Introduction
Osteosarcoma (OS) is the most common mesenchymal
sarcoma with high morbidity, mainly arising from the metaphysis of
the long bones of adolescents and young adults (1). Despite tumor excision combin ed with
chemotherapy and radiotherapy, the five-year survival rate of
patients with recurrent or metastatic OS has remained as low as
~30% (1). As aberrant upregulation
of oncogenes is closely associated with the progression of OS, the
identification of novel oncogenes is crucial for developing
effective therapeutic targets for OS (2).
Galectin-3, a member of the galectin family, is an
endogenous β-galactoside-binding lectin. It has been well
established that galectin-3 has a role in the regulation of cell
recognition, adhesion, chemoattraction, proliferation, apoptosis,
cell cycle, differentiation, immunomodulation and angiogenesis
(3,4). Accumulating evidence has demonstrated
that galectin-3 participates in cancer aggressiveness and is
closely associated with tumor cell transformation, migration,
invasion and metastasis (5–7).
Recently, galectin-3 was found to be associated with the
progression of OS; Zhou et al (8) reported that the serum levels of
galectin-3 were markedly elevated in patients with OS when compared
with those in healthy controls, and that increased serum levels of
galectin-3 were significantly associated with the stage of OS.
Furthermore, galectin-3 was upregulated in OS tissues compared to
that in non-malignant tissues, and its expression in OS tissues was
correlated with the OS stage and metastasis (8). These findings suggested that
galectin-3 may act as an oncogene in OS. However, the exact role of
galectin-3 in the regulation of OS cells has remained to be
determined.
The present study aimed to explore the role of
galectin-3 in the regulation of OS cell proliferation, apoptosis,
migration and invasion. In addition, the underlying molecular
mechanism was investigated.
Materials and methods
Agents
RPMI 1640 medium, fetal bovine serum (FBS),
Lipofectamine 2000, TRIzol reagent, First Strand cDNA Synthesis kit
(cat. no. K1612), DyNAmo ColorFlash SYBR Green qPCR assay kit (cat.
no. F-416L) and gentian violet were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). MTT was purchased from Biosharp
(Hefei, Anhui, China). Monoclonal mouse anti-human galectin-3 (cat.
no. ab2785; 1:200; incubation, 3 h at room temperature), monoclonal
mouse anti-human matrix metalloproteinase (MMP)2 (cat. no. ab86607;
1:200; incubation, 3 h at room temperature), monoclonal mouse
anti-human MMP9 (cat. no. ab119906; 1:50; incubation, 3 h at room
temperature), polyclonal rabbit anti-human phosphorylated
extracellular signal-regulated kinase (p-ERK; cat. no. ab131438;
1:200; incubation, 3 h at room temperature), monoclonal mouse
anti-human ERK (cat. no. ab119933; 1:100; incubation, 3 h at room
temperature) and monoclonal mouse anti-GAPDH (cat. no. ab8245;
1:50; incubation, 3 h at room temperature) antibodies, as well as
rabbit anti-mouse immunoglobulin G (IgG; cat. no. ab46540;
incubation, 40 min at room temperature) and mouse anti-rabbit IgG
(cat. no. ab99700; incubation, 40 min at room temperature)
secondary antibody were purchased from Abcam (Cambridge, UK). The
washing steps between all antibody treatments were as follows:
Three washes with Dulbecco's phosphate buffered saline (DPBS), each
for 5 min. A SuperSignal West Pico Chemiluminescent Substrate kit
(cat. no. 34080) was purchased from Pierce (Rockford, IL, USA).
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(cat. no. 556547) was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). A Transwell chamber was obtained from Corning,
Inc. (Corning, NY, USA).
Tissue specimens
The present study was approved by the Ethics
Committee of Central South University (Changsha, China). Written
informed consent was obtained from each patient. Fifteen primary OS
samples and their normal matched adjacent tissues were collected at
the Department of Orthopedics, Xiangya Hospital of Central South
University (Changsha, China). Tissues were immediately snap-frozen
in liquid nitrogen after surgical removal.
