Introduction
Nasopharyngeal carcinoma is a relatively rare head
and neck cancer worldwide, but is common in southern China and
Southeast Asia without a defined etiology. At present, although
radiotherapy combined with chemotherapy has enhanced the
therapeutic effect of nasopharyngeal carcinoma treatments, the
metastatic and relapse rate remain high (1,2).
Therefore, novel strategies for the treatment of nasopharyngeal
carcinoma are urgently required.
MicroRNAs (miRNAs) are small non-coding RNAs, which
can bind to the 3′-untranslated region (3′-UTR) of target mRNA and
cause RNA degradation or translation inhibition (3). It has been demonstrated that miRNAs
are important in multiple biological processes, including cell
survival, proliferation, differentiation and apoptosis (4). miRNAs have been demonstrated to
promote or inhibit the development and progression of
nasopharyngeal carcinoma, suggesting that miRNAs may function as
tumor suppressors or oncogenes (5).
The deregulation of miRNA-214 (miR-214) has been
found in multiple types of cancer (6–11).
miR-214 has been reported to be upregulated in ovarian cancer,
gastric cancer and melanoma, but downregulated in breast cancer,
cervical cancer and hepatocellular carcinoma (6–11).
In addition, Zhang et al revealed that miR-214 is important
in nasopharyngeal carcinoma and found that miR-214 was upregulated
in nasopharyngeal carcinoma (12).
Silencing of miR-214 promoted apoptosis and suppressed
proliferation in nasopharyngeal carcinoma cells, and suppressed
tumor growth in nude mice (12).
Furthermore, Bim was identified as a direct target of miR-214 and
low expression of Bim in nasopharyngeal carcinoma tissues
correlated with a poor survival rate of nasopharyngeal carcinoma
patients (12). Since one miRNA
can have numerous targets, other targets of miR-214 may also exist
in nasopharyngeal carcinoma cells.
The present study aimed to investigate the role of
miR-214 in the regulation of nasopharyngeal carcinoma cell
proliferation and apoptosis, in addition to the underlying
molecular mechanisms.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), TRIzol reagent, MTT, SYBRH Green Master mix,
Superscript II Reverse Transcriptase kit and Lipofectamine 2000
were purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). The miRNA reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) detection kit was purchased from GeneCopoeia
(Rockville, MD, USA). The bicinchoninic acid (BCA) Protein Assay
kit and enhanced chemiluminescence (ECL) kit were purchased from
Pierce Biotechnology, Inc. (Rockford, IL, USA). The Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was
purchased from BD Pharmingen (San Diego, CA, USA). The PsiCHECK™2
vector was purchased from Promega Corporation (Madison, WI, USA). A
Quick-Change Site-Directed Mutagenesis kit was purchased from
Stratagene (La Jolla, CA, USA). Mouse anti-Bax monoclonal antibody
(1:50; ab5714) and mouse anti-GAPDH monoclonal antibody (1:100;
ab8245) were purchased from Abcam (Cambridge, UK).
Tissue specimen collection
The present study was approved by the Ethical
Committee of Central South University (Changsha, China). In total,
16 nasopharyngeal carcinoma tissues as well as their matched normal
adjacent tissues were obtained from the Department of
Otorhinolaryngology Head and Neck Surgery, Xiangya Hospital of
Central South University. Written informed consent from patients
with nasopharyngeal carcinoma was obtained. Tissues were frozen in
liquid nitrogen following surgical removal and stored at −70°C
prior to use.
Cell culture
Four human nasopharyngeal carcinoma cell lines,
including CNE1, HONE1, C666-1 and CNE2, as well as the normal
nasopharyngeal epithelial cell line NP69 were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in DMEM with 10% FBS and 1%
penicillin/streptomycin at 37°C with 5% CO2.
RNA isolation and RT-qPCR
Total RNA was isolated from tissues and cells using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer's instructions. The concentration of RNA was
calculated by measuring the OD260. Reverse transcription of miR-214
and its specific amplification were performed using an miRNA
RT-qPCR Detection kit. The relative expression of miRNA was
analyzed by the 2−ΔΔCt method. The U6 small nuclear RNA
was used for normalization. For determining the expression level of
mRNA, total RNA was reverse-transcribed using a Superscript II
Reverse Transcriptase kit according to the manufacturer's
instructions. Subsequently, PCR was performed with SYBRH Green
Master mix using a real-time PCR detection system (ABI 7500;
Invitrogen Life Technologies). The following specific primers were
used: Bax, forward 5′-CCCGAGAGGTCTTTTTCCGAG-3′ and reverse
5′-CCAGCCCATGATGGTTCTGAT-3′; GAPDH, forward
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative expression of Bax mRNA
was calculated by normalization to the GAPDH mRNA level.
