carlsbad, CA, USA), antibiotics (100 mg/ml streptomycin and 100
U/ml penicillin; North China Pharmaceutical Co., Ltd, Shijiazhuang,
China) and 10 nmol/l dihydrotestosterone (Sigma-Aldrich, St. Louis,
MO, USA). The cells under normoxia were incubated at 37°C, with 5%
CO2. The U2 OS cells were obtained from the Cell
Resource Center of Chinese Academy of Medical Sciences (Beijing,
China), and cultured in Eagle's minimum essential medium or McCoy's
5a modified medium supplemented with 10% FBS, and were incubated at
37°C with 5% CO2. For hypoxia treatment, cells were
placed in a hypoxia incubator (HERAcell 150i CO2
incubator; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
infused with 5% CO2, 3% O2 and N. Small
interference (si) HIF-1α and siRNA control oligomers were
synthesized by GenePharma Technology (Shanghai, China) and were
transfected into the stromal cells at a concentration of 5 nM with
Lipofectamine® 2000 (Invitrogen Life Technologies) to
suppress HIF-1α expression. The overexpression of HIF-1α was
induced with HIF-1α-pcDNA3.1 plasmid (Sino Biological, Inc.,
Beijing, China) transfection (48).
RNA extraction and RT-qPCR
A mirVana miRNA Isolation kit (Ambion, Austin, TX,
USA) was used to extract miRNA from clinical specimens or cultured
cells. The mirVana qRT-PCR miRNA Detection kit (Ambion) was used to
quantify miR-210 expression, and U6 small nuclear RNA was used as
an internal control. The ΔΔCt method was used to calculate the
relative quantification (49). The
RNeasy Mini kit (Qiagen, Valencia, CA, USA) was used to extract
total messenger (m)RNA from clinical specimens or cell samples, and
RT-qPCR analysis of Drosha, Dicer and HIF-1α mRNA expression was
performed using SYBR Green RT-PCR kit (Takara Bio, Inc., Tokyo,
Japan) with the LightCycler 2.0 (Roche Diagnostics GmbH, Mannheim,
Germany). The qPCR was performed as follows: 42°C for 5 min, 95°C
for 10 sec, followed by 40 cycles at 95°C for 15 sec and 60°C for
30 sec. All mRNA expression levels were normalized to GAPDH and
quantified using the ΔΔCt method (49).
Western blot analysis
Tissue samples for immunoblotting were placed in
ice-cold isolation solution and homogenized. Following
homogenization, total protein concentration was measured using the
bicinchoninic acid assay protein assay reagent kit (Thermo Fisher
Scientific, Inc.) and was adjusted to 2 mg/ml with isolation
solution. Equal quantities of protein and sample buffer were
separated by 12% SDS-PAGE, and subsequently transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
Tris-buffered saline containing 5% milk at 4°C for 3 h, and
incubated with Dicer (cat. no. sc-30226), Drosha (cat. no.
sc-33778), HIF-1α (cat. no. sc-10790) or GAPDH (cat. no. sc-25778)
rabbit polyclonal antibodies (1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), followed by incubation with horseradish