Cell culture
The human OS cell line Saos-2, MG63 and U2OS, and
the human osteoblast cell line hFOB1.19 were obtained from the Cell
Bank of Central South University (Changsha, China). Cells were
cultured in RPMI 1640 medium supplemented with 10% FBS at 37°C in a
humidified incubator containing 5% CO2.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from tissues or cells using
TRIzol reagent in accordance with the manufacturer's instructions.
Total RNA was reverse-transcribed into cDNA by using a First Strand
cDNA Synthesis kit, in accordance with the manufacturer's
instructions. Expression of mRNA was examined using the SYBR green
qPCR assay kit, in accordance with the manufacturer's instructions.
The specific primer pairs (Shanghai Shenggong Co., Ltd., Shanghai,
China) were as follows: Galectin-3 sense,
5′-GTGAAGCCCAATGCAAACAGA-3′ and anti-sense,
5′-AGCGTGGGTTAAAGTGGAAGG-3′; GAPDH (internal reference) sense,
5′-CTGGGCTACACTGAGCACC-3′ and anti-sense,
5′-AAGTGGTCGTTGAGGGCAATG-3′. The PCR was conducted using using an
ABI 7500 thermocycler (Invitrogen Life Technologies) with the
following cycling conditions: 95°C for 10 min, 40 cycles of
denaturation at 95°C for 15 sec, and an annealing/elongation step
at 60°C for 1 min. The experiments were independently repeated
three times. The PCR products were separated by SDS-PAGE, and
analyzed using SDS Relative Quantification Software 2.2.2 (Applied
Biosystems Life Technologies, Carlsbad, CA, USA). The same baseline
and cycle threshold (CT) were set for each target. The relative
mRNA expression was analyzed using the 2−ΔΔCt method
(9).
Transfection
Lipofectamine 2000 was used to perform transfection
according to the manufacturer's instructions. Briefly, cells were
cultured to 70% confluence and re-suspended in serum-free medium.
Small interfering RNA (siRNA) specific for galectin-3 (Nlunbio,
Changsha, China) and Lipofectamine 2000 were diluted, mixed and
incubated for 20 min at room temperature, followed by addition to
the cell suspension. After incubation at 37°C for 6 h, the medium
was replaced with normal serum-containing medium. Cells were then
cultured for 24 h prior to being subjected to the following assays.
The MG63 cells transfected with galectin-3 siRNA were treated with
10 mM curcumin for 3 h.
Western blot analysis
Tissues or cells were solubilized in cold
radioimmunoprecipitation assaylysis buffer (Sigma-Aldrich, St.
Louis, MO, USA). Proteins were separated by 12% SDS-PAGE (Nlunbio)
and transferred onto a polyvinylidene difluoride (PVDF) membrane.
The PVDF membrane (Invitrogen Life Technologies) was then incubated
with Tris-buffered saline containing Tween 20 (Sigma-Aldrich)
containing 5% skimmed milk at room temperature for 3 h and then
incubated with mouse anti-galectin-3, MMP2, MMP9, p-ERK, ERK and
GAPDH primary antibodies, respectively, at room temperature for 3
h. After incubation with the rabbit anti-mouse secondary antibodies
at room temperature for 40 min, ECL detection was performed using
an ECL kit and an ECL detection apparatus with an X-ray exposure
cartridge (MitoScience, Eugene, OR, USA) and film (Kodak, Tokyo,
Japan). Relative protein expression was analyzed using Image-Pro
plus software 6.0 (Media Cybernetics, Rockville, MD, USA), and
expressed as the density ratio vs. GAPDH.
Cell proliferation assay
The MTT assay was used to assess cell proliferation.
The cells in each group were cultured in 96-well plates at a
density of 1×105 cells/well, at 37°C for 0, 24, 48 and
72 h. A total of 100 µl fresh serum-free medium supplemented
with 0.5 g/l MTT was subsequently added to each well, and incubated
at 37°C for 4 h. The medium was then removed by aspiration, and 50
µl dimethylsulfoxide (Sigma-Aldrich) was added to each well.
After incubation at 37°C for a further 10 min, the absorbance at
570 nm of each sample was measured using a PHERAstar FS microplate
reader (Invitrogen Life Technologies).
