Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors in children and adults (1,2). It
occurs predominantly around regions with active bone growth and
repair. Emerging evidence suggests that OS is caused by genetic and
epigenetic changes, which interrupt osteoblast differentiation from
mesenchymal stem cells (3).
Advances in OS therapy over the past decade have improved patient
outcomes (4), and the 5-year
survival rate of patients with OS has markedly improved. However,
the outcome remains poor and the majority of patients succumbed to
pulmonary metastases (5).
MicroRNAs (miRNAs), a class of small non-coding RNA
molecules, result in translational repression or degradation, and
contribute to the inhibition of gene expression (6,7).
miRNAs exert a significant role in a wide range of physiological
and pathological processes, including tumorigenesis (8). Increasing evidence implicates miRNAs
in cancer progression, including tumor growth, differentiation,
invasion, metastasis and angiogenesis (9). It has been demonstrated that several
miRNAs are dysregulated in OS tissues or cell lines (10–15).
For instance, miR-15a and miR-16-1 induce apoptosis and cell cycle
arrest in OS by inhibiting the expression of cyclin D1 (16), while miR-20a increases the
metastatic potential of OS cells by regulating the expression of
Fas (17). The expression of
miR-21 was revealed to be significantly upregulated in OS tissues,
and miR-21 deficiency markedly reduced the invasion and migration
ability of MG-63 cells by negatively regulating RECK (10).
Previous studies have demonstrated that miR-214 can
act as a tumor suppressor or an oncogene in different cancer types,
which may be dependent on cellular context (18–24).
For example, miR-214 as a tumor suppressor may promote cell
proliferation in nasopharyngeal carcinoma (25). It was demonstrated that miR-214
regulates gastric cancer cell proliferation, migration and invasion
by targeting PTEN (26), and
suppresses the growth and invasiveness of cervical cancer cells by
targeting UDP-N-acetyl-D-galactosamine:polypeptide
N-Acetylgalactosaminyltransferase 7 (27). However, the role of miR-214 in the
progression and metastasis of OS remains to be elucidated.
Therefore, the present study investigated the expression of miR-214
and its role in OS tissues and cell lines, aiming to demonstrate
that miR-214 is a potential therapeutic target for the treatment of
OS.
Materials and methods
Cell culture and tissue samples
A total of 22 paired OS and matched normal non-tumor
tissues were obtained from the Department of Orthopedics, Tangshan
Gongren Hospital (Tangshan, China). The tissues were immediately
stored in liquid nitrogen until use. All samples were derived from
patients who had not received adjuvant treatment, including
radiotherapy or chemotherapy, prior to surgery. Written informed
consent was obtained from all patients and this study was approved
by the Ethics Committee of Tangshan Gongren Hospital.
Human OS cell lines, U2OS, Saos-2 and MG-63, were
obtained from American Type Culture Collection (Rockville, MD, USA)
and were cultured in Dulbecco's modified Eagle's medium (DMEM;
Hyclone, Beijing, China), RPMI-1640 (Hyclone) and DMEM,
respectively, supplemented with 10% fetal bovine serum (FBS;
Hyclone), 100 mg/ml streptomycin and 100 IU/ml penicillin, at 37°C
with 5% CO2. The human hFOB 1.19 osteoblast cell line
was maintained in DMEM/F12 medium (Hyclone), supplemented with 10%
FBS, 100 mg/ml streptomycin and 100 IU/ml penicillin (Hyclone) at
37°C with 5% CO2. TargetScan 6.2 software (www.targetscan.org/) was used to assess miR-214, then
from a number of candidates, PTEN was selected for validation.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA and miRNA (miR) were isolated using
RNeasy Mini and miRNeasy Mini kits (Qiagen, Valencia, CA, USA),
according to the manufacturer's instructions. The expression of
miR-214 was determined by RT-qPCR, using TaqMan MicroRNA assay kits
(ABI, Foster City, CA, USA) on a LightCycler 480 system II (Roche,
Basel, Switzerland). The primer sequences were as follows: PTEN,
forward: 5′-CCAGGACCAGAGGAAACCT-3′ and reverse:
5′-GCTAGCCTCTGGATTTGA-3′; GAPDH, forward:
5′-ATGTCGTGGAGTCTACTGGC-3′ and reverse: 5′-TGACCTTGCCCACAGCCTTG-3′.