peroxidase-conjugated mouse anti-rabbit secondary antibody
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA). The
proteins were detected using enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.). All immunoblots are representative of at
least three independent experiments.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The differences in Dicer,
Drosha and HIF-1α expression between the two groups were analyzed
by Student's t-test. Correlations between miR-210 and HIF-1α
expression were analyzed using the Spearman's rank correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-210 is upregulated in GCTB
specimens
miR-210, the most significantly upregulated miRNA in
cancer under hypoxic conditions, was selected for investigation in
GCTB tissues in the present study. RT-qPCR was used to evaluate
miR-210 expression levels in 42 GCTB tissues and 11 osteochondroma
tissues. The clinical information regarding the patients with GCTB
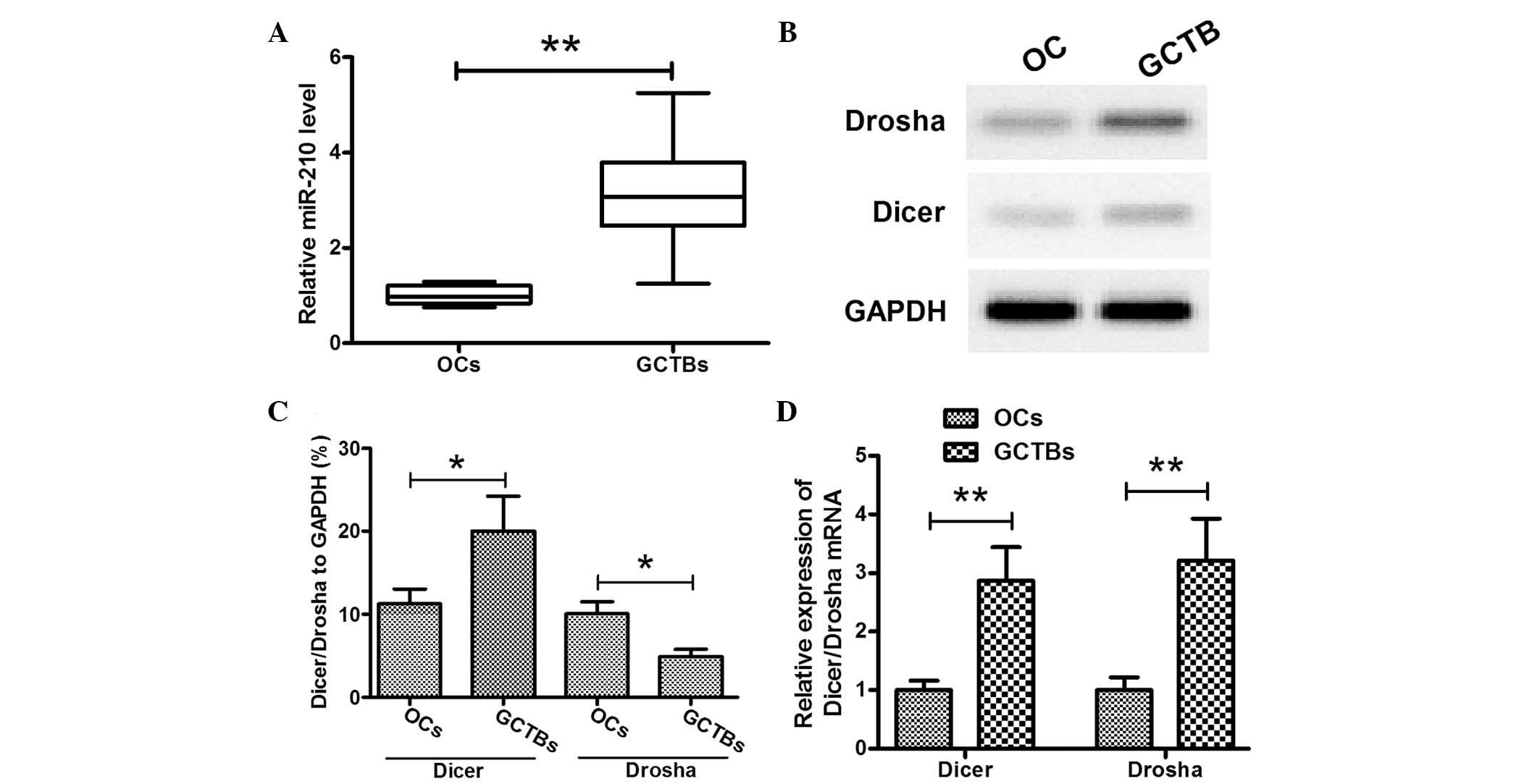
evaluated in the present study is listed in Table I. The results demonstrated that the
relative miR-210 expression levels in GCTB were 3.105±1.002,
significantly higher than those in the osteochondroma tissues
(1.010±0.194) (P<0.01; Fig.
1A). In order to confirm the overexpression of miR-210, the
miR-210 protein expression levels in the above specimens were also
assessed by western blotting. The results of the western blot
analysis also demonstrated significantly higher expression of
miR-210 in GCTB specimens than those in the osteochondroma
specimens (35.939±9.033 vs. 22.791±4.217) (P<0.05; Fig. 1B). It was therefore concluded that
miR-210 was upregulated in GCTB specimens. To further evaluate the
upregulation of miR-210 in GCTBs, the expression of key
miRNA-processing enzymes, Dicer and Drosha, was evaluated in the
GCTB specimens. The results of western blotting (Fig. 1C and D) and RT-qPCR (Fig. 1E) analyses demonstrated that the
protein and mRNA expression levels of Dicer and Drosha were
upregulated in GCTB specimens. The association between miR-210
expression and clinicopathological parameters was statistically
evaluated, and a significant difference in miR-210 level was
detected amongst patients with varying Jaffe grades, as well as
patients with or without recurrence (P=0.031 and 0.004,
respectively). There was no significant association detected
between miR-210 expression and the other clinicopathological
parameters evaluated.
 | Table ICorrelation between miR-210 and
HIF-1α expression levels and clinicopathological parameters in
giant cell tumor of bone specimens. |
Table I
Correlation between miR-210 and
HIF-1α expression levels and clinicopathological parameters in
giant cell tumor of bone specimens.
