Introduction
Glioblastoma is the most aggressive type of
malignant brain tumor (1).
Therapeutic outcomes have improved with the development of
comprehensive treatments, including surgery, radiation and
chemotherapy; however, patients with glioblastoma grade IV have a
poor long-term prognosis, with an overall survival rate of 1 year
(2,3). Recent studies have extensively
investigated the cellular and genetic mechanisms underlying
glioblastoma progression (4,5).
Although the molecular mechanisms underlying glioma radioresistance
have yet to be elucidated, they are thought to involve dysregulated
gene expression (6).
MicroRNAs (miRNAs) are 19–23 nucleotide RNA
molecules, which bind the 3′-untranslated region (3′-UTR) of genes,
and regulate gene expression by inhibiting protein translation
(7). A previous study demonstrated
that miRNAs exert post-transcriptional gene silencing effects
(8). In addition, miRNAs mediate
the degradation of RNA transcripts (9). Due to the important role of miRNAs in
the development, invasion, and metastasis of malignant tumors,
previous studies have focused on the role of miRNAs in tumors
(10,11). Numerous miRNAs are overexpressed in
malignant tumors, as compared with normal tissues (12), and the dysregulation of miRNAs may
result in increased tumorigenesis (13).
miRNAs have a significant role in glioma. miRNA-21
has been shown to be overexpressed in glioblastoma, and following
the suppression of miRNA-21 expression, glioblastoma growth was
inhibited (14). miRNA-184
regulates cell cycle-associated proteins, thereby regulating the
proliferation of glioma cells (15). Furthermore, miRNA-23a is
upregulated in glioma and promotes the invasion of glioma cells
(16).
Our previous study investigated the role of BMI1
polycomb ring finger oncogene (Bmi-1) in the response of U-87 MG
cells to radiation exposure (17).
The results indicated that miRNA-128a was able to bind to the
3′-UTR of Bmi-1 in order to suppress its expression. miRNA-128a is
a brain-specific miRNA, which is significantly downregulated in
glioma (18). It has previously
been demonstrated that, as compared with normal adjacent brain
tissue, the expression levels of miRNA-128a in glioblastoma tissue
are decreased. The role of miRNA-128a may be associated with the
proliferation and self-renewal of glioma stem cells (19). Although the role of miRNA-128a in
glioma is well understood, its association with radioresistance in
glioblastoma has yet to be elucidated. The present study aimed to
investigate the role of miRNA-128a in the growth inhibition of U-87
MG cells exposed to X-ray radiation. The present study hypothesized
that U-87 MG cell radioresistance results from suppression of the
Bmi-1 oncogene by miRNA-128a.
Materials and methods
Cell culture
The U-87 MG glioblastoma cell line was obtained from
the Cell Bank of Type Culture Collection of Chinese Academy
Sciences (Shanghai, China). The U-87 MG cells were cultured in
Minimum Essential Medium (MEM; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences) according to the manufacturer's
instructions, and incubated in a humidified atmosphere containing
5% CO2 at 37°C.
Radiation
The U-87 MG cells were exposed to X-ray radiation
using a linear accelerator source (Elekta Synergy®
Platform; Elekta, Stockholm, Sweden) at a dose rate of 300 Gy/min.
Prior to radiation, a radiation plan was designed, and the accuracy
of the X-ray radiation doses were verified by a radiation therapy
physicist using I'mRT MatriXX 2D-ion chamber array (IBA Dosimetry,
Schwarzenbruck, Germany).
To investigate the effect of radiation, the cells
were exposed on day 0 to various doses of X-ray radiation,
including 0 Gy (control group) and 1, 2, 4, 6 and 8 Gy. All groups
of cells were continuously incubated following radiation until the
experiments were finished.
Following equivalent doses of radiation, different
modes of fractionation may have different effects on tumor cells.
To determine the effects of different modes of fractionation, three
radiation groups with equivalent radiation doses were assessed in
the present study, and compared with the control group, which was
administered a 0 Gy dose: The conventional fractionation group was
exposed to a 2 Gy X-ray dose daily from day 0 to day 4; the
hypofractionation group was exposed to an 8 Gy X-ray dose on day 0;
the hyperfractionation group was exposed at 1.1 Gy X-ray dose twice
a day from day 0 to day 4, and the interval between the two
treatments was >6 h.