Western blot analysis
Tissues or cells were lysed in RIPA buffer
containing 1 mM phenylmethylsulfonyl fluoride. Protein was
quantified using the BCA Protein Assay kit. The protein sample was
diluted, heated for denaturation and then subjected to SDS-PAGE
electrophoresis. After the proteins were transferred onto a
polyvinylidene difluoride (PVDF) membrane (Millipore Corporation,
Billerica, MA, USA), 5% skimmed milk powder was applied for 2 h at
37°C. Subsequently, mouse monoclonal anti-Bax or anti-GAPDH
antibodies were incubated for 3 h at room temperature with the PVDF
membrane. The PVDF membrane was rinsed in Tris-Buffered saline and
Tween 20 three times and then incubated with a fluorescent
secondary rabbit anti-mouse IgG antibody (1:10,000; ab46540; Abcam)
for 2 h. The PVDF membrane was scanned using an infrared imaging
system (Odyssey® CLx Infrared Imaging System; LI-COR
Biosciences, Lincoln, NE, USA) and the band intensity was detected
using Odyssey analysis software, version 3.0 (LI-COR
Biosciences).
Transfection
In accordance with the manufacturer's instructions,
transfection was performed using Lipofectamine® 2000
(Invitrogen Life Technologies). For miR-214 functional analysis,
cells were transfected with the scrambled miRNA as a negative
control, miR-214 mimics or the miR-214 inhibitor. For Bax
functional analysis, cells were transfected with Bax-specific
siRNA.
Bioinformatics analysis
Bioinformatics analysis was conducted in order to
analyze the putative target of miR-214. TargetScan online software