| Clinicopathological
parameter | Cases (n) | miR-210 levels
| HIF-1α mRNA levels
|
|---|
| Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| Gender | | | 0.365 | | 0.640 |
| Male | 20 | 2.985 ± 0.547 | | 3.136 ± 0.703 | |
| Female | 22 | 3.214 ± 0.623 | | 3.076 ± 0.672 | |
| Age (years) | | | 0.134 | | 0.093 |
| ≤20 | 12 | 3.327 ± 0.606 | | 3.323 ± 0.574 | |
| 21–40 | 23 | 2.926 ± 0.502 | | 3.042 ± 0.620 | |
| >41 | 7 | 3.312 ± 0.622 | | 2.937 ± 0.663 | |
| Jaffe grade | | | 0.031a | | 0.022a |
| I | 14 | 2.468 ± 0.381 | | 2.994 ± 0.525 | |
| II | 23 | 3.336 ± 0.533 | | 3.102 ± 0.578 | |
| III | 5 | 3.826 ± 0.420 | | 3.431 ± 0.626 | |
| Recurrence | | | 0.004b | | 0.009b |
| No | 33 | 2.915 ± 0.436 | | 2.968 ± 0.564 | |
| Yes | 9 | 3.802 ± 0.562 | | 3.608 ± 0.731 | |
HIF-1α is upregulated in GCTB specimens
and positively correlated with miR-210 overexpression
miR-210 has previously been determined to be induced
by hypoxia via HIF-1α (50,51).
To evaluate the hypoxic regulation of miR-210 in GCTB specimens,
HIF-1α mRNA expression in the GCTB and osteochondroma specimens was
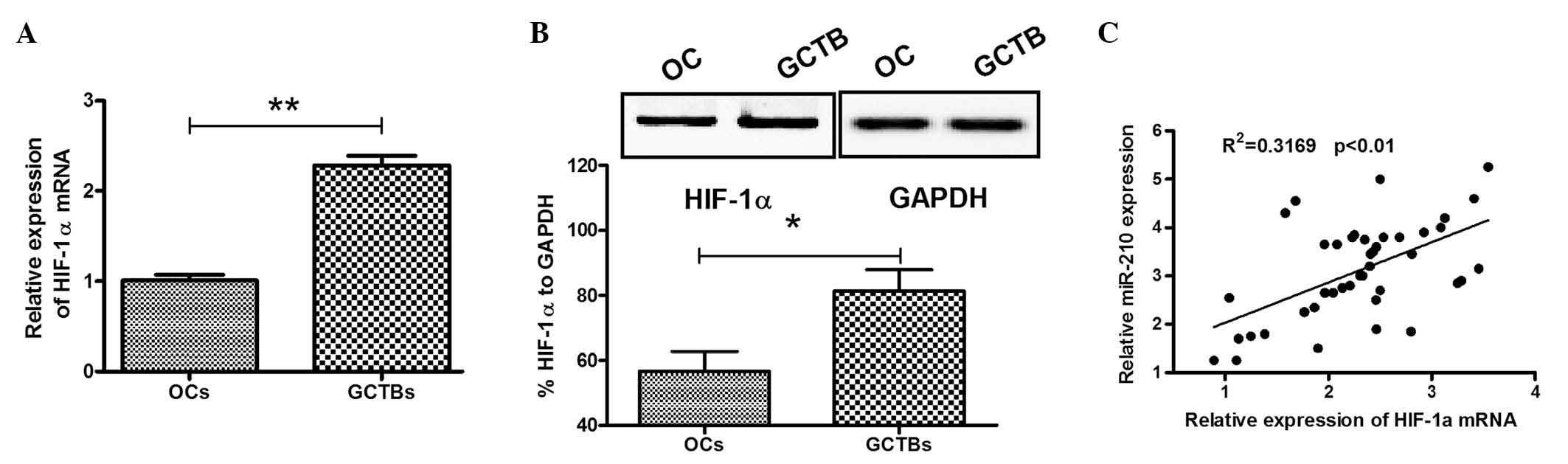
determined by RT-qPCR. As shown in Fig. 2A, the relative HIF-1α mRNA
expression was 2.286±0.677 in the GCTB specimens, significantly
higher than 1.010±0.194 in the osteochondroma specimens
(P<0.01). HIF-1α overexpression was also evaluated in the
aforementioned specimens by western blotting, and the HIF-1α
protein expression relative to GAPDH was 81.333±11.590%, also
significantly higher than 56.667±10.599% in the osteochondroma
specimens (P<0.05; Fig. 2B). To
further examine the correlation between miR-210 overexpression and
HIF-1α upregulation, Spearman's rank analysis was conducted on the
miR-210 and HIF-1α mRNA expression levels in each GCTB specimen. It
was revealed that the HIF-1α mRNA expression was positively
correlated with miR-210 expression in GCTB specimens.
(R2=0.3169, P<0.01; Fig.
2C). Statistical evaluation also revealed an association
between HIF-1α overexpression and Jaffe grade and recurrence. There
were higher HIF-1α mRNA levels in patients with higher Jaffe grade
or recurrence (P=0.022 and 0.009, respectively). No association was
detected between HIF-1α mRNA expression and any other
clinicopathological parameter evaluated.
miR-210 and HIF-1α are upregulated in
primary GCTB stromal cells under hypoxia
To further confirm the correlation between miR-210
and HIF-1α expression and hypoxia in GCTBs, primary stromal cells
were isolated from GCTB tissues and the miR-210 and HIF-1α
expression levels were examined under hypoxia in the primary
stromal and U2 OS human osteosarcoma cells. The miRNA and mRNA
samples from the two types of cell under normoxia or hypoxia, were
analyzed using RT-qPCR. In agreement with the results of the
analysis of the clinical specimens, there was a significant
induction of miR-210 expression under hypoxia in the primary
stromal and U2 OS cells. The miR-210 expression began increasing
from 4 h post hypoxia treatment, reaching the highest levels at 12
h post treatment (P<0.05 or P<0.01; Fig. 3A and B). Analogous results were
observed in the HIF-1α expression levels in primary stromal and U2
OS cells. Under hypoxic conditions, HIF-1α expression was
significantly upregulated in primary stromal cells from 8–24 h post
treatment, and in U2 OS cells from 4–24 h post treatment (P<0.05
or P<0.01; Fig. 3C and D).