Cell growth curve and inhibition
ratio
The U-87 MG glioblastoma cells were harvested during
the logarithmic growth phase and seeded into 24-well dishes at a
density of 5×104/well in a final volume of 500
µl/well. The cells were subsequently exposed to various
doses or fractions of X-ray radiation. Every 24 h, the number of
cells in three random wells was counted using a cell counter
(Inno-Alliance Biotech, Inc., Wilmington, NC, USA), and the mean
number of cells were calculated for each time point. A cell growth
curve was generated with cell number on the x-axis and the various
time points on the y-axis. The inhibition ratio was calculated from
the number of radiation group cells divided by the number of
control group cells. The results were presented as the mean ±
standard error of three independent experiments.
Cell cycle analysis
To detect the cell cycle phase of the cells
following 24 h exposure to X-ray radiation, the U-87 MG cells were
fixed with 70% ethanol for 30 min. Each sample of the U-87 MG cells
was re-suspended in 1 ml phosphate-buffered saline (PBS)
supplemented with 1% FBS, and treated with 500 µl PBS
containing with 100 µg/ml ribonuclease (Beyotime Institute
of Biotechnology, Haimen, China) and 50 µg/ml propidium
iodide (Sigma-Aldrich, St. Louis, MO, USA). The samples were
subsequently analyzed by flow cytometry (FCM) using the LSR
Fortessa Cell Analyzer (BD Biosciences, Franklin Lakes, NJ, USA). A
cell cycle profile analysis of the DNA histograms of integrated red
fluorescence was performed using Modfit I 2.0 for Windows (Verity
Software House, Inc., Topsham, ME, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated using the Ultrapure RNA kit
(CwBio, Inc., Beijing, China). RNA quality was assessed by 1%
agarose gel electrophoresis (AGE). The RNA was reverse transcribed
using the HiFi-MMLV cDNA kit (CwBio-Electron, Inc.). The cDNA was
subsequently amplified using UltraSYBR Mixture (with ROX; CwBio,
Inc.). β-actin (ACTB) mRNA expression levels were used as an
internal control to normalize the data. The primers were purchased
from CwBio, Inc. (cat. no. CW0900). The Bmi-1 primers used in the
present study were as follows: Bmi-1, forward
5′-TCCACCTCTTCTTGTTTGCCT-3′, and reverse
5′-GAAGAAGTTGCTGATGACCCA-3′. All methods were conducted according
to the manufacturer's instructions.
For miRNA-128a analysis, total RNA was isolated
using the miRNApure Mini kit (CwBio, Inc.). RNA quality was
assessed by AGE. The RNA was reverse transcribed using a miRNA cDNA
kit (CwBio, Inc.). The cDNA was then amplified using a Real-Time
PCR Assay kit (CwBio, Inc.). Small nuclear RNA U6 expression levels
were used as an internal control to normalize the data. The primers
were synthesized by CwBio, Inc. The sequences of the primers used
were as follows: U6, forward 5′-GCTTCGGCAGCACATATACTAAAAT-3′; and
miRNA-128a, forward 5′-CACAGTGAACCGGTCTCTTT-3′.
The mRNA and miRNA expression levels were measured
by RT-qPCR using an LC-480II thermal cycler (Roche Diagnostics,
Basel, Switzerland). The cycling conditions were as follows: 95°C
for 10 min, followed by 40 cycles of amplification at 95°C for 15
sec and 60°C for 60 sec. The relative miRNA and mRNA expression
levels were calculated using the ΔΔCT method (20).