Wound healing assay
A wound healing assay was performed to evaluate the
cell migratory capacity. In brief, cells were cultured to full
confluence. Wounds of ~1 mm width were created with a pipette tip
(Gilson, Inc., Middleton, WI, USA), and cells were washed and
incubated in serum-free medium. After incubation for 24 h in
serum-free medium, cells were incubated in medium containing 10%
fetal bovine serum. Cultures at 0 and 48 h were observed under a
CX22 microscope (Olympus, Tokyo, Japan).
Cell invasion assay
The invasive ability of OS cells was determined in
24-well plates. Transwell chambers containing a layer of Matrigel.
A cell suspension (density, 1×106)was added in the upper
chamber, and RPMI 1640 containing 10% FBS was added into the lower
chamber. After incubation for 24 h, non-invading cells as well as
the Matrigel on the interior of the inserts was removed using a
cotton-tipped swab. Invasive cells on the lower surface of the
membrane were stained with gentian violet, rinsed with water and
air-dried. Five fields were randomly selected and the cell number
was counted under a CX22 microscope.
Apoptosis analysis
The levels of cell apoptosis were determined using
an Annexin V-FITC Apoptosis Detection kit (BD Biosciences)
according to the manufacturer's instructions, and analyzed using a
C6 flow cytometer (BD Biosciences). A total of 24 h
post-transfection, the cells were harvested and washed twice with
cold PBS. Subsequently, 1×106 cells were resuspended in
200 µl binding buffer supplemented with 10 µl
Annexin-V-FITC and 5 µl propidium iodide-PE, and incubated
in the dark for 30 min. Finally, 300 µl binding buffer was
added, and the cells were analyzed by flow cytometry.
Statistical analysis
Values are expressed as the mean ± standard
deviation. One-way analysis of variance was used to statistically
analyze data. SPSS 17 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analyses. P<0.05 was considered to indicate
a statistically significant difference between values.
Results
Galectin-3 is overexpressed in OS tissues
and cell lines
To explore the role of galectin-3 in OS, the mRNA
and protein expression of galectin-3 in OS tissues as well as their
matched normal adjacent tissues was determined by RT-qPCR and
western blot analyses. As shown in Fig. 1A and B, the mRNA and protein
expression levels of galectin-3 were frequently upregulated in OS
tissues when compared with those in normal adjacent tissues.
Furthermore, the mRNA and protein expression of galectin-3 were
assessed in the three OS cell lines Saos-2, MG63 and U2OS, as well
as in the human osteoblast cell line hFOB1.19. As shown in Fig. 1C and D, the mRNA and protein
expression of galectin-3 in OS cells was higher than that in
hFOB1.19 cells (P<0.01). Furthermore, MG63 cells showed the
highest expression of galectin-3 amongst all cell lines tested.
Accordingly, this cell line was used in the subsequent experiments
of the present study.
Galectin-3 knockdown inhibits OS cell
proliferation
To investigate the role of galectin-3 in the
regulation of OS-cell proliferation, MG63 cells were transfected
with galectin-3 siRNA. To confirm knockdown of galectin, the mRNA
and protein expression of galectin-3 in MG63 cells was then
assessed. As shown in Fig. 2A and
B, transfection with galectin-3 siRNA significantly inhibited
the mRNA and protein expression of galectin-3 in MG63 cells
(P<0.01). Subsequently, an MTT assay was performed to determine
the effect of galectin-3 silencing on cell proliferation. As shown
in Fig. 2C, after transfection
with galectin-3 siRNA, the cell proliferation was significantly
downregulated compared to that in the control group (P<0.01),
suggesting that siRNA-induced knockdown of galectin-3 inhibited
OS-cell proliferation.