Western blot analysis was utilized to evaluate the upregulation of
HIF-1α protein expression in the primary stromal cells under
hypoxia. As shown in Fig. 3E, from
8 to 48 h post hypoxia treatment, HIF-1α was significantly
upregulated (P<0.05 or P<0.01). Taken together, these in
vitro results confirmed the upregulation in miR-210 and HIF-1α
expression under hypoxia.
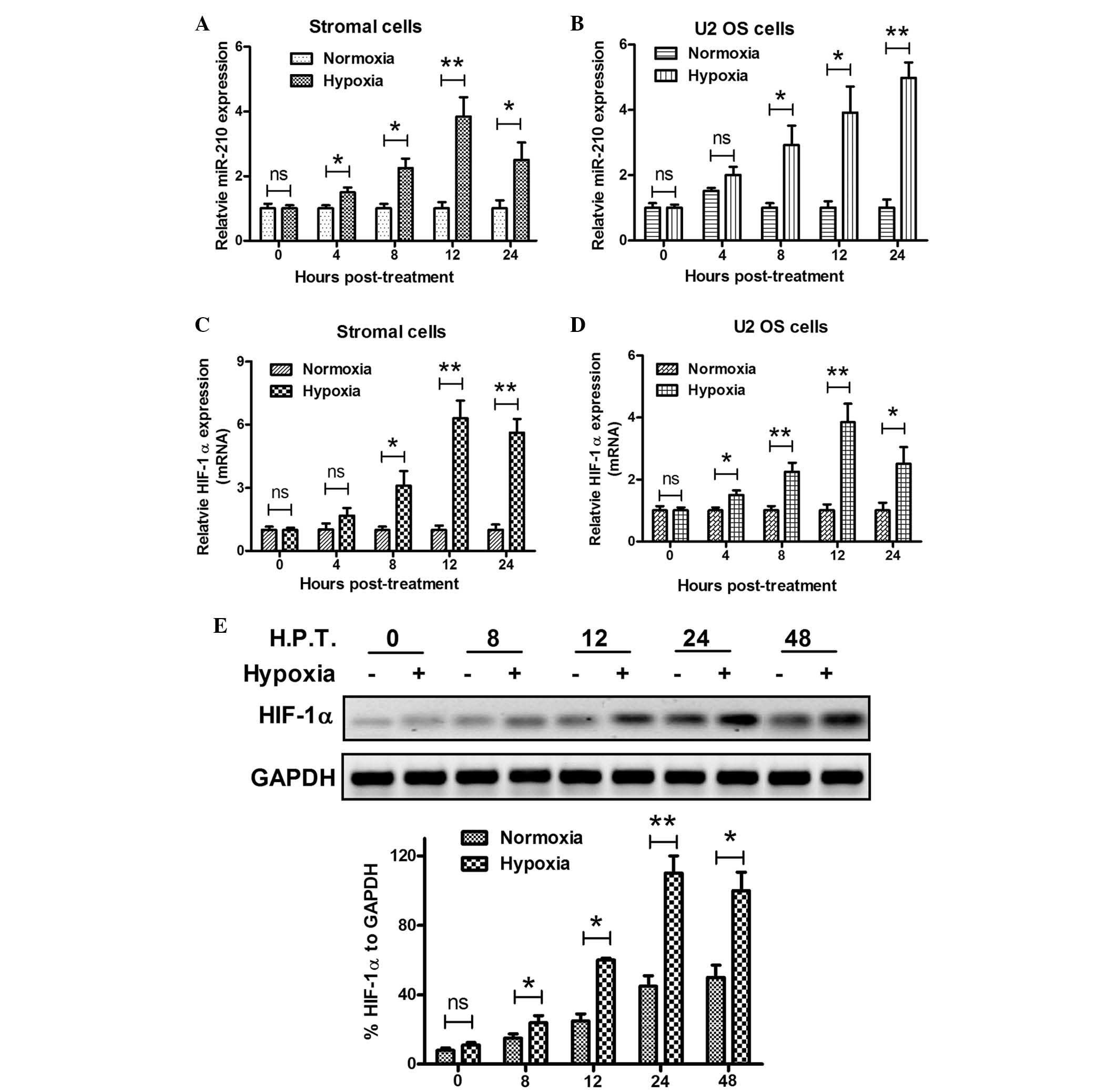 | Figure 3Overexpression of miR-210 and HIF-1α
in primary stromal cells under hypoxia. (A and B) Hypoxia
upregulated miR-210 expression in primary stromal and U2 OS human
osteosarcoma cells, as determined by RT-qPCR. (C and D) Hypoxia
upregulated HIF-1α mRNA expression in primary stromal and U2 OS
cells, as determined by RT-qPCR. (E) Hypoxia upregulated HIF-1α
protein expression levels in primary stromal cells relative to
GAPDH, as determined by western blot analysis. All experiments were
performed in triplicate. Values are expressed as the mean ±
standard error; *P<0.05, **P<0.01.