Analysis of the levels of intracellular
reactive oxygen species (ROS)
The fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich)
is able to pass through the cytomembrane, and is cleaved by
nonspecific intracellular esterases in order to form
2′,7′-dichlorodihydrofluorescein. DCFH-DA is also oxidized by ROS
to form highly fluorescent 2′,7′-dichlorofluorescein (DCF). The
fluorescence intensity of DCF is therefore proportional to the
levels of ROS in the cells. The U-87 MG glioma cells were exposed
to various doses of X-ray radiation. After 6 h, the cells were
digested by 0.25% trypsin (Sigma-Aldrich) and harvested. The cells
were washed twice with PBS, and incubated with 10 µM DCFH-DA
for 20 min in the dark at 37°C. During incubation, the cells were
gently agitated every 3–5 min. The cells were then washed three
times with MEM in the absence of FBS, prior to being resuspended in
1 ml PBS. The levels of ROS were assessed by FCM using an LSR
Fortessa cell analyzer (BD Biosciences) at an excitation wavelength
of 488 nm and emission wavelength of 530 nm. The DCF fluorescence
intensity data were evaluated by CellQuest-Pro 5.1™ (BD
Biosciences) and presented as the mean fluorescence intensity.
NAC treatment
The U87-MG cells were divided into control group, 8
Gy group and 8 Gy group with NAC treatment. Prior to 1 h exposure
to an 8 Gy dose of X-ray radiation, the culture medium in the
groups subjected to NAC treatment was replaced with the MEM
supplemented with 10% FBS and 1 mM NAC. The culture medium in the
control group and 8 Gy group were changed with MEM with 10% FBS
(without NAC). All groups were incubated until being exposed to
radiation, Following a 12 h exposure to radiation, the medium in
all groups was replaced by MEM supplemented with 10% FBS.
Statistical analysis
Each experiment was conducted in triplicate. The
results were presented as the mean ± standard error. Student's
t-test (unpaired) was used to evaluate the statistically
significant differences. Statistical analysis was performed using
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Growth of U-87 MG glioma cells following
X-ray exposure
In our previous study, the number of U-87 MG glioma
cells did not decrease following exposure to various doses of X-ray
radiation (17). To investigate
whether various X-ray radiation fractions affected cellular growth,
the U-87 MG cells were divided into three experimental groups: A
conventional group, a hypofraction group, and a superfraction
radiation group, which were exposed to X-ray radiation at 2 Gy qd
for 5 days, 1.1 bid Gy for 5 days and 8 Gy for 1 day, respectively
(Fig. 1). Cell growth was
significantly inhibited in the hypofraction radiation group. Cell
growth of the conventional and super-fraction radiation groups was
not inhibited until day 5, and no significant difference was
observed between the two groups. Cell numbers increased in a
time-dependent manner in all radiation groups, which suggests that
the U-87 MG glioma cells exhibit radioresistance.
Inhibition ratio in U-87 MG glioma cells
following X-ray exposure
To further investigate the inhibitory effects of
X-ray radiation on the U-87 MG cells, the cellular inhibition
ratios were calculated following exposure to various radiation
fractions (Fig. 2A) and various
X-ray radiation doses (Fig. 2B). A
significant increase in cell growth was detected in both the
conventional and superfraction groups between days 1 and 4, and the
same effects were observed in the 1 and 2 Gy groups. In the
radiation groups exposed to ≥4 Gy radiation doses, the inhibitory
effects on cellular growth were significant, and occurred in a
dose-dependent manner. These results suggest that X-ray radiation
is able to suppress the growth of U-87 MG glioma cells. Our
previous study demonstrated that the mechanism underlying radiation
inhibition was cellular senescence, as opposed to apoptosis
(17).
Cell cycle distribution of U-87 MG glioma
cells following X-ray exposure
Our previous study investigated the cell cycle
distribution of U-87 MG cells following 72 h exposure to X-ray
radiation. The number of cells in G2/M phase was
significantly increased, and the number of cells in
G0/G1 phase were significantly decreased in
the 6 and 8 Gy groups (17). Cell
senescence is characterized by an irreversible cell cycle arrest in
G0/G1 phase. In our previous study, in the 6
and 8 Gy groups following 72 h exposure to radiation, the number of
senescent cells was markedly increased; however, the number of
cells in G0/G1 phase was markedly decreased
(17). These results suggest that
U-87 MG glioma cells exhibit cellular senescence evasion. To
investigate whether the evasion of senescence occurred at earlier
time points, the cell cycle distribution of the U-87 MG cells was
analyzed following a 24 h exposure to X-ray radiation (Fig. 3). The results indicate that the
proportion of cells in S phase was significantly increased in the 1
and 2 Gy groups, and significantly decreased in the 6 and 8 Gy
groups (P<0.05). The proportion of cells in G2/M
phase was significantly decreased in all radiation groups. The
proportion of cells in G0/G1 phase increased
in a dose-dependent manner. These results suggest that evasion of
senescence may not occur following 24 h exposure to X-ray
radiation.