Primers were purchased from Invitrogen Life Technologies (Shanghai,
China). The expression of PTEN was determined using SYGR green real
time PCR (Takara Bio, Inc., Tokyo, Japan). The RT-qPCR data were
normalized using the 2−ΔΔCt method relative to GAPDH as
described previously (28).
Plasmid construction and cell
transfection
Anti-miR-214 (product ID: AM12124), anti-miR
negative control (product ID: AM17010), miR-214 (product ID:
PM12124) and miR negative control (product ID: AM17110) were
purchased from Ambion (Austin, TX, USA). For the luciferase
reporter assay, the following primer was used: Forward,
5′-CGAGCTCGGACGAACTGGTGTAATG-3′ and reverse,
5′-CGACGCGTGTCCAGAGTCCAGCATAA-3′. The PCR fragment was inserted
into a pMir-Report vector (Ambion) between the SacI and
MluI restriction sites. The mutation was performed using a
fast mutation kit (New England Biolabs, Ipswich, Canada), according
to the manufacturer's instructions. The transfection was performed
when cells were grown to 80% confluence, using Lipofectamine 3000
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. This work was in agreement with the
National Health and Family Planning Commission Guidelines.
Luciferase activity assay
The luciferase activity assay was performed, as
previously described (29).
Briefly, the MG63 cells were cultured in 12-well plates
(1×105 cells/well) and were cotransfected with wild-type
(WT) or mutated (Mut) 3′-untranslated regions (UTRs) of PTEN
luciferase reporter constructs, and miR-214 or control mimic using
Lipofectamine 3000. Following incubation for 24 h, the cells were
harvested and luciferase activity was determined using a
Dual-Luciferase Reporter assay kit (Promega, Madison, WI, USA).
Cell viability assay
The Cell Counting kit-8 (CCK-8) assay was used as a
qualitative index of cell viability, which is based on the
conversion of a water-soluble tetrazolium salt,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
to a water soluble formazan dye upon reduction by dehydrogenases in
the presence of an electron carrier (30). The cells were plated into 96-well
microplates and subsequently the cell count was performed using
CCK-8 (Dojindo Molecular Technologies, Inc., Beijing, China),
according to the manufacturer's instructions. Briefly, 10 µl
CCK-8 solution was added to each well and the samples were
incubated for 1 h prior to the absorbance being measured at 450 nm
using the SpectraMax M5 (Molecular Devices, Sunnyvale, CA,
USA).
Colony formation assay
A total of 500 transfected MG-63 cells were seeded
into 6-well plates and maintained in DMEM, containing 10% FBS for
14 days. The cells were subsequently fixed and stained with 100%
methanol for 20 min, followed by 0.5% crystal violet for 15 min at
room temperature. Visible colonies were quantified using an
inverted microscope (IX73; Olympus, Tokyo, Japan).
In vitro migration and invasion
assay
Migration and invasion assays were performed using
Transwell chambers. For the migration assay, 5×104 cells
were seeded into the upper chamber of Transwell plates (BD
Bioscience, San Jose, CA, USA). For the invasion assay,
1×105 cells were added into the upper chamber precoated
with matrigel (BD Bioscience). In each assay, the cells were
maintained in medium without serum in the upper chamber and medium,
containing 10% FBS, was added to the lower chamber as a
chemoattractant. Following incubation for 24 h, the cells failing
to migrate or invade through the membrane were wiped away. The
membranes were subsequently fixed and stained with 0.5% crystal
violet. A total of four random fields were counted per chamber
using an inverted microscope (Olympus) and each experiment was
repeated three times.
Western blotting assay
The cells were harvested and resuspended in
phosphate-buffered saline. Following centrifugation at 100 × g for
5 min at room temperature, the pellet was lysed in ice-cold Lysis
Buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% TritonX-100, 1% sodium
deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4,
0.5 µg/ml leupeptin and PMSF], containing 1% Halt Protease
and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific,
Waltham, MA, USA) for 30 min. The supernatant was collected
following centrifugation at 4°C for 10 min at 10,142 × g and the
protein concentration was determined using a BCA protein assay kit
(Tiangen Biotech Co., Ltd., Beijing, China) with the SpectraMax M5
spectrophotometer at 562 nm. The protein samples were denatured
using sample loading buffer (Beyotime Institute of Biotechnology,
Haimen, China) for 10 min at 95°C and stored at 4°C for future use.