GCTB, giant cell tumor of bone; OS, osteosarcoma; miR-210,
microRNA-210; mRNA, messenger RNA; HIF-1α, hypoxia-inducible
factor-1α; H.P.T, hours post-treatment. |
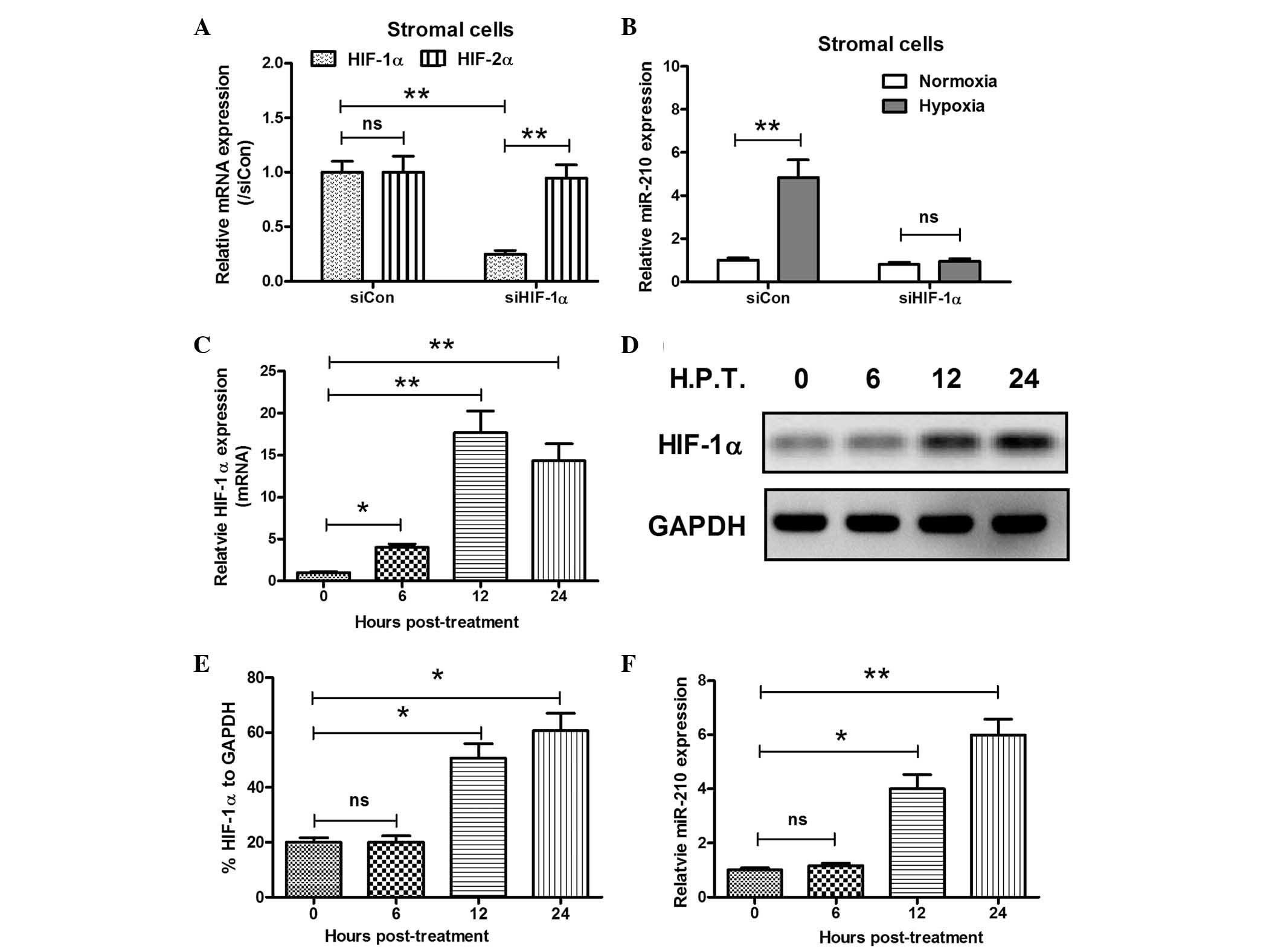
Promotion of miR-210 expression in
primary GCTB stromal cells by hypoxia is HIF-1α-dependent
In order to explore the correlation between miR-210
expression and the production of HIF-1α, the regulatory effect of
HIF-1α on miR-210 overexpression was determined in primary stromal
cells. HIF-1α-specific siRNA was used to block HIF-1α expression
and, as shown in Fig. 4A, siHIF-1α
specifically inhibited HIF-1α expression, without altering HIF-2α
expression (P<0.01). Subsequently, miR-210 levels in stromal
cells under hypoxic conditions, with or without HIF-1α blockage by
siHIF-1α were evaluated. Hypoxia promoted miR-210 expression levels
to ~5-fold greater than those under normoxia in primary stromal
cells transfected with control siRNA (P<0.01), while this
promotion was blocked by siHIF-1α (no significant difference
compared with the normoxia group; Fig.