mRNA expression levels of Bmi-1 in U-87
MG glioma cells following exposure to X-ray radiation
Our previous study determined that the protein
expression levels of Bmi-1 were significantly increased in the 6
and 8 Gy groups following 72 h exposure to X-ray radiation
(17), which suggested that an
increase in Bmi-1 expression may underlie evasion of senescence. To
investigate whether the expression of Bmi-1 was affected by X-ray
radiation, the mRNA expression levels of Bmi-1 were detected in the
U-87 MG cells following 72 h exposure to X-ray radiation by RT-qPCR
(Fig. 4A). The mRNA expression
levels of Bmi-1 were significantly decreased in the 1 and 2 Gy
groups, and significantly increased in the 6 and 8 Gy groups. No
significant difference was detected in the mRNA expression levels
of Bmi-1 in the 4 Gy group.
miRNA-128a expression in U-87 MG glioma
cells following exposure to X-ray radiation
To investigate the hypothesis that Bmi-1 expression
is regulated by miRNA-128a, the expression levels of miRNA-128a
were detected in the U-87 MG glioma cells by RT-qPCR, following 72
h exposure to X-ray radiation (Fig.
4B). The expression levels of miRNA-128a were significantly
decreased in the 8Gy group, and increased in the 1 and 2 Gy groups.
No significant difference was observed in the expression levels of
miRNA-128a in the 4 and 6 Gy groups. These results indicate that
the expression levels of miRNA-128a are negatively correlated with
those of Bmi-1.
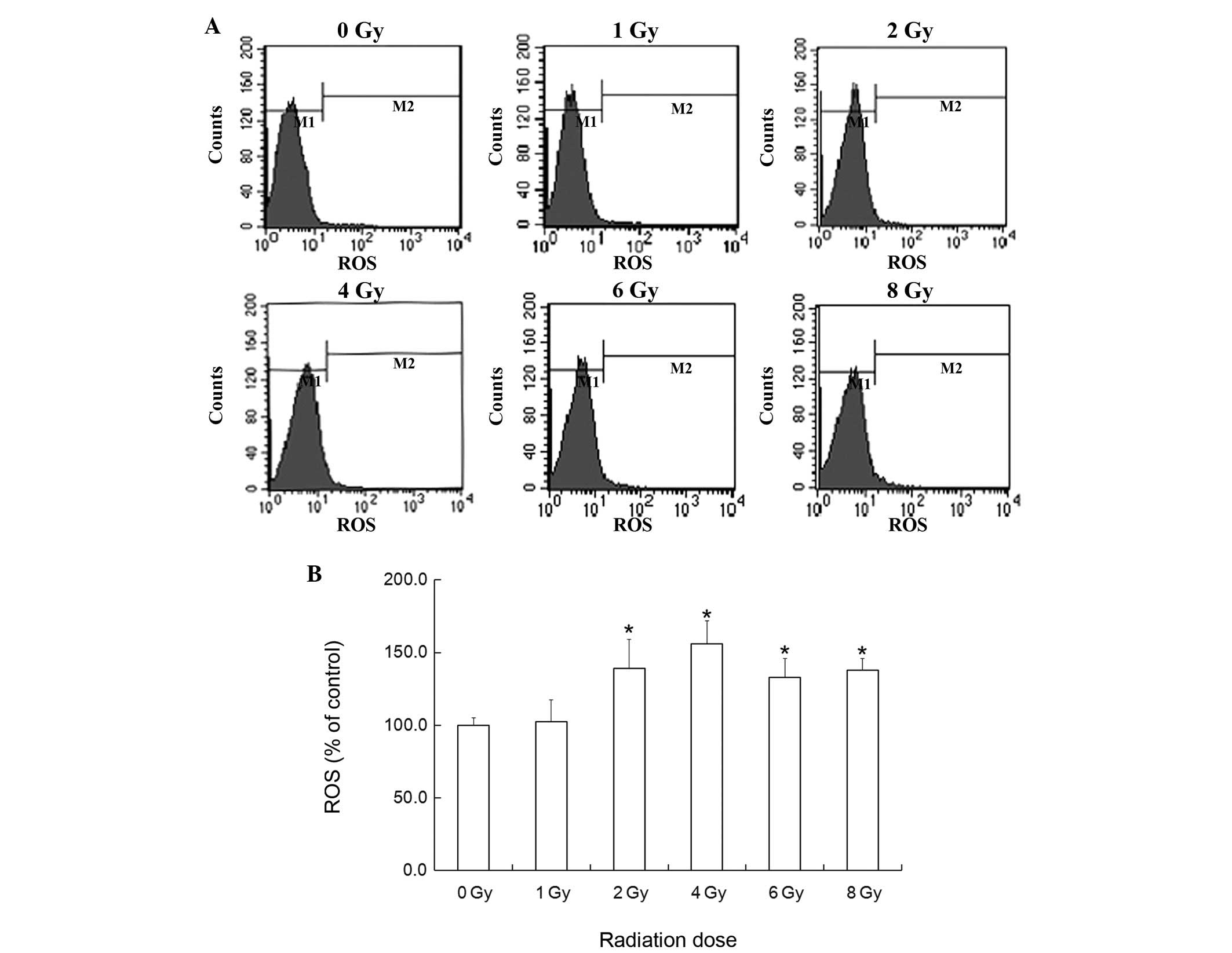
Effects of ROS on U-87 MG glioma cells
following exposure to X-ray radiation
The intracellular levels of ROS in U-87 MG glioma
cells were detected following 6 h exposure to X-ray radiation. The
ROS levels in the 2, 4, 6 and 8 Gy groups were significantly
increased, but no significant difference was identified in the 1 Gy
group (Fig. 5).
N-acetyl cysteine (NAC) is an active oxygen