(www.targetscan.org) was used for this
process.
Luciferase reporter assay
A luciferase reporter assay was performed to
determine whether Bax was a direct target of miR-214. In brief,
Lipofectamine 2000 reagent was used to transfect CNE1 cells with
miR-214 mimics or scrambled miRNA, with either a wild type or
mutant type of Bax 3′UTR plasmid, respectively. Renilla and
firefly luciferase activities were measured at 48 h
post-transfection using the Dual-Glo™ Luciferase Reporter Assay
System (Promega Corporation) according to the manufacturer's
instructions. The ratio of Renilla to firefly luciferase
value was determined.
Cell proliferation analysis
MTT (Invitrogen Life Technologies) was used to
perform cell proliferation analysis according to the manufacturer's
instructions. Briefly, for each group, 1×104 cells per
well were plated in a 96-well plate and incubated for 0, 12, 24 or
48 h at 37°C and 5% CO2. To assess cell proliferation,
10 µl of MTT (5 mg/ml) was added to each well and then
incubated for 4 h at 37°C and 5% CO2. The supernatant
was removed and 100 µl dimethyl sulfoxide was added to
dissolve the precipitation. The absorbance was detected at 492 nm
using a microplate reader (FLx800; Biotek Instruments, Inc.,
Winooski, VT, USA).
Apoptosis analysis
Cell apoptosis analysis was performed using an
Annexin V-FITC Apoptosis Detection kit, in accordance with the
manufacturer's instructions. In brief, 1×106 cells were
resuspended in binding buffer. Following that, 2.5 µl of