Equal concentrations (1.5–2.0 µg/µl) of the proteins
were separated on 10% SDS-PAGE gels (Beyotime Institute of
Biotechnology) and transferred onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked with 5% non-fat milk in Tris-buffered saline with 20%
Tween-20 (TBST) for 1 h at room temperature, and was subsequently
incubated with primary antibodies at 4°C overnight. The membrane
was incubated with the appropriate horseradish peroxidase
(HRP)-conjugated secondary antibody, at a dilution of 1:10,000 in
blocking buffer, for 1 h at room temperature. The protein bands
were observed using enhanced chemiluminescence (HRP substrate; EMD
Millipore) and exposed in a dark room. All antibodies were diluted
in 5% skimmed milk in TBST. The antibodies against PTEN
(monoclonal, rabbit anti-human, cat. no. ab32199, 1:1,000), pAkt
(monoclonal, rabbit anti-human, cat. no. ab81283, 1:7,000), Akt
(monoclonal, rabbit anti-human, cat. no. ab32505, 1:5,000), GAPDH
(monoclonal, rabbit anti-human, cat. no. ab181602, 1:10,000),
β-actin (monoclonal, rabbit anti-human, cat. no. ab6276, 1:10,000),
HRP-secondary antibody (goat anti-rabbit, cat. no. ab6721,
1:10,000), were purchased from Abcam (Cambridge, UK). The protein
expression level of PTEN protein was analyzed using Quantity One
software version 4.4 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and normalized against that of β-actin.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analysis was performed by analysis of
variance or two-tail Student's t-test using SPSS version 14 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
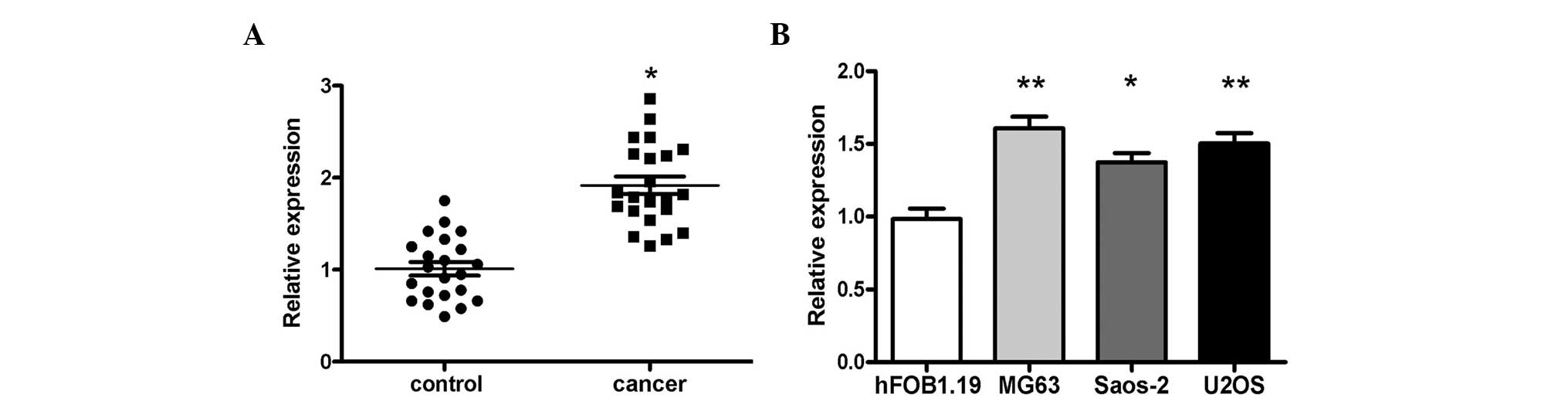
Expression of miR-214 was increased in
human OS tissues and cell lines
In order to determine the expression levels of
miR-214 in OS tissues, RT-qPCR detection was performed in 22 pairs
of OS tissues and normal tissues. The data demonstrated that
miR-214 was significantly increased in the OS tissues compared with
the matched normal tissues (Fig.