scavenger, which was used in the present study to investigate
whether the levels of ROS were associated with the anti-tumorigenic
effects of X-ray radiation. Prior to 1 h exposure to 8 Gy dose
X-ray radiation, NAC was added to the MEM at a final concentration
of 1 mM. The cell growth curve indicates that NAC is able to
promote proliferation of U-87 MG glioma cells following exposure to
X-ray radiation, and the growth curve of the NAC group was similar
to that of the control group (Fig.
6A). The inhibition ratio of the NAC + 8 Gy group was markedly
decreased, as compared with the 8 Gy group (Fig. 6B). These results suggest that ROS
has a significant role on the anti-tumorigenic effects of X-ray
radiation, and reducing the levels of ROS may result in
radioresistance.
Discussion
Glioma is the most prevalent type of primary
(21) and malignant tumor
(22) of the nervous system
worldwide. Patients with glioblastoma grade IV have the shortest
overall survival (23). Our
previous study demonstrated that the U-87 MG human glioblastoma
cell line is resistant to apoptosis and radiation (17). Evasion of cellular senescence may
be the primary mechanism underlying the radioresistance of U-87 MG
cells following exposure to X-ray radiation. In addition, Bmi-1 may
have an important role in the radioresistance of U-87 cells by
promoting senescence evasion.
Radiotherapy is an important therapeutic strategy
for the treatment of malignant tumors. Approximately 50% of
patients with malignant tumors require radiotherapy at a certain
stage of their treatment, and this proportion increases to 92% in
patients with nervous malignant tumors (24). The radioresistance of gliomas often
decreases the efficacy of radiation (3); therefore, further research into
increasing the radiosensitivity of gliomas may prove useful to
prolong the overall survival time of patients with glioma.