Annexin V and 5 µl of propidium iodide were added. Following
incubation for 15 min in the dark, 400 µl of binding buffer
was added. Cell apoptosis was determined by flow cytometry
(FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis of differences was performed by one-way analysis of
variance using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-214 is upregulated in nasopharyngeal
carcinoma tissues and cell lines
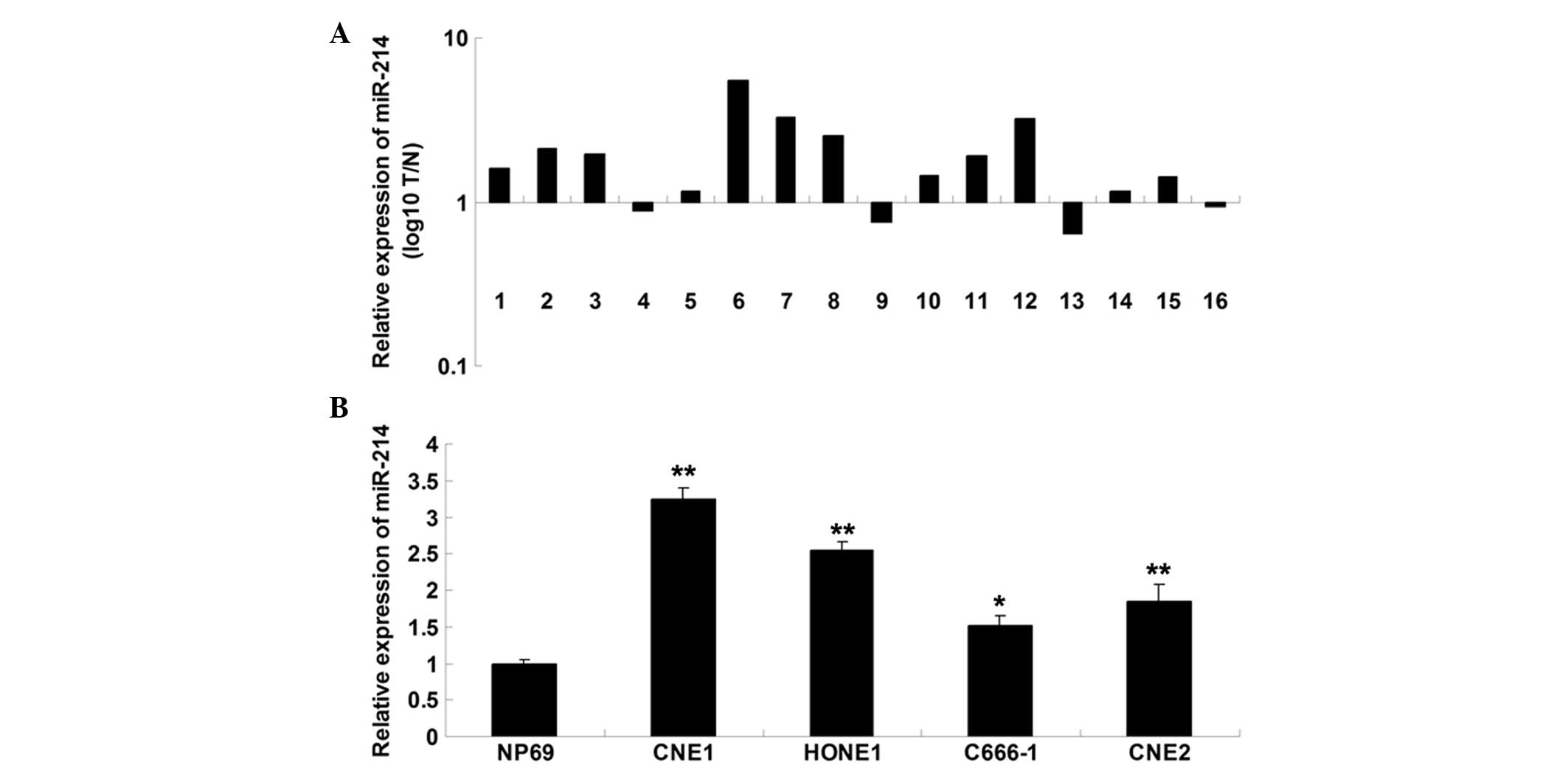
The expression level of miR-214 was initially
examined in nasopharyngeal carcinoma tissues and their matched
normal adjacent tissues using RT-qPCR. As shown in Fig. 1A, miR-214 was significantly
upregulated in nasopharyngeal carcinoma tissues, when compared with
their matched normal adjacent tissues. In addition, miR-214 was
also upregulated in nasopharyngeal carcinoma cell lines compared
with normal nasopharyngeal epithelial NP69 cells (Fig. 1B).
Inhibition of miR-214 suppresses
proliferation and induces apoptosis in nasopharyngeal carcinoma
CNE1 cells
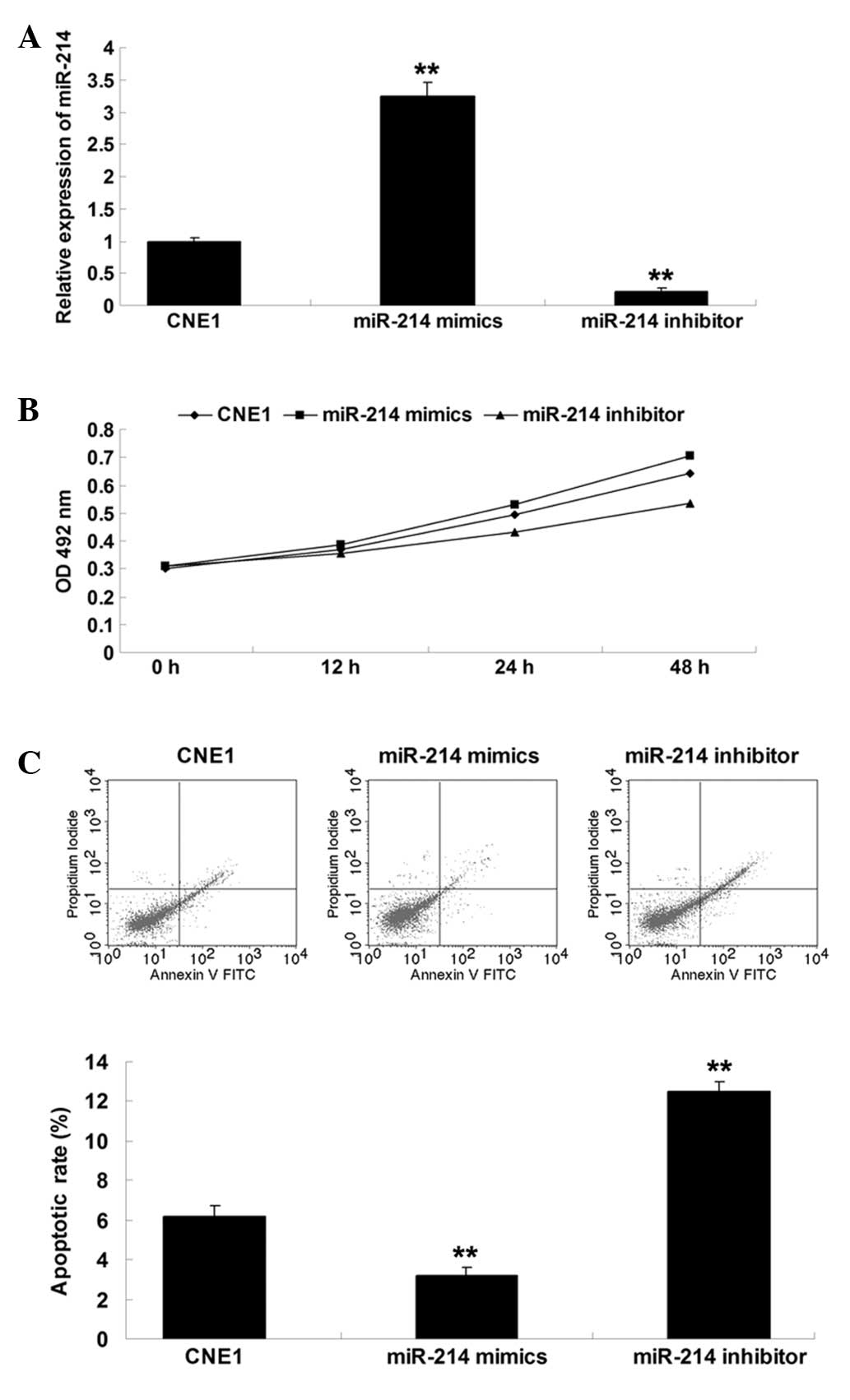
As miR-214 was upregulated in nasopharyngeal
carcinoma CNE1 cells, miR-214 mimics and an miR-214 inhibitor were
used to perform functional analysis of miR-214 in CNE1 cells. As
shown in Fig. 2A, the expression
of miR-214 was notably decreased following transfection with the
miR-214 inhibitor, however, it was increased following transfection
with miR-214 mimics. Subsequently, a cell proliferation and