1A). Additionally, the expression of miR-214 in three OS cell
lines, U2OS, Saos-2 and MG-63, was markedly increased compared with
that in the human hFOB 1.19 osteoblast cell line (Fig. 1B).
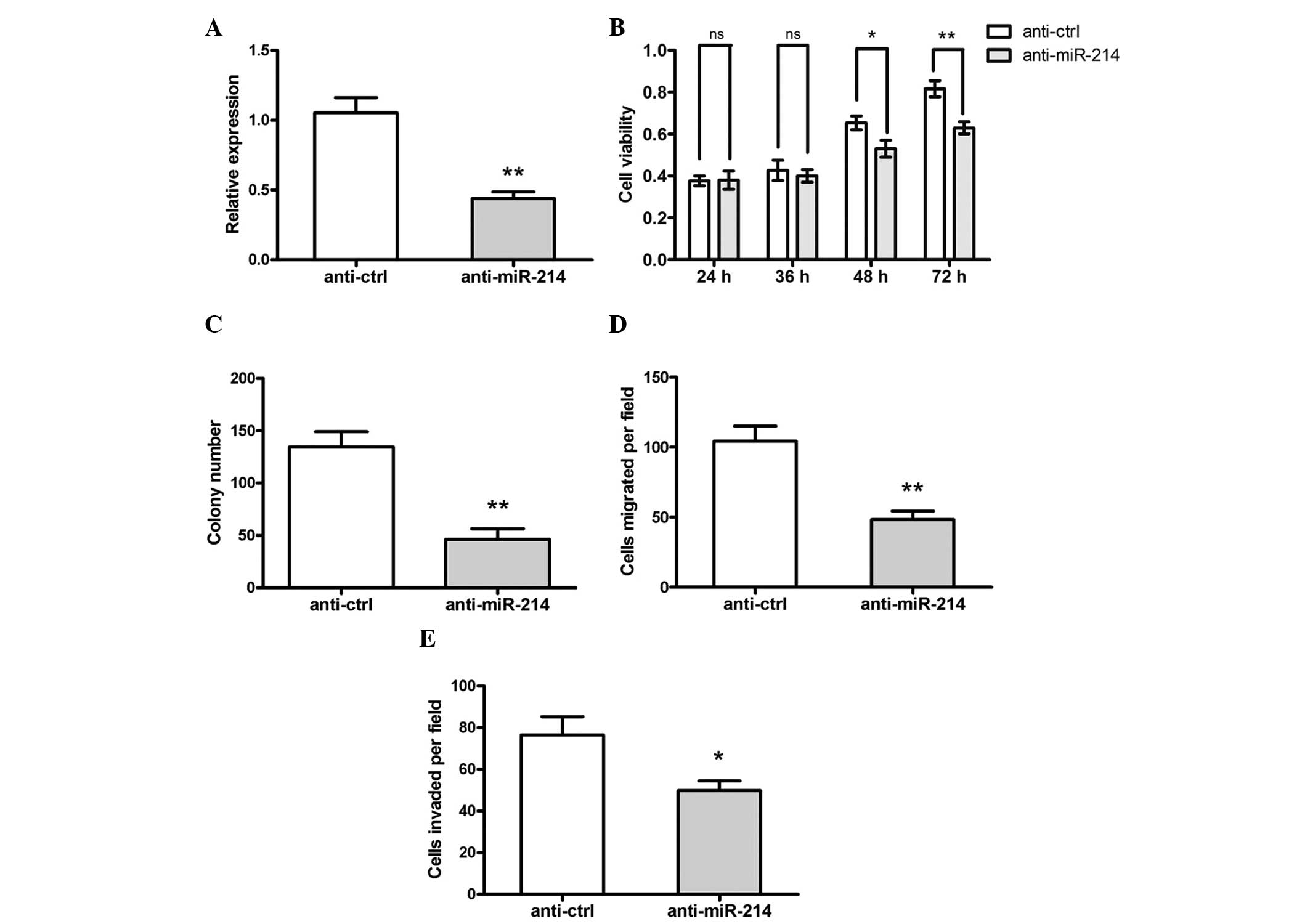
Inhibition of miR-214 suppresses OS cell
proliferation and migration
To investigate the role of miR-214 in OS cell
proliferation, MG-63 cells were transfected with the miR-214
inhibitor, anti-miR-214, or control inhibitor, anti-ctrl. RT-qPCR
demonstrated that the expression of miR-214 in the cells
transfected with anti-miR-214 was significantly decreased compared
with the control group (Fig. 2A).