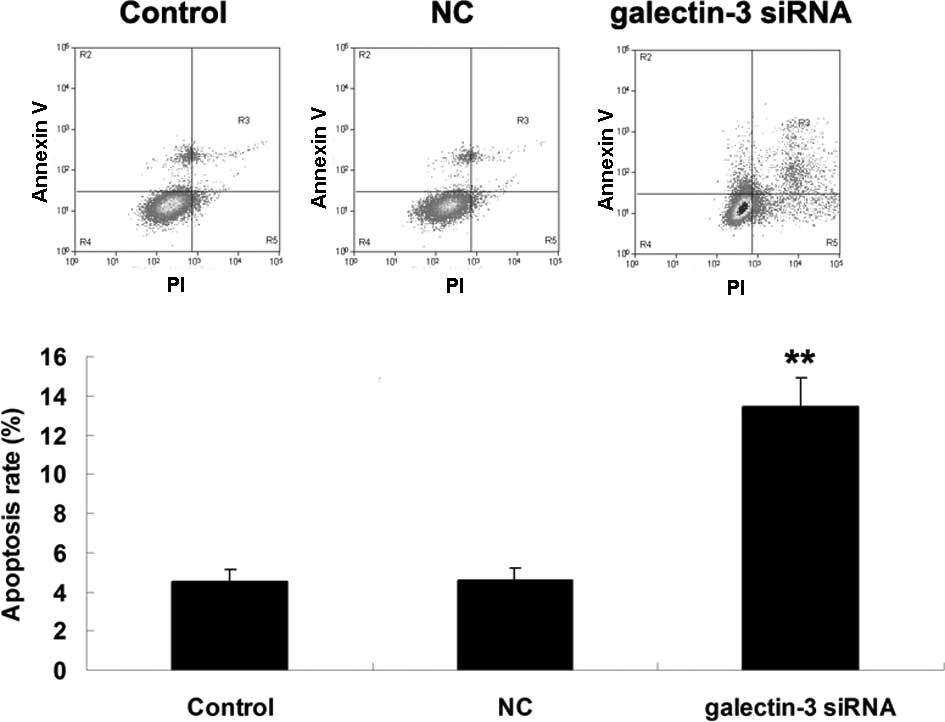
Knockdown of galectin-3 enhances
apoptosis of MG63 cells
The effect of siRNA-induced downregulation of
galectin-3 on MG63-cell apoptosis was assessed. As shown in
Fig. 3, the apoptotic rate of MG63
cells was markedly upregulated after transfection with
galectin-3-specific siRNA (P<0.01), suggesting that
siRNA-induced galectin-3 downregulation promoted OS-cell
apoptosis.
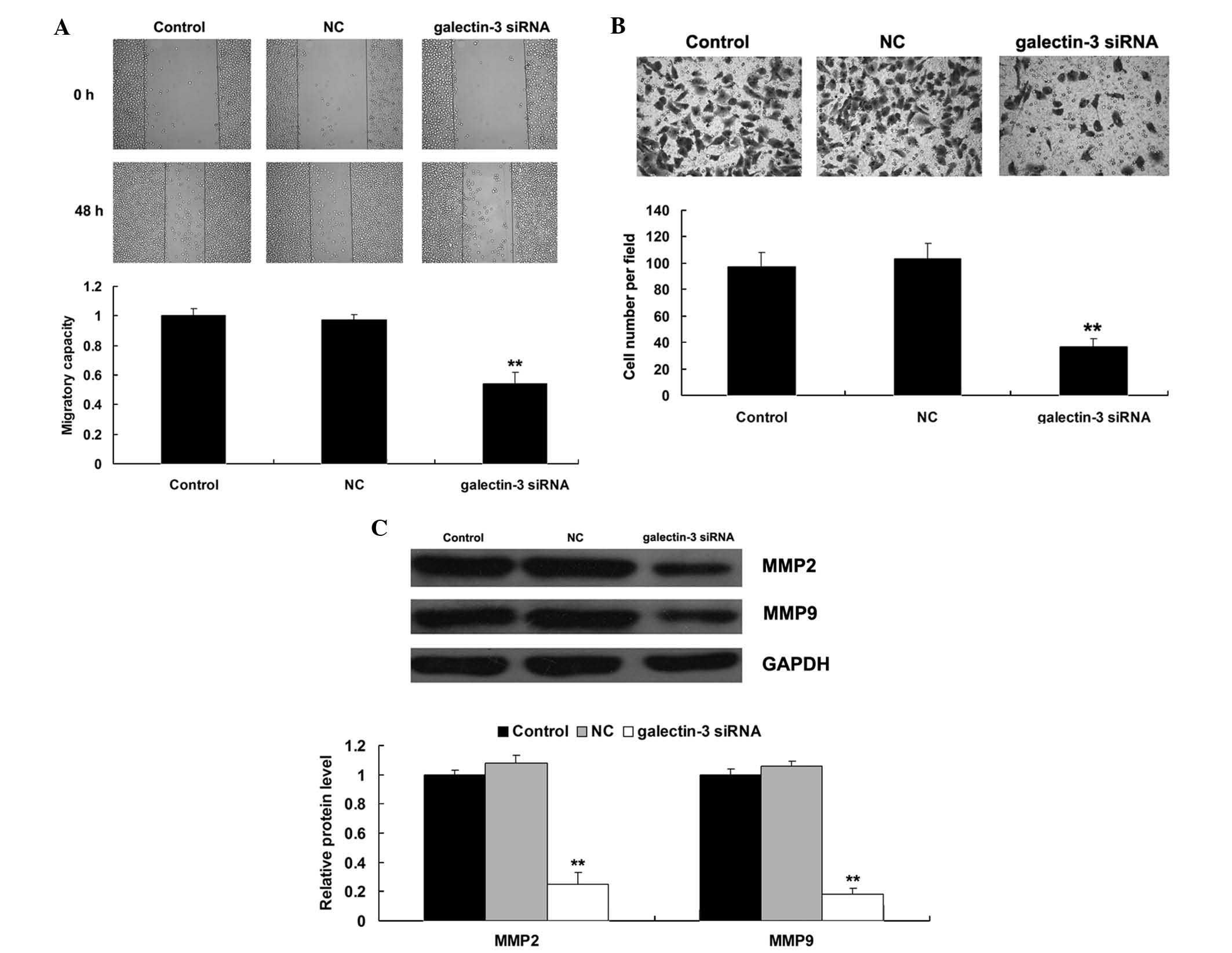
Knockdown of galectin-3 suppresses
migration and invasion of MG63 cells
The present study then investigated the effect of
siRNA-induced downregulation of galectin-3 on the migration and
invasion of MG63 cells. As shown in Fig. 4A and B, the cell migration and
invasion were significantly decreased in MG63 cells transfected
with galectin-3 siRNA compared to those in the control group
(P<0.01), suggesting that galectin-3 has a promoting role in the
regulation of OS-cell migration and invasion. As MMP2 and MMP9 are
two crucial regulators involved in tumor cell migration and
invasion (10), the present study
then examined the protein levels of MMP2 and MMP9 in MG63 cells
with or without galectin-3 knockdown. As shown in Fig. 4C, siRNA-induced galectin-3
knockdown led to a marked decrease in MMP2 and MMP9 expression in
MG63 cells (P<0.01).
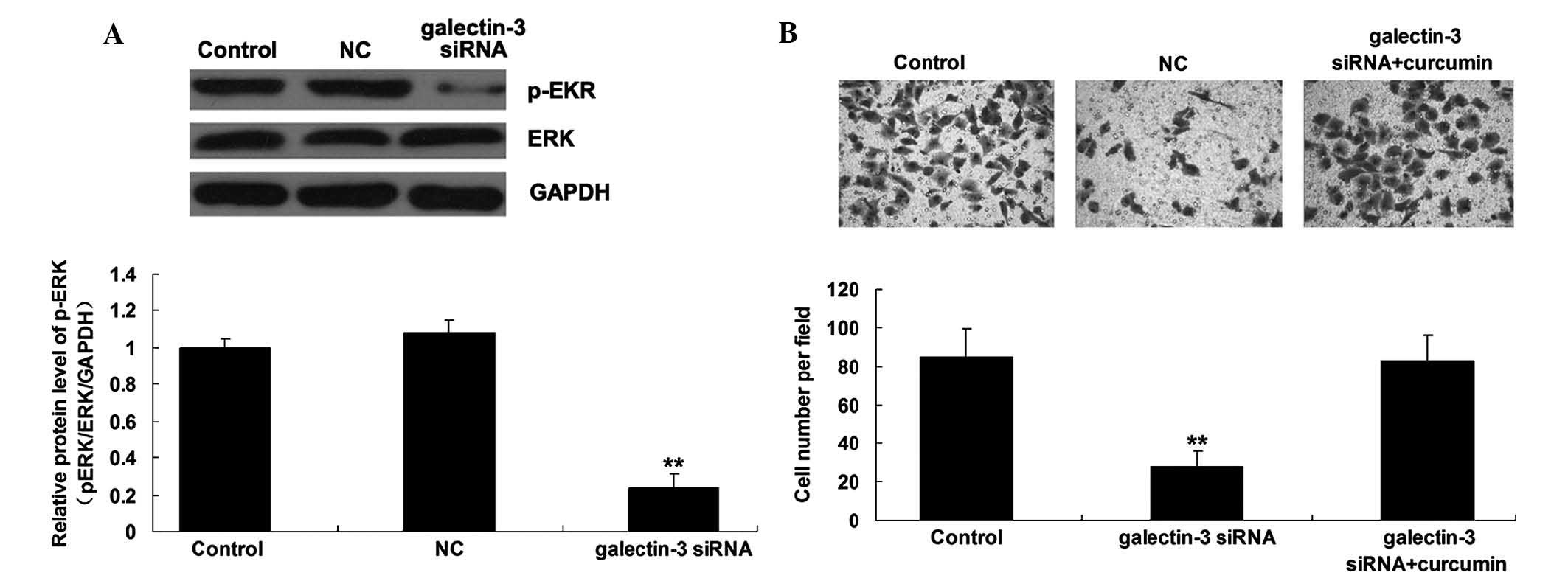
The mitogen-activated protein kinase
kinase (MEK)/ERK signaling pathway is involved in the