4B). To verify that the promotion of miR-210 expression by
hypoxia was HIF-1α-dependent, a HIF-1α-pcDNA3.1 recombinant plasmid
was constructed and transfected into the primary stromal cells.
HIF-1α expression was significantly upregulated following
recombinant plasmid transfection at the mRNA (P<0.05 or
P<0.01; Fig. 4C) and protein
levels (P<0.05 or P<0.01; Fig.
4D and E). Furthermore, the high expression of HIF-1α induced
by plasmid transfection significantly enhanced miR-210 expression
in the stromal cells, 12 and 24 h post recombinant plasmid
transfection (P<0.05 or P<0.01; Fig. 4F).
Discussion
The oncogenic or tumor suppressive roles of miRNAs
have previously been identified in primary malignant bone tumors,
particularly in osteosarcomas (38–43)
and Ewing sarcoma cancers (52,53),
but not in primary benign bone tumors, including osteomas,
osteochondromas and giant cell tumors. Zuntini et al
(54) initially identified the
deregulated miRNA profiling in multiple osteochondromas, indicating
disease-specific miRNA expression in cartilage. A significantly
decreased level of miR-136 in metastatic versus non-metastatic
GCTBs was also confirmed by RT-qPCR analysis (55). Whether other miRNAs were involved
in the GCTBs had remained to be elucidated. In the present study,
it was revealed that miR-210 was overexpressed in GCTB specimens,
and that this miR-210 overexpression was associated with HIF-1α
overexpression in clinical GCTB specimens, as well as primary GCTB
stromal cells. Furthermore, the upregulated miR-210 expression in
GCTB specimens was correlated with the tumor Jaffe grade and
recurrence. Thus, a significant deregulation of miR-210 expression
was confirmed in the GCTB stromal cells and specimens, and the
miR-210 expression levels may function as a clinical marker for the
degree of tumor growth in vivo.
The deregulated growth of solid tumors always
involves a hypoxic microenvironment, which drives tumor cells to
undergo genetic and phenotypic adaptations that allow them to
survive and sustain this deregulated growth under hypoxic
conditions. The present study provided evidence indicating that
miR-210 was involved in a crucial hypoxia-response network in
GCTBs. In order to aid the elucidation of the regulatory networks
involved in mediating the hypoxia response, the potential
regulators of miR-210 expression were determined, Drosha and Dicer,
which are also upregulated under hypoxia. The in vitro
results in GCTB stromal cells indicated that HIF-1α inhibition by
siRNA blocked the hypoxia-induced upregulation of miR-210 in
primary GCTB stromal cells, while the manipulated overexpression of
HIF-1α significantly enhanced miR-210 expression under normoxia.
Therefore, these results revealed that the upregulation of miR-210
induced by hypoxia was HIF-1α-dependent.
Oxygen-dependent regulation of HIF-1α is dependent
on a family of prolyl hydroxylases (PHDs) that hydroxylate HIF-1α
protein at two prolines under normoxic conditions, resulting in the
degradation of HIF-1α (56).
Conversely, the expression of PHDs is selectively controlled by
HIF-1 and -2 proteins (57). There
is a positive feedback loop between the PHDs and HIF-1α (58,59)
under hypoxic conditions. During hypoxia, these prolines are not
hydroxylated and therefore HIF-1α degradation is blocked. It has
previously been demonstrated that miR-210 targets
glycerol-3-phosphate dehydrogenase 1-like, resulting in the
stabilization of HIF-1α (59).
Furthermore, in certain types of cell it has been demonstrated that
hypoxia-induced miR-210 is an HIF-1α target (60), and therefore a positive feedback
loop between miR-210 and HIF-1α has been identified. However, to
the best of our knowledge there has been no previous study that has
identified the deregulation of miR-210 and HIF-1α expression in
GCTBs, let alone a positive feedback loop between them. The present
study demonstrated that the hypoxia-induced miR-210 overexpression
in primary GCTB stromal cells was induced by HIF-1α. Whether
miR-210 in turn upregulates HIF-1α under hypoxic conditions in
GCTBs remains to be elucidated and requires further
investigation.