Following exposure to various X-ray radiation
fractions, the number of U-87 MG cells did not decrease in all
experimental groups, which indicated that the U-87 MG glioma cells
exhibited radioresistance. Analysis of inhibition ratios following
exposure to X-ray radiation demonstrated a signifi-cant inhibition
of U-87 MG glioma cell growth at an X-ray radiation dose ≥4 Gy,
indicating that the mechanism underlying cell growth inhibition was
senescence, as opposed to apoptosis. At an X-ray dose ≤2 Gy, the
growth of U-87 MG cells was markedly increased in the initial few
days, and after 5 days inhibition of growth occurred in response to
both single and constant doses of radiation. In clinical settings,
X-ray radiation doses ≤2 Gy are usually used to avoid normal brain
tissue injury. Therefore, patients with glioma, specifically
glioblastoma, may safely undergo constant radiation for five
days.
Senescence is an irreversible cell cycle arrest
characterized by anti-apoptotic gene expression (25). It is widely accepted that tumor
cells are able to evade senescence in order to maintain their
proliferative ability. Therefore, research into the stimulation of
cellular senescence in tumor cells merits further study (26). The cell cycle usually arrests
permanently at G0/G1 phase during senescence.
The results of the present study indicated that the proportion of
cells in G2/M phase decreased significantly in all
radiation groups, and the proportion of cells in
G0/G1 phase increased significantly in a
dose-dependent manner, following 24 h exposure to X-ray radiation.
These results were concordant with the changes observed in the cell
cycle during senescence. The results of the present study
determined that senescence evasion may not occur following 24 h
exposure to X-ray radiation. However, following 72 h exposure to
radiation, the proportion of cells in the
G0/G1 phase was significantly decreased in
the 6 and 8 Gy groups, whereas the protein expression levels of
Bmi-1 were significantly increased (17). The occurrence of senescence may
induce Bmi-1 expression resulting in senescence evasion.
Bmi-1 belongs to the polycomb group gene family,
which regulates cellular proliferation. Bmi-1 acts on the Ink4a-Arf
genetic locus, resulting in the downregulation of p16Ink4a and
p19Arf (p14Arf in human) (27).
The P16/retinoblastoma (Rb)/E2F signaling pathway has an important
role in tumor development, and promotes glioma progression. Bmi-1
is able to suppress p16Ink4a, thereby increasing the activity
levels of E2F1 (28). P16Ink4a is
a specific inhibitor of cyclin-dependent kinase (CDK), which
inhibits Rb via phosphorylation. CDK regulates passage from the G
phase to the S phase of the cell cycle. A previous study
demonstrated that when Rb expression is downregulated, the protein
expression levels of E2F significantly increased resulting in
abnormal cell proliferation (29).
P16Ink4a expression is an indicator of cellular senescence
(30). The P19Arf/mouse double
minute 2 homolog (MDM2)/p53 is another significant signaling
pathway responsible for tumor suppression. P19Arf is able to
stabilize p53 by suppressing MDM2, and activating p53-dependent
transcription, as a result, the cell cycle may be arrested in
G1 or G2/M phases prior to apoptosis
(31). Due to the fact that U-87
MG glioma cells did not undergo apoptosis following exposure to
X-ray radiation, Bmi-1-induced senescence evasion may act via the
P16/Rb/E2F signaling pathway, as opposed to the P19Arf/MDM2/P53
signaling pathway. Our previous study demonstrated that the
expression levels of Bmi-1 were significantly increased following
exposure to X-ray radiation in the 6 and 8 Gy groups (17), and the present study demonstrated
that the mRNA expression levels of Bmi-1 also significantly
increased, which indicated that the expression levels of Bmi-1 may
be increased at the transcription level, as opposed to the
translation level. Therefore, senescence evasion may be suppressed
via the inhibition of Bmi-1 gene transcription. However, the
mechanism underlying the upregulation of Bmi-1 expression in U-87
MG glioma cells following ≥6 Gy dose X-ray radiation remains to be
fully elucidated.