In addition, cell viability of the cells, which were transfected
with anti-miR-214 were also markedly reduced (Fig. 2B) between 48 and 72 h following
transfection. Transfection of anti-miR-214 also clearly suppressed
colony formation in the MG-63 cells (Fig. 2C).
To investigate the effect of miR-214 on the motility
of OS cells, in vitro migration and invasion assays were
performed. It was revealed that transfection of anti-miR-214
significantly suppressed the in vitro migration and invasion
abilities of the MG-63 cells (Fig. 2D
and E).
miR-214 directly targets PTEN in OS
cells
TargetScan 6.2 software was used to search the
potential target gene of miR-214. PTEN was predicted to be a target
of miR-214 (Fig. 3A). A luciferase
activity assay revealed that miR-214 significantly suppressed the
activity of PTEN luciferase in the WT 3′-UTR, however, not the Mut
3′-UTR in HEK293 cells (Fig. 3B).
In addition, overexpression of miR-214 significantly suppressed the
protein expression of PTEN. The protein expression of pAKT was
significantly increased and the total AKT protein remained
identical, while inhibition of miR-214 exhibited the opposite
effects (Fig. 3C and D).
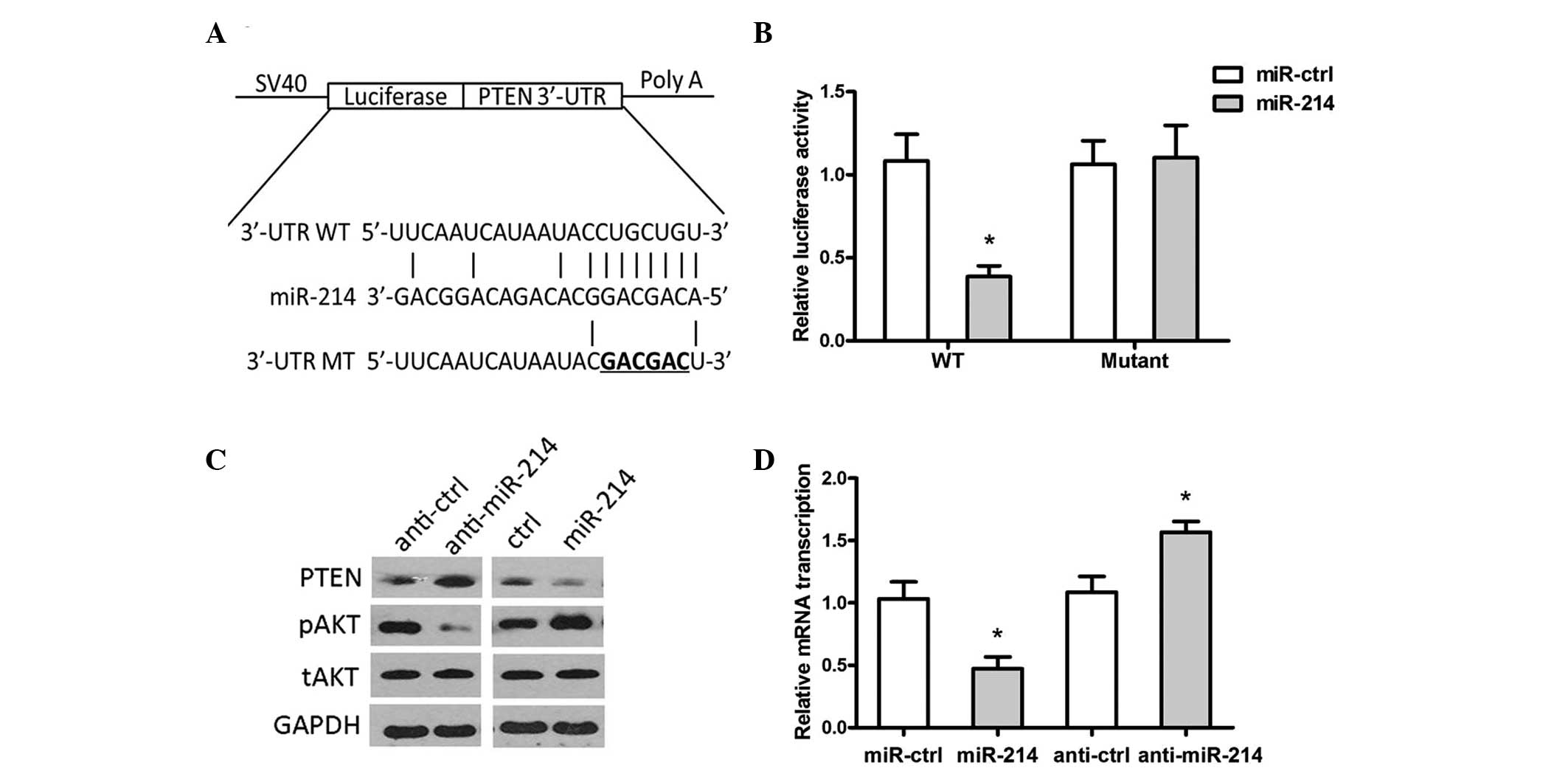 | Figure 3PTEN is a direct target of miR-214.