apoptosis assay was performed to investigate the role of miR-214 in
nasopharyngeal carcinoma CNE1 cells. The data demonstrated that
inhibition of miR-214 suppressed proliferation and induced
apoptosis in nasopharyngeal carcinoma CNE1 cells (Fig. 2B and C). By contrast,
overexpression of miR-214 promoted nasopharyngeal carcinoma cell
proliferation and inhibited cell apoptosis (Fig. 2B and C).
Bax is a target of miR-214 in
nasopharyngeal carcinoma CNE1 cells
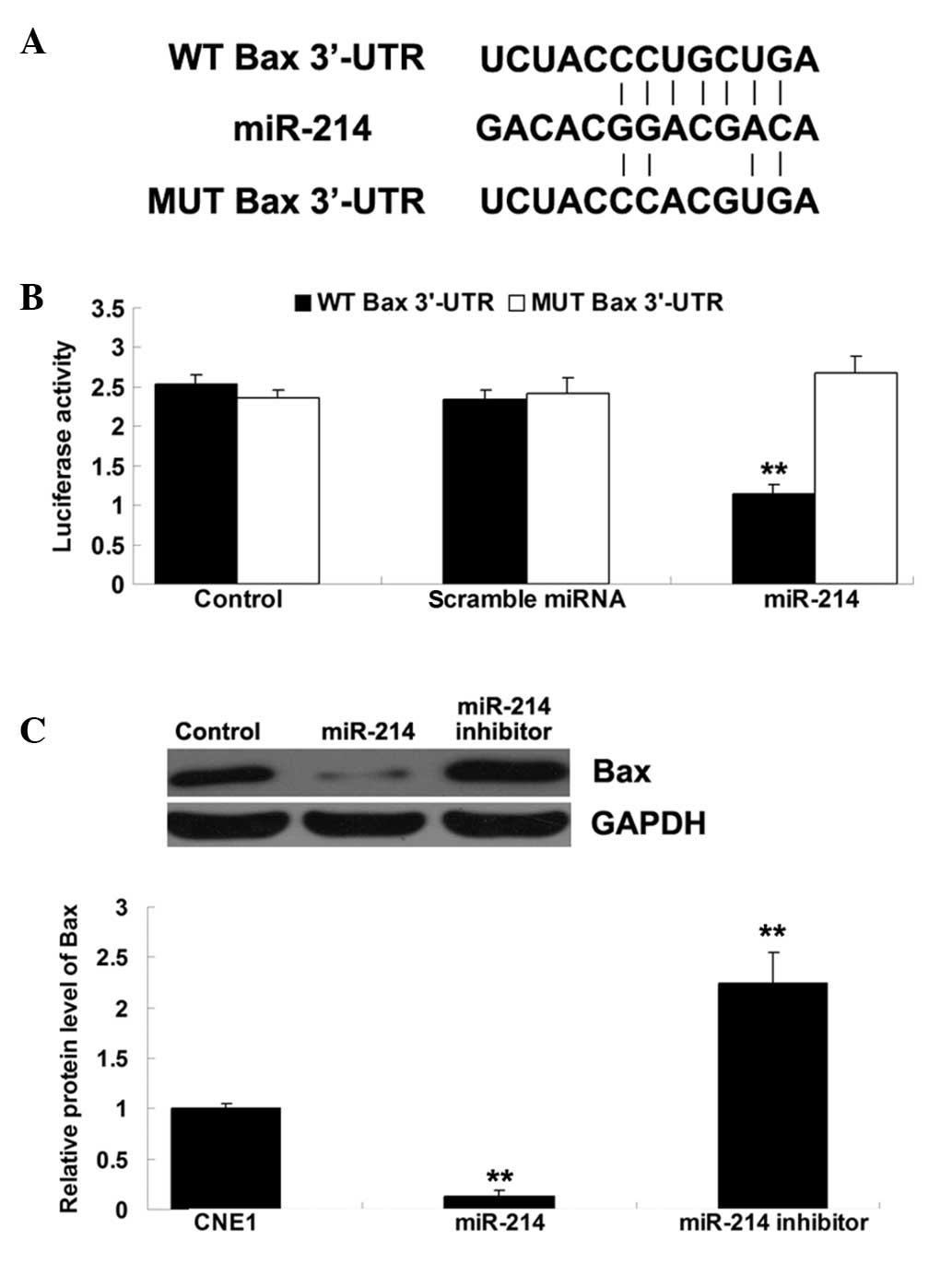
Based on bioinformatical analysis, Bax was found to
be a putative target of miR-214 and the putative seed sequences for
miR-214 at the 3′UTR of Bax are indicated (Fig. 3A). To further clarify whether Bax
was a target of miR-214, plasmids containing the wild and mutant
types of the 3′-UTR of Bax were generated (Fig. 3A). A luciferase reporter assay was
further performed in nasopharyngeal carcinoma CNE1 cells. The data
demonstrated that the luciferase activity was only reduced in
nasopharyngeal carcinoma CNE1 cells co-transfected with miR-214
mimics and the wild type 3′-UTR of Bax, but was unaltered in CNE1
cells co-transfected with miR-214 mimics and the mutant 3′-UTR of
Bax, indicating that Bax is a target of miR-214 in nasopharyngeal
carcinoma CNE1 cells (Fig.
3B).
Following that, the present study investigated
whether miR-214 could negatively regulate the expression of Bax in
nasopharyngeal carcinoma CNE1 cells. CNE1 cells were transfected
with miR-214 mimics or an miR-214 inhibitor, and the protein level