miRNA-128a is able to suppress Bmi-1 expression. To
investigate whether miRNA-128a was involved in the response of
glioma cells following exposure to X-ray radiation by targeting
Bmi-1, the expression levels of miRNA-128a were evaluated by
RT-qPCR. The results demonstrated that miRNA-128a expression was
affected by X-ray radiation. The expression levels of miRNA-128a
were significantly increased in the 1 and 2 Gy groups, and
significantly decreased in the 8 Gy group. No significant
difference was observed in the expression levels of miRNA-128a
between the 4 and 6 Gy groups, and the control group; however,
miRNA-128a expression was suppressed by X-ray radiation in a
dose-dependent manner. Thus suggesting that miRNA-128a may
participate in the regulation of Bmi-1 expression in U-87 MG cells
following exposure to X-ray radiation.
miRNA-128a was able to regulate the intracellular
levels of ROS by targeting Bmi-1, thereby promoting senescence and
inhibiting the growth of medulloblastoma cells (32). ROS are able to modulate various
cellular functions, including tumor cell biology (33). Radiation stimulates the generation
of ROS in tumor cells resulting in apoptosis, senescence, or cell
death (34). The present study
demonstrated that ROS levels in the U-87 MG cells were
significantly increased following ≥2 Gy dose X-ray radiation.
Following treatment with the ROS scavenger NAC, the growth of the
U-87 MG cells was markedly increased, and the inhibitory effects of
X-ray radiation were markedly decreased. Therefore, the decrease in
the levels of ROS may induce radioresistance in U-87MG glioma
cells. One of the possible mechanisms underlying the senescence
evasion of U-87MG glioma cells is a decrease in miRNA-128a
expression following exposure to X-ray radiation, which results in
Bmi-1 gene transcription upregulation. This would then lead to
reduced levels of intracellular ROS, resulting in senescence
evasion.
The present study demonstrated the radioresistance
of U-87 MG cells. Exposure to radiation was able to increase the
mRNA expression levels of Bmi-1 resulting in senescence evasion,
which may function via the p16/Rb/E2F signaling pathway. miRNA-128a
and its downstream gene Bmi-1 may be important for the
radioresistance of U-87 MG glioma cells. In addition, ROS may be
important for the inhibitory effects induced by X-ray radiation in
U-87 MG cells. However, the present study had the following
limitations: (i) The study investigated U-87 MG glioma cells only,
and therefore the conjectures obtained from the results may not be
significant for other types of gliomas; (ii) the mechanism
underlying the downregulated generation of ROS, which resulted in
radioresistance remains to be elucidated; (iii) no conclusive
association was observed between ROS levels and the expression
levels of Bmi-1 and miRNA-128a; and (iv) although the expression
levels of Bmi-1 and miRNA-128a were altered in the U-87 MG cells
following exposure to X-ray radiation, whether radiosensitivity is
increased if the expression levels of Bmi-1 or miRNA-128a are
downregulated remains to be determined. Further research is
required in order to elucidate these mechanisms.
Acknowledgments
The present study was supported by the Youth
Foundation in the Second Hospital of Shandong University (grant no.
2013010016), the Jinan University Institute Independent Innovation
Plan (grant no. 201401263) and the Shandong Provincial Natural
Science Foundation (grant no. R2013HL027).
References
|
1
|
Haris K, Ismail S, Idris Z, Abdullah JM
and Yusoff AA: Expression profile of genes modulated by Aloe emodin
in human U87 glioblastoma cells. Asian Pac J Cancer Prev.
15:4499–4505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JH, Jung TY, Jung S, Kim IY, Jang WY,
Moon KS and Jeong EH: Performance status during and after
radiotherapy plus concomitant and adjuvant temozolomide in elderly
patients with glioblastoma multiforme. J Clin Neurosci. 20:503–508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wick W, Weller M, van den Bent M, Sanson
M, Weiler M, von Deimling A, Plass C, Hegi M, Platten M and
Reifenberger G: MGMT testing - the challenges for biomarker-based
glioma treatment. Nat Rev Neurol. 10:372–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Errafiy R, Aguado C, Ghislat G, Esteve JM,
Gil A, Loutfi M and Knecht E: PTEN increases autophagy and inhibits
the ubiquitin-proteasome pathway in glioma cells independently of
its lipid phosphatase activity. PLoS One. 8:e833182013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim YC, Roberts TL, Day BW, Harding A,
Kozlov S, Kijas AW, Ensbey KS, Walker DG and Lavin MF: A role for
homologous recombination and abnormal cell-cycle progression in
radio-resistance of glioma-initiating cells. Mol Cancer Ther.