(A) Computational analysis revealed that miR-214 potentially
targeted PTEN. (B) HEK293 cells were cotransfected with miR-214 and
WT or Mut 3′-UTR luciferase reporter construct. (C) The protein
expression levels of PTEN, pAKT, tAKT and GAPDH were detected by
western blotting in MG-63 cells transfected with miR-214/ctrl or
anti-miR-214/anti-ctrl. (D) The protein expression of PTEN was
quantified using Quality One software. (*P<0.05,
compared with the control group). miR, microRNA; UTR, untranslated
region, WT, wild-type; Mut, mutant; p, phosphorylated; t, total;
ctrl, control; PTEN, phosphatase and tensin homolog. |
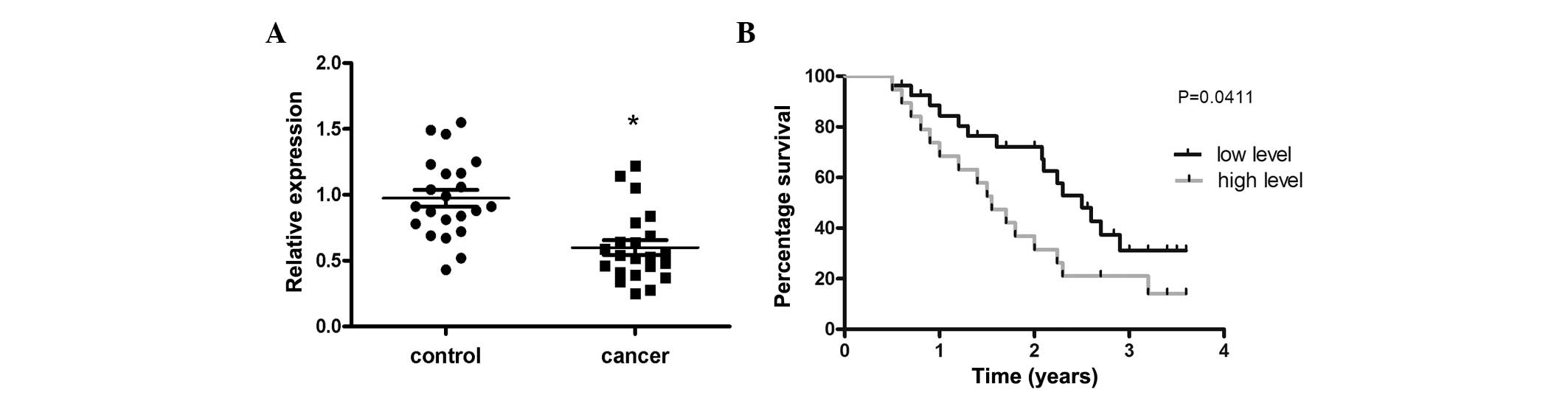
miR-214 is negatively correlated with
PTEN in OS tissues
The mRNA expression of PTEN in the 22 OS and the
corresponding normal tissues was measured. The results revealed
that the mRNA expression of PTEN was significantly decreased in OS
tissues compared with the corresponding normal tissues (Fig. 4A). In addition, 35 OS cases were
investigated and revealed that those patients expressing low levels
of miR-214 exhibited a longer survival time compared with those
expressing a high level (Fig.