In conclusion, the overexpression of miR-210 and
HIF-1α was identified in GCTB specimens and primary GCTB stromal
cells, and a correlation between the two was observed. The in
vitro experiments also indicated that the hypoxia-induced
miR-210 upregulation was mediated by HIF-1α. The identified
overexpression of miR-210 and HIF-1α in GCTB specimens in the
present study suggests an adaptive response to hypoxia in GCTB,
thus implying that hypoxia-associated molecules may be an effective
target for treatment of GCTB.
References
|
1
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.PubMed/NCBI
|
|
2
|
Anract P, De Pinieux G, Cottias P,
Pouillart P, Forest M and Tomeno B: Malignant giant-cell tumours of
bone. Clinico-pathological types and prognosis: a review of 29
cases. Int Orthop. 22:19–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gamberi G, Serra M, Ragazzini P, et al:
Identification of markers of possible prognostic value in 57 giant
cell tumors of bone. Oncol Rep. 10:351–356. 2003.PubMed/NCBI
|
|
4
|
Miszczyk L, Wydmanski J and Spindel J:
Efficacy of radiotherapy for giant cell tumor of bone: given either
postoperatively or as sole treatment. Int J Radiat Oncol Biol Phys.
49:1239–1242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olivera P, Perez E, Ortega A, et al:
Estrogen receptor expression in giant cell tumors of the bone. Hum
Pathol. 33:165–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melillo G: Inhibiting hypoxia-inducible
factor 1 for cancer therapy. Mol Cancer Res. 4:601–605. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin SC, Liao WL, Lee JC and Tsai SJ:
Hypoxia-regulated gene network in drug resistance and cancer
progression. Exp Biol Med (Maywood). 239:779–792. 2014. View Article : Google Scholar
|
|
8
|
Cummins EP and Taylor CT:
Hypoxia-responsive transcription factors. Pflugers Arch.
450:363–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Licausi F, Weits DA, Pant BD, Scheible WR,
Geigenberger P and van Dongen JT: Hypoxia responsive gene
expression is mediated by various subsets of transcription factors
and miRNAs that are determined by the actual oxygen availability.
New Phytol. 190:442–456. 2011. View Article : Google Scholar
|
|
10
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
13
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and HIF-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.
|
|
16
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
17
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pralhad T, Madhusudan S and Rajendrakumar
K: Concept, mechanisms and therapeutics of angiogenesis in cancer
and other diseases. J Pharm Pharmacol. 55:1045–1053. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor RM, Kashima TG, Knowles HJ and
Athanasou NA: VEGF, FLT3 ligand, PlGF and HGF can substitute for
M-CSF to induce human osteoclast formation: implications for giant
cell tumour pathobiology. Lab Invest. 92:1398–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knowles HJ and Athanasou NA:
Hypoxia-inducible factor is expressed in giant cell tumour of bone
and mediates paracrine effects of hypoxia on monocyte-osteoclast
differentiation via induction of VEGF. J Pathol. 215:56–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wülling M, Delling G and Kaiser E: The
origin of the neoplastic stromal cell in giant cell tumor of bone.
Hum Pathol. 34:983–993. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morgan T, Atkins GJ, Trivett MK, et al:
Molecular profiling of giant cell tumor of bone and the
osteoclastic localization of ligand for receptor activator of
nuclear factor kappaB. Am J Pathol. 167:117–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skubitz KM, Cheng EY, Clohisy DR, Thompson
RC and Skubitz AP: Gene expression in giant-cell tumors. J Lab Clin
Med. 144:193–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atkins GJ, Haynes DR, Graves SE, et al:
Expression of osteoclast differentiation signals by stromal
elements of giant cell tumors. J Bone Miner Res. 15:640–649. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cowan RW, Singh G and Ghert M: PTHrP
increases RANKL expression by stromal cells from giant cell tumor
of bone. J Orthop Res. 30:877–884. 2012. View Article : Google Scholar
|
|
27
|
Xu M, Song ZG, Xu CX, et al: IL-17A
stimulates the progression of giant cell tumors of bone. Clin
Cancer Res. 19:4697–4705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao VH, Singh RK, Delimont DC, et al:
Transcriptional regulation of MMP-9 expression in stromal cells of
human giant cell tumor of bone by tumor necrosis factor-alpha. Int
J Oncol. 14:291–300. 1999.PubMed/NCBI
|
|
29
|
Kumta SM, Huang L, Cheng YY, Chow LT, Lee
KM and Zheng MH: Expression of VEGF and MMP-9 in giant cell tumor
of bone and other osteolytic lesions. Life Sci. 73:1427–1436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kudo N, Ogose A, Ariizumi T, et al:
Expression of bone morphogenetic proteins in giant cell tumor of
bone. Anticancer Res. 29:2219–2225. 2009.PubMed/NCBI
|
|
31
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao L, Chen X and Cao Y: New role of
microRNA: carcinogenesis and clinical application in cancer. Acta
Biochim Biophys Sin (Shanghai). 43:831–839. 2011. View Article : Google Scholar
|
|
38
|
Fan L, Wu Q, Xing X, Wei Y and Shao Z:
MicroRNA-145 targets vascular endothelial growth factor and
inhibits invasion and metastasis of osteosarcoma cells. Acta
Biochim Biophys Sin (Shanghai). 44:407–414. 2012. View Article : Google Scholar
|
|
39
|
Duan Z, Choy E, Harmon D, et al:
MicroRNA-199a-3p is down-regulated in human osteosarcoma and
regulates cell proliferation and migration. Mol Cancer Ther.