galectin-3-mediated invasiveness of OS cells
The present study further investigated the activity
of MEK/ERK pathway-associated signaling in MG63 cells with or
without transfection with galectin-3 siRNA. As shown in Fig. 5A, downregulation of galectin-3
markedly inhibited the activity of MEK/ERK signaling (P<0.01),
suggesting that the MEK/ERK signaling pathway may act as a
downstream effector of the malignant properties of MG63 cells. As
the MEK/ERK signaling pathway has been demonstrated to be involved
in the regulation of OS-cell invasion (11,12),
the present study used curcumin, an agonist of MEK, to upregulate
the activity of the MEK/ERK signaling pathway. As shown in Fig. 5B, the suppressive effect of
galectin-3 knockdown on MG63 cell invasion was markedly reversed by
treatment with curcumin (P<0.01), suggesting that the MEK/ERK
signaling pathway is involved in the galectin-3-mediated OS cell
invasion.
Discussion
As most OS have been shown to display a marked
alternation of their gene expression profile compared with that of
normal osteoblasts, understanding of the de-regulation of these
oncogenes or tumor suppressors may aid in the development of
effective therapeutic strategies for OS (13). The present study reported that
galectin-3 was significantly upregulated in OS tissues and cells.
In addition, the present study suggested that galectin-3 acts as an
oncogene in OS cells and that the MEK/ERK signaling pathway is
involved in galectin-3-mediated OS-cell invasion.
Galectin-3, a versatile 29-35 kDa protein, is the
only chimera galectin found in vertebrates. Galectin-3 has been
found to participate in several biological processes, including
cell adhesion (3), cell activation
and chemoattraction (3), growth
and differentiation (14), and
apoptosis and cell cycle progression (15). Accumulating evidence has suggested
that galectin-3 has a role in multiple types of cancer (16), including gastric carcinoma
(17), parathyroid cancer
(18), multiple myeloma (19), ovarian cancer (19) and OS (8). Furthermore, galectin-3 has emerged as
a useful biomarker in the diagnosis and/or prognosis of certain
malignancies (20). However, the
detailed role of galectin-3 in the regulation of cellular
biological processes of OS cells has remained to be fully
elucidated. The present study showed that the expression of
galectin-3 was significantly increased in OS tissues when compared
with that in normal adjacent tissues. In addition, galectin-3 was
also upregulated in three OS cell lines compared to a human
osteoblast cell line. These results were consistent with those of a
previous study, which reported that the serum levels as well as the
tissue expression levels of galectin-3 were increased in patients
with OS compared to those in healthy controls (8). These findings suggested that
galectin-3 is involved in the development and progression of
OS.