4B).
Discussion
Emerging studies have revealed that miRNAs are
involved in the progression of various types of cancer, including
OS, via the regulation of the expression of multiple target genes
involved in cancer progression and metastasis. Therefore,
identification of specific miRNAs and their targets involved in
tumorigenesis may provide valuable insight for the diagnosis and
therapy of patients with human malignancies. The present study
demonstrated that the expression of miR-214 was upregulated in OS
tissues. Forced overexpression of miR-214 enhanced cell
proliferation in MG-63 and U2OS cells, while miR-214 inhibition by
its antisense oligonucleotides repressed cell proliferation.
Therefore, the present study demonstrated that miR-214 may be an
oncomicroRNA in the progression of OS. However, further studies are
required to elucidate the roles in vivo.
In addition, at the molecular level, these results
revealed that PTEN is a direct target of miR-214 in OS cells. PTEN
was originally identified as the tumor suppressor gene frequently
lost on chromosome 10q23. The relevance of PTEN in cancer was
addressed through the generation of germline knockout PTEN mice in
several previous studies (31).
These previous studies revealed the requirement of PTEN for
embryonic development. Notably, heterozygous loss of this tumor
suppressor in the mouse resulted in the development of cancer of
multiple origins, as well as a lethal lymphoproliferative disease.
In humans, germline loss and mutation of PTEN is observed in a
group of autosomal dominant syndromes, PTEN hamartoma tumor
syndromes, which are characterized by neurological disorders,
multiple hamartomas and cancer susceptibility. Upregulation of PTEN
can increase the expression of Caspase 3 to cause dysregulation of
apoptosis in tumor cells, which forms the molecular mechanisms of
PTEN contribution to tumorigenesis and progression of malignant
tumors (32).
In conclusion, the present study described a novel
miR-214/PTEN association. The data also provided a mechanism for
PTEN dysregulation and its contribution to OS cell growth,
migration and invasion. These results suggested that miR-214 may
act as an oncogene in OS and represents a potential molecular
target for OS therapy.
References
|
1
|
Klein MJ and Siegal GP: Osteosarcoma:
Anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma: Conventional treatment vs. gene therapy. Cancer Biol
Ther. 8:106–117. 2009. View Article : Google Scholar
|
|
3
|
Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q
and Ma B: MicroRNA-34a inhibits the proliferation and metastasis of
osteosarcoma cells both in vitro and in vivo. PLoS One.
7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amankwah EK, Conley AP and Reed DR:
Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol.
5:147–162. 2013.PubMed/NCBI
|
|
5
|
Rainusso N, Wang LL and Yustein JT: The
adolescent and young adult with cancer: State of the art - bone
tumors. Curr Oncol Rep. 15:296–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang W, Gao B, Fu P, Xu S, Qian Y and Fu
Q: The miRNAs in the pathogenesis of osteosarcoma. Front Biosci
(Landmark Ed). 18:788–794. 2013. View
Article : Google Scholar
|
|
10
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar
|
|
11
|
He C, Xiong J, Xu X, Lu W, Liu L, Xiao D
and Wang D: Functional elucidation of MiR-34 in osteosarcoma cells
and primary tumor samples. Biochem Biophys Res Commun. 388:35–40.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song B, Wang Y, Xi Y, Kudo K, Bruheim S,
Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, et al:
Mechanism of chemoresistance mediated by miR-140 in human
osteosarcoma and colon cancer cells. Oncogene. 28:4065–4074. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao G, Cai C, Yang T, Qiu X, Liao B, Li
W, Ji Z, Zhao J, Zhao H, Guo M, et al: MicroRNA-221 induces cell
survival and cisplatin resistance through PI3K/Akt pathway in human
osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li G, Cai M, Fu D, Chen K, Sun M, Cai Z
and Cheng B: Heat shock protein 90B1 plays an oncogenic role and is
a target of microRNA-223 in human osteosarcoma. Cell Physiol
Biochem. 30:1481–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao JH, Zhou RP, Peng AF, Liu ZL, Huang
SH, Long XH and Shu Y: microRNA-195 suppresses osteosarcoma cell
invasion and migration in vitro by targeting FASN. Oncol Lett.