10:1337–1345
|
|
40
|
Pan Z, Sun X, Shan H, et al: MicroRNA-101
inhibited postinfarct cardiac fibrosis and improved left
ventricular compliance via the FBJ osteosarcoma
oncogene/transforming growth factor-beta1 pathway. Circulation.
126:840–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan J, Chen L, Chen X, Sun W and Zhou X:
Identification of serum microRNA-21 as a biomarker for
chemosensitivity and prognosis in human osteosarcoma. J Int Med
Res. 40:2090–2097. 2012. View Article : Google Scholar
|
|
42
|
van der Deen M, Taipaleenmaki H, Zhang Y,
et al: MicroRNA-34c inversely couples the biological functions of
the runt-related transcription factor RUNX2 and the tumor
suppressor p53 in osteosarcoma. J Biol Chem. 288:21307–21319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mao JH, Zhou RP, Peng AF, et al:
microRNA-195 suppresses osteosarcoma cell invasion and migration in
vitro by targeting FASN. Oncol Lett. 4:1125–1129. 2012.PubMed/NCBI
|
|
44
|
Fasanaro P, Greco S, Lorenzi M, et al: An
integrated approach for experimental target identification of
hypoxia-induced miR-210. J Biol Chem. 284:35134–35143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mizuno Y, Tokuzawa Y, Ninomiya Y, et al:
miR-210 promotes osteoblastic differentiation through inhibition of
AcvR1b. Febs Lett. 583:2263–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ramakrishnan A, Torok-Storb B and Pillai
MM: Primary marrow-derived stromal cells: isolation and
manipulation. Methods Mol Biol. 1035:75–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang XH, Zhao FJ, Han BM, et al: Primary
stromal cells isolated from human various histological/pathological
prostate have different phenotypes and tumor promotion role. Chin
Med J (Engl). 124:1700–1707. 2011.
|
|
48
|
Zhao JH, Luo Y, Jiang YG, He DL and Wu CT:
Knockdown of β-Catenin through shRNA cause a reversal of EMT and
metastatic phenotypes induced by HIF-1α. Cancer Invest. 29:377–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
50
|
Mutharasan RK, Nagpal V, Ichikawa Y and
Ardehali H: microRNA-210 is upregulated in hypoxic cardiomyocytes
through Akt- and p53-dependent pathways and exerts cytoprotective
effects. Am J Physiol Heart Circ Physiol. 301:H1519–H1530. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Biswas S, Roy S, Banerjee J, et al:
Hypoxia inducible microRNA 210 attenuates keratinocyte
proliferation and impairs closure in a murine model of ischemic
wounds. Proc Natl Acad Sci USA. 107:6976–6981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Riggi N, Suvà ML, De Vito C, et al:
EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate
mesenchymal stem cell reprogramming toward Ewing sarcoma cancer
stem cells. Genes Dev. 24:916–932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ban J, Jug G, Mestdagh P, et al:
Hsa-mir-145 is the top EWS-FLI1-repressed microRNA involved in a
positive feedback loop in Ewing's sarcoma. Oncogene. 30:2173–2180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zuntini M, Salvatore M, Pedrini E, et al:
MicroRNA profiling of multiple osteochondromas: identification of
disease-specific and normal cartilage signatures. Clin Genet.
78:507–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mosakhani N, Pazzaglia L, Benassi MS, et
al: MicroRNA expression profiles in metastatic and non-metastatic
giant cell tumor of bone. Histol Histopathol. 28:671–678. 2013.
|
|
56
|
Vogel S, Wottawa M, Farhat K, et al:
Prolyl hydroxylase domain (PHD) 2 affects cell migration and
F-actin formation via RhoA/rho-associated kinase-dependent cofilin
phosphorylation. J Biol Chem. 285:33756–33763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fujita N, Markova D, Anderson DG, et al:
Expression of prolyl hydroxylases (PHDs) is selectively controlled
by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the
intervertebral disc: distinct roles of PHD2 and PHD3 proteins in
controlling HIF-1alpha activity in hypoxia. J Biol Chem.
287:16975–16986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Demidenko ZN and Blagosklonny MV: The
purpose of the HIF-1/PHD feedback loop: to limit mTOR-induced
HIF-1alpha. Cell Cycle. 10:1557–1562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kelly TJ, Souza AL, Clish CB and
Puigserver P: A hypoxia-induced positive feedback loop promotes
hypoxia-inducible factor 1alpha stability through miR-210
suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell
Biol. 31:2696–2706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Krock BL, Skuli N and Simon MC:
Hypoxia-induced angio-genesis: good and evil. Genes Cancer.
2:1117–1133. 2011. View Article : Google Scholar
|