of Bax was examined using western blotting. As shown in Fig. 3C, the protein level of Bax was
increased following inhibition of miR-214, while it was decreased
following upregulation of miR-214, indicating that the protein
expression of Bax was negatively regulated by miR-214 in
nasopharyngeal carcinoma CNE1 cells.
Inhibition of Bax reverses the effect of
miR-214 downregulation on cell proliferation and apoptosis in
nasopharyngeal carcinoma CNE1 cells
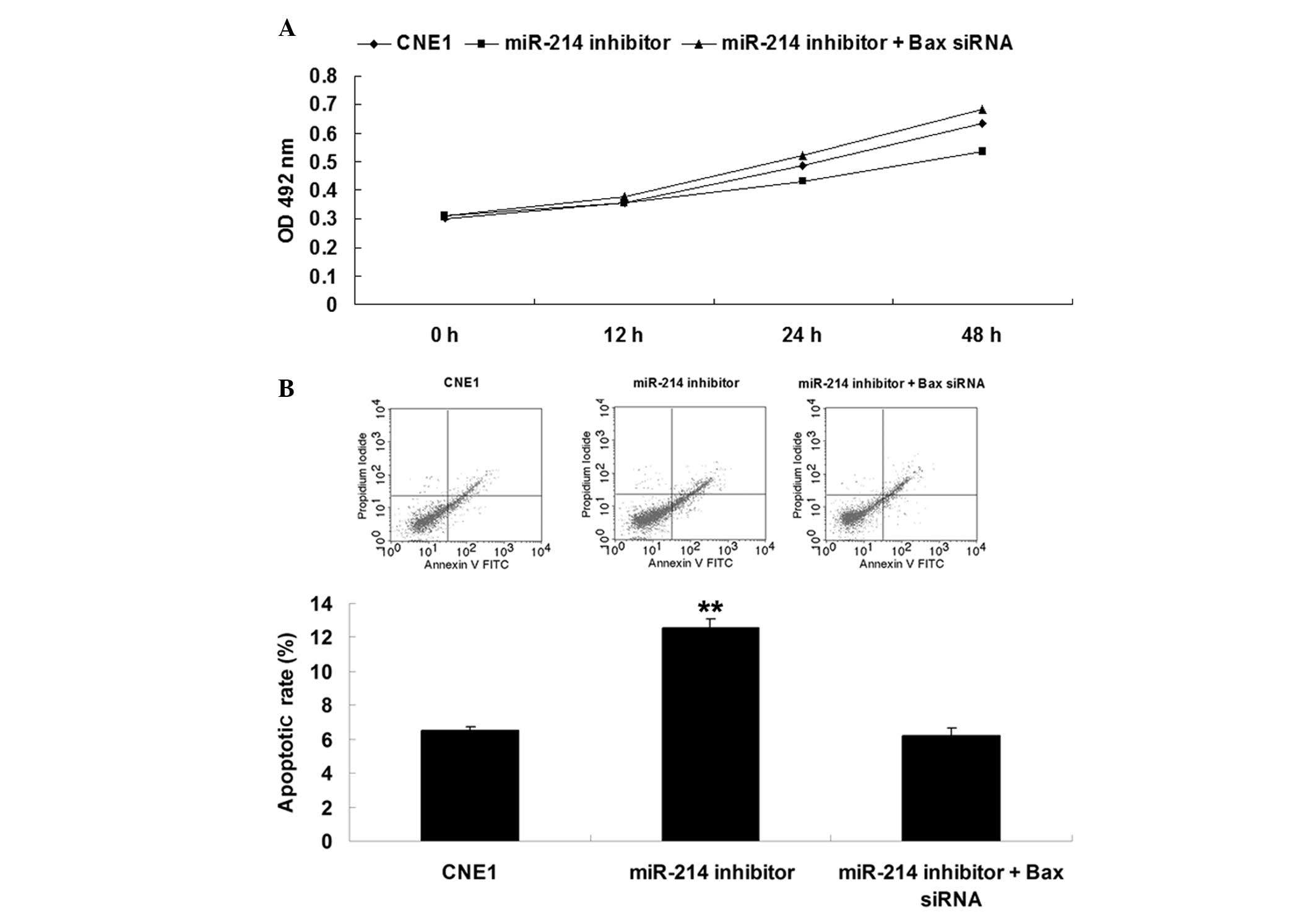
The present study further determined whether miR-214
had an oncogenic role in nasopharyngeal carcinoma cells through
directly targeting Bax. As shown in Fig. 4A, inhibition of Bax notably
reversed the inhibitory effect of miR-214 downregulation on CNE1
cell proliferation. In addition, siRNA-mediated Bax inhibition also
reversed the promoting effect of miR-214 downregulation on CNE1
cell apoptosis (Fig. 4B). Based on
these data, it was suggested that Bax is a downstream effector in
miR-214-mediated cell proliferation and apoptosis in nasopharyngeal
carcinoma CNE1 cells.
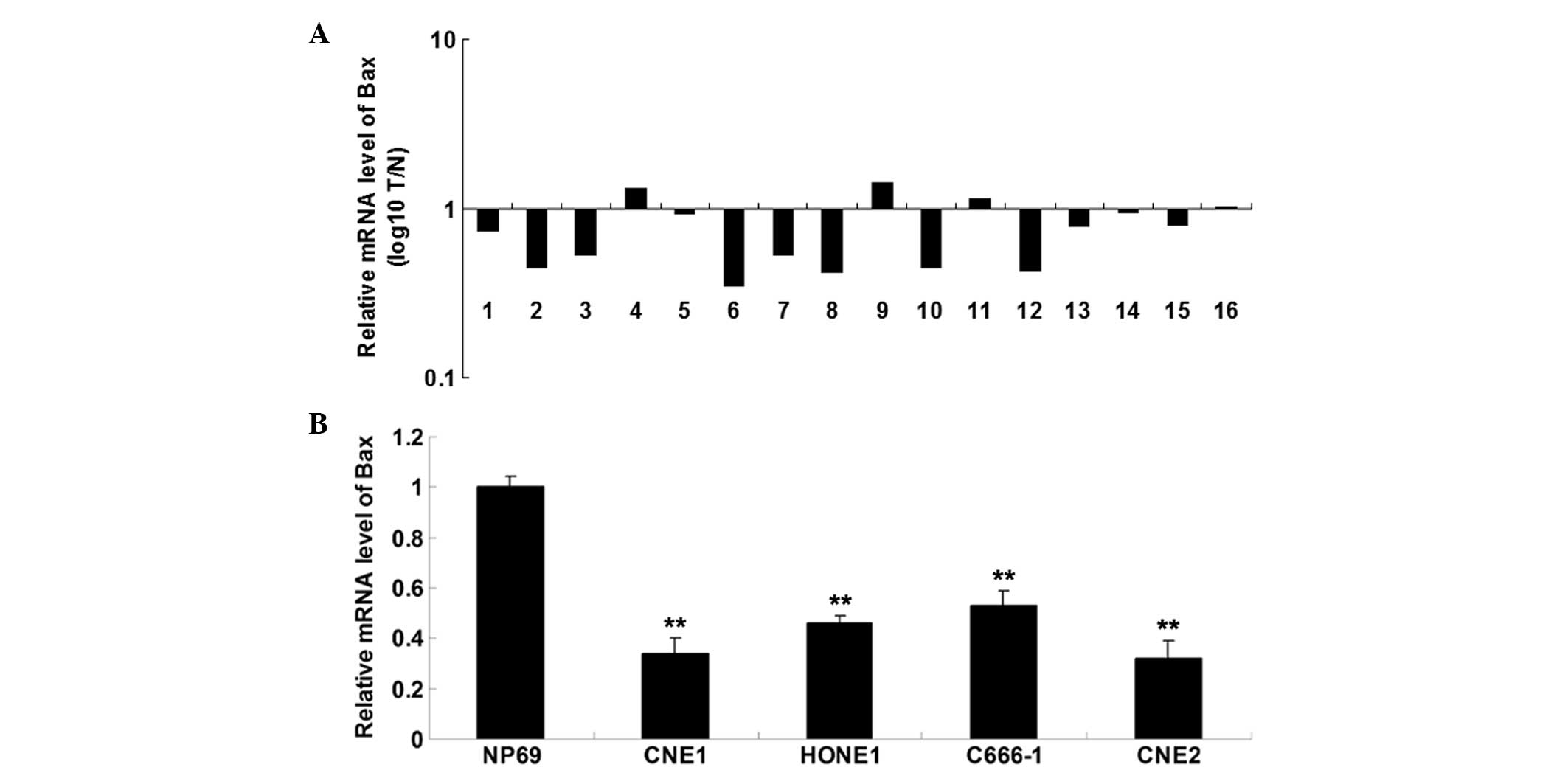
Bax is downregulated in nasopharyngeal
carcinoma tissues
The mRNA expression level of Bax was determined. As
shown in Fig. 5A, the mRNA level
of Bax was decreased in nasopharyngeal carcinoma tissues, compared
with their matched normal adjacent tissues. In addition, the mRNA
level of Bax was also reduced in nasopharyngeal carcinoma cell
lines, when compared with normal nasopharyngeal epithelial cells
(Fig. 5B).