4:1125–1129. 2012.PubMed/NCBI
|
|
16
|
Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma
YL, Ji ZW, Li XX, Han K, Gao J, et al: miR-15a and miR-16-1
downregulate CCND1 and induce apoptosis and cell cycle arrest in
osteosarcoma. Oncol Rep. 28:1764–1770. 2012.PubMed/NCBI
|
|
17
|
Huang G, Nishimoto K, Zhou Z, Hughes D and
Kleinerman ES: miR-20a encoded by the miR-17-92 cluster increases
the metastatic potential of osteosarcoma cells by regulating Fas
expression. Cancer Res. 72:908–916. 2012. View Article : Google Scholar :
|
|
18
|
MicroRNA-214 suppresses osteoblast
differentiation by binding to Osterix. Bonekey Rep. 2:3932013.
|
|
19
|
Chen YF, Dong Z, Xia Y, Tang J, Peng L,
Wang S and Lai D: Nucleoside analog inhibits microRNA-214 through
targeting heat-shock factor 1 in human epithelial ovarian cancer.
Cancer Sci. 104:1683–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong X, Liu H, Chen F, Li D and Zhao Y:
MiR-214 promotes the alcohol-induced oxidative stress via
down-regulation of glutathione reductase and cytochrome P450
oxidoreductase in liver cells. Alcohol Clin Exp Res. 38:68–77.
2014. View Article : Google Scholar
|
|
21
|
Yang T, Zhang GF, Chen XF, Gu HH, Fu SZ,
Xu HF, Feng Q and Ni YM: MicroRNA-214 provokes cardiac hypertrophy
via repression of EZH2. Biochem Biophys Res Commun. 436:578–584.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC,
Huang CH, Lee YS, Yen TC and Hsieh SY: MicroRNA-214 down-regulation
contributes to tumor angiogenesis by inducing secretion of the
hepatoma-derived growth factor in human hepatoma. J Hepatol.
57:584–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YS, Wang YH, Xia HP, Zhou SW,
Schmid-Bindert G and Zhou CC: MicroRNA-214 regulates the acquired
resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant
cell lines. Asian Pac J Cancer Prev. 13:255–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Luo XJ, Xiong AW, Zhang ZD, Yue S,
Zhu MS and Cheng SY: MicroRNA-214 promotes myogenic differentiation
by facilitating exit from mitosis via down-regulation of
proto-oncogene N-ras. J Biol Chem. 285:26599–26607. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZC, Li YY, Wang HY, Fu S, Wang XP,
Zeng MS, Zeng YX and Shao JY: Knockdown of miR-214 promotes
apoptosis and inhibits cell proliferation in nasopharyngeal
carcinoma. PLoS One. 9:e861492014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cell Int. 13:682013.
View Article : Google Scholar
|
|
27
|
Peng RQ, Wan HY, Li HF, Liu M, Li X and
Tang H: MicroRNA-214 suppresses growth and invasiveness of cervical
cancer cells by targeting UDP-N-acetyl-α-D-galactosamine:pol
ypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem.
287:14301–14309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bubner B and Baldwin IT: Use of real-time
PCR for determining copy number and zygosity in transgenic plants.
Plant Cell Rep. 23:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H and Yang BB: Stress response of
glioblastoma cells mediated by miR-17-5p targeting PTEN and the
passenger strand miR-17-3p targeting MDM2. Oncotarget. 3:1653–1668.
2012. View Article : Google Scholar
|
|
30
|
Han SB, Shin YJ, Hyon JY and Wee WR:
Cytotoxicity of voriconazole on cultured human corneal endothelial
cells. Antimicrob Agents Chemother. 55:4519–4523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aguissa-Touré AH and Li G: Genetic
alterations of PTEN in human melanoma. Cell Mol Life Sci.
69:1475–1491. 2012. View Article : Google Scholar
|
|
32
|
Zheng HC, Li YL, Sun JM, Yang XF, Li XH,
Jiang WG, Zhang YC and Xin Y: Growth, invasion, metastasis,
differentiation, angiogenesis and apoptosis of gastric cancer
regulated by expression of PTEN encoding products. World J
Gastroenterol. 9:1662–1666. 2003.PubMed/NCBI
|