11:1863–1872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ketting RF: microRNA biogenesis and
function: An overview. Adv Exp Med Biol. 700:1–14. 2011. View Article : Google Scholar
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giraldez AJ, Cinalli RM, Glasner ME,
Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP and
Schier AF: MicroRNAs regulate brain morphogenesis in zebrafish.
Science. 308:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Png KJ, Halberg N, Yoshida M and Tavazoie
SF: A microRNA regulon that mediates endothelial recruitment and
metastasis by cancer cells. Nature. 481:190–194. 2012. View Article : Google Scholar
|
|
12
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar
|
|
13
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Corsten MF, Miranda R, Kasmieh R,
Krichevsky AM, Weissleder R and Shah K: MicroRNA-21 knockdown
disrupts glioma growth in vivo and displays synergistic
cytotoxicity with neural precursor cell delivered S-TRAIL in human
gliomas. Cancer Res. 67:8994–9000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui QK, Liu WD, Zhu JX, Wang YH and Wang
ZG: MicroRNA-184 promotes proliferation ability of glioma cells by
regulating FOXO3. Asian Pac J Trop Med. 7:776–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu X, Chen D, Cui Y, Li Z and Huang J:
Targeting microRNA-23a to inhibit glioma cell invasion via HOXD10.
Sci Rep. 3:34232013.PubMed/NCBI
|
|
17
|
Ye L, Wang C, Yu G, Jiang Y, Sun D, Zhang
Z, Yu X, Li X, Wei W, Liu P, et al: Bmi-1 induces radioresistance
by suppressing senescence in human U87 glioma cells. Oncol Lett.
8:2601–2606. 2014.PubMed/NCBI
|
|
18
|
Ciafrè SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Murayama S, Kawai R, Hirabuki N, Miura T,
Mitomo M, Kozuka T and Usio Y: Intra-arterial ACNU chemotherapy of
malignant glioma. Nihon Igaku Hoshasen Gakkai Zasshi. 48:144–153.
1988.In Japanese. PubMed/NCBI
|
|
22
|
Taylor LP: Diagnosis, treatment, and
prognosis of glioma: Five new things. Neurology. (18 Suppl
1)75:S28–S32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delaney G, Jacob S, Featherstone C and
Barton M: The role of radiotherapy in cancer treatment: Estimating
optimal utilization from a review of evidence-based clinical
guidelines. Cancer. 104:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitt CA: Cellular senescence and cancer
treatment. Biochim Biophys Acta. 1775:5–20. 2007.
|
|
26
|
Roninson IB: Tumor cell senescence in
cancer treatment. Cancer Res. 63:2705–2715. 2003.PubMed/NCBI
|
|
27
|
Molofsky AV, He S, Bydon M, Morrison SJ
and Pardal R: Bmi-1 promotes neural stem cell self-renewal and
neural development but not mouse growth and survival by repressing
the p16Ink4a and p19Arf senescence pathways. Genes Dev.
19:1432–1437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara E, Smith R, Parry D, Tahara H, Stone
S and Peters G: Regulation of p16CDKN2 expression and its
implications for cell immortalization and senescence. Mol Cell
Biol. 16:859–867. 1996.PubMed/NCBI
|
|
31
|
Zhang Y, Xiong Y and Yarbrough WG: ARF
promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus
deletion impairs both the Rb and p53 tumor suppression pathways.
Cell. 92:725–734. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venkataraman S, Alimova I, Fan R, Harris
P, Foreman N and Vibhakar R: MicroRNA 128a increases intracellular
ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer
cell growth by promoting senescence. PLoS One. 5:e107482010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Finkel T: Signal transduction by reactive
oxygen species in non-phagocytic cells. J Leukoc Biol. 65:337–340.
1999.PubMed/NCBI
|
|
34
|
Bauer G: Low dose radiation and
intercellular induction of apoptosis: Potential implications for
the control of oncogenesis. Int J Radiat Biol. 83:873–888. 2007.
View Article : Google Scholar : PubMed/NCBI
|