Discussion
It is well established that miRNAs are involved in
the progression of multiple types of cancer via negatively
regulating the protein expression of various target genes (13,14).
However, few studies have investigated the deregulation of miRNAs
in nasopharyngeal carcinoma. The present study found that miR-214
was upregulated, while Bax was downregulated in nasopharyngeal
carcinoma tissues and cell lines. Further investigation suggested
that miR-214 promoted proliferation and inhibited apoptosis in
nasopharyngeal carcinoma cells via directly inhibiting the protein
expression of Bax, a novel target of miR-214 identified in
nasopharyngeal carcinoma CNE1 cells.
Aberrant expression of miRNAs has been found to be
involved in the development and progression of human nasopharyngeal
carcinoma (15,16). The present study reported that the
expression level of miR-214 was notably increased in nasopharyngeal
carcinoma tissues and cell lines, when compared with their matched
normal adjacent tissues and normal nasopharyngeal epithelia cells.
Zhang et al also demonstrated that miR-214 was upregulated
in nasopharyngeal carcinoma tissues and cell lines (12). In addition, Deng et al
reported that miR-214 induced tumorigenesis in nasopharyngeal
carcinoma by directly targeting lactotransferrin (17). The present study also revealed that
miR-214 had an oncogenic role in nasopharyngeal carcinoma cells.
However, to the best of our knowledge, no other target of miR-214
has previously been identified in nasopharyngeal carcinoma
cells.
In the present study, it was found that Bax acted as
a downstream effector in miR-214-mediated cell proliferation and
apoptosis in nasopharyngeal carcinoma CNE1 cells. Bax belongs to
the B-cell lymphoma 2 (Bcl-2) protein family, the members of which
form hetero or homodimers and act as regulators of cell apoptosis.
Bax forms a heterodimer with Bcl-2 and promotes cell apoptosis
(18,19). In addition, Bax can increase the
opening of the mitochondrial voltage-dependent anion channel and
cause a loss in membrane potential, leading to the release of
cytochrome c, which is important in cell apoptosis (20). Furthermore the expression of Bax
has been demonstrated to be regulated by the tumor suppressor p53
(21). In addition, downregulation
of Bax has been demonstrated to be involved in the progression of
multiple types of cancer by promoting cancer cell survival
(22,23). Bax was also found to be involved in
nasopharyngeal carcinoma (24). Li
et al demonstrated that overexpression of Bax could induce
the apoptosis of nasopharyngeal carcinoma HNE1 cells and prevent
growth of implanted tumors (24).
Zhang et al reported that Bax inhibitor 1 protein (BI-1), a
novel inhibitor of Bax, could induce apoptosis-resistance in
nasopharyngeal carcinoma cells and inhibition of BI-1 decreased the
ratio of Bcl-X (L)/Bcl-2 with Bax protein and increased the
activity of caspase-3, thus leading to a significant increase in
nasopharyngeal carcinoma cell apoptosis (23). In the present study, an inverse
correlation between miR-214 and Bax expression was found in the
nasopharyngeal carcinoma tissues and cell lines. The data also
demonstrated that Bax was negatively regulated by miR-214 at the
post-transcriptional level, via a specific target site within the
3′-UTR. Furthermore, miR-214 was demonstrated to promote cell
proliferation and inhibit cell apoptosis through directly targeting
Bax in nasopharyngeal carcinoma cells.
The association between miR-214 and Bax has been
reported in hippocampal neurons (25,26).
Yan et al found that isoflurane increased vulnerability to
intracellular or extracellular amyloid β, which was mediated by the
downregulation of miR-214 that leads to an upregulation of Bax
(25). Zhang et al also
demonstrated that halothane induced neuronal cell death
vulnerability by inhibiting miR-214 and upregulating Bax (26). However, to the best of our
knowledge, their association in cancer has never been reported. Bax
has been demonstrated to be a target of other miRNAs. For instance,
miR-128 was found to target Bax and induce apoptosis in human
embryonic kidney cells (27). Li
et al demonstrated that miR-886-5p could inhibit cervical
carcinoma cell apoptosis via targeting Bax (28). In addition, Ji et al found
that inhibition of miR-128 sensitized breast cancer cells to
chemodrugs by targeting Bax (29).
These data suggest that deregulation of Bax by miRNAs is a key
mechanism underlying cancer progression.
In conclusion, the present study suggests that the
oncogenic role of miR-214 in the regulation of nasopharyngeal
carcinoma cell proliferation and apoptosis involves its inhibitory
effect on the protein expression of Bax. Thus, miR-214 may be a
potential therapeutic target for nasopharyngeal carcinoma.
Acknowledgments
This study was supported by the Fundamental Research
Funds for the Central Universities of Central South University
(grant no. 2014zzts082).
References
|
1
|
Xu T, Tang J, Gu M, Liu L, Wei W and Yang
H: Recurrent nasopharyngeal carcinoma: a clinical dilemma and
challenge. Curr Oncol. 20:e406–e419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braccini AL, Haberer-Guillerm S, Azria D,
et al: Radioanatomy of rhinopharyngeal carcinoma. Cancer Radiother.
17:715–723. 2013.In French. View Article : Google Scholar
|
|
3
|
Wright CM, Dan T, Dicker AP and Simone NL:
microRNAs: The short link between cancer and RT-induced DNA damage
response. Front Oncol. 4:1332014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen HC, Chen GH, Chen YH, et al: MicroRNA
deregulation and pathway alterations in nasopharyngeal carcinoma.
Br J Cancer. 100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu CX, Xu M, Tan L, et al: MicroRNA
miR-214 regulates ovarian cancer cell stemness by targeting
p53/Nanog. J Biol Chem. 287:34970–34978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Zhao C, Shi H, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penna E, Orso F, Cimino D, et al: miR-214
coordinates melanoma progression by upregulating ALCAM through
TFAP2 and miR-148b downmodulation. Cancer Res. 73:4098–4111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Derfoul A, Juan AH, Difilippantonio MJ,
Palanisamy N, Ried T and Sartorelli V: Decreased microRNA-214
levels in breast cancer cells coincides with increased cell
proliferation, invasion and accumulation of the Polycomb Ezh2
methyltransferase. Carcinogenesis. 32:1607–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Li J, Wang X, Zheng C and Ma W:
Downregulation of microRNA-214 and overexpression of FGFR-1
contribute to hepatocellular carcinoma metastasis. Biochem Biophys
Res Commun. 439:47–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ZC, Li YY, Wang HY, et al: Knockdown
of miR-214 promotes apoptosis and inhibits cell proliferation in
nasopharyngeal carcinoma. PLoS One. 9:e861492014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JT, Wang J, Srivastava V, Sen S and
Liu SM: MicroRNA machinery genes as novel biomarkers for cancer.
Front Oncol. 4:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He ML, Luo MX, Lin MC and Kung HF:
MicroRNAs: potential diagnostic markers and therapeutic targets for
EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta.
1825:1–10. 2012.
|
|
16
|
Yu BL, Peng XH, Zhao FP, et al:
MicroRNA-378 functions as an onco-miR in nasopharyngeal carcinoma
by repressing TOB2 expression. Int J Oncol. 44:1215–1222.
2014.PubMed/NCBI
|
|
17
|
Deng M, Ye Q, Qin Z, et al: miR-214
promotes tumorigenesis by targeting lactotransferrin in
nasopharyngeal carcinoma. Tumour Biol. 34:1793–1800. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar
|
|
19
|
Renault TT and Manon S: Bax: Addressed to
kill. Biochimie. 93:1379–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumarswamy R and Chandna S: Putative
partners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or
none of them? Mitochondrion. 9:1–8. 2009. View Article : Google Scholar
|
|
21
|
Wu X and Deng Y: Bax and BH3-domain-only
proteins in p53-mediated apoptosis. Front Biosci. 7:d151–d156.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Li X, Zhang Y and Zhou K: Bax
inhibitor-1 mediates apoptosis-resistance in human nasopharyngeal
carcinoma cells. Mol Cell Biochem. 333:1–7. 2010. View Article : Google Scholar
|
|
24
|
Li H, Xie M, Xu G and Li Y: Effect of
transduction bax gene on experimental nasopharyngeal carcinoma.
Zhonghua Er Bi Yan Hou Ke Za Zhi. 36:430–432. 2001.In Chinese.
|
|
25
|
Yan H, Xu T, Zhao H, Lee KC, Wang HY and
Zhang Y: Isoflurane increases neuronal cell death vulnerability by
downregulating miR-214. PLoS One. 8:e552762013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L and Zhang Y: Halothane increases
neuronal cell death vulnerability by downregulating miR-214 and
upregulating Bax. Int J Clin Exp Med. 6:452–460. 2013.PubMed/NCBI
|
|
27
|
Adlakha YK and Saini N: MicroRNA-128
downregulates Bax and induces apoptosis in human embryonic kidney
cells. Cell Mol Life Sci. 68:1415–1428. 2011. View Article : Google Scholar
|
|
28
|
Li JH, Xiao X, Zhang YN, et al: MicroRNA
miR-886-5p inhibits apoptosis by down-regulating Bax expression in
human cervical carcinoma cells. Gynecol Oncol. 120:145–151. 2011.
View Article : Google Scholar
|
|
29
|
Ji S, Shao G, Lv X, et al: Downregulation
of miRNA-128 sensitises breast cancer cell to chemodrugs by
targeting Bax. Cell Biol Int. 37:653–658. 2013. View Article : Google Scholar : PubMed/NCBI
|