To further determine whether galectin-3 is relevant
to OS progression, galectin-3-specific siRNA was used to knockdown
the expression of galectin-3 in OS cells. It was found that
silencing of galectin-3 expression significantly suppressed OS-cell
proliferation and promoted OS-cell apoptosis. Similar findings have
been reported in other studies; Zheng et al (21) demonstrated that downregulation of
galectin-3 inhibited the proliferation of hepatocellular carcinoma
cells. Furthermore, Huang et al (22) found that inhibition of galectin-3
expression by siRNA suppressed cell proliferation and induced cell
apoptosis of pituitary tumor cells. In addition, galectin-3 was
found to be involved in the regulation of cell cycle progression;
Wang et al (23) showed
that galectin-3 was involved in the regulation of prostate cancer
cell growth and apoptosis by modulating the expression of p21, an
important regulator in cell cycle progression. Furthermore,
galectin-3 may be involved in the regulation of cyclin-D, which
regulates the G1-to-S phase transition, in non-small cell lung
cancer cells (24). Accordingly,
it is hypothesized that the inhibition of cell proliferation caused
by galectin-3 knockdown observed in the present study may due to
cell cycle arrest.
It has been well established that galectin-3
participates in the regulation of tumor-cell migration and
invasion. For instance, Zhang et al (25) reported that silencing of galectin-3
inhibited migration and invasion of human tongue cancer cells via
inhibition of β-catenin. However, to the best of our knowledge, the
effect of galectin-3 on OS-cell migration and invasion has never
been studied. The present study showed that siRNA-induced
galectin-3 inhibition markedly inhibited OS-cell migration and
invasion; furthermore, MMP2 and MMP9 were also downregulated after
silencing of galectin-3 in OS cells. In addition, several studies
have shown that MEK/ERK signaling is involved in
galectin-3-mediated cell invasion. Song et al (26) reported that inhibition of
galectin-3 downregulated the activity of Ras, as well as its
downstream ERK signaling. Curcumin is an agonist of MEK, which can
further activate ERK. Curcumin was used in the present study to
determine whether galectin-3 exerted its effects on OS cell
invasion via MEK signaling. However, it remains unclear whether
curcumin exhibits anti-carcinogenic properties. The results
demonstrated that pre-treatment with curcumin effectively reversed
the suppressive effect of galectin-3 knockdown on OS-cell invasion,
suggesting that MEK/ERK signaling participates in
galectin-3-mediated OS-cell invasion.
In conclusion, the present study showed that the
expression of galectin-3 was significantly increased in OS tissues
and cell lines. siRNA-induced inhibition of galectin-3
significantly inhibited proliferation, migration and invasion, and
induced apoptosis of OS cells. In addition, MEK/ERK signaling was
found to be involved in the galectin-mediated OS-cell invasion.
These results suggested that galectin-3 may be a promising target
for the treatment of OS.
References
|
1
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92:288–290. 2013.PubMed/NCBI
|
|
2
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fortuna-Costa A, Gomes AM, Kozlowski EO,
Stelling MP and Pavão MS: Extracellular galectin-3 in tumor
progression and metastasis. Front Oncol. 4:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viguier M, Advedissian T, Delacour D,
Poirier F and Deshayes F: Galectins in epithelial functions. Tissue
Barriers. 2:e291032014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laderach DJ, Gentilini L, Jaworski FM and
Compagno D: Galectins as new prognostic markers and potential
therapeutic targets for advanced prostate cancers. Prostate Cancer.
2013:5194362013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisa NH, Ebrahim MA, Ragab M, Eissa LA and
El-Gayar AM: Galectin-3 and matrix metalloproteinase-9: Perspective
in management of hepatocellular carcinoma. J Oncol Pharm Pract.
2014.Epub ahead of print. PubMed/NCBI
|
|
7
|
von Klot CA, Kramer MW, Peters I,
Hennenlotter J, Abbas M, Scherer R, Herrmann TR, Stenzl A, Kuczyk
MA, Serth J and Merseburger AS: Galectin-1 and Galectin-3 mRNA
expression in renal cell carcinoma. BMC Clin Pathol. 14:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou X, Jing J, Peng J, Mao W, Zheng Y,
Wang D, Wang X, Liu Z and Zhang X: Expression and clinical
significance of galectin-3 in osteosarcoma. Gene. 546:403–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou F, Wang L, Wang H, Gu J, Li M, Zhang
J, Ling X, Gao X and Luo C: Elevated gene expression of S100A12 is
correlated with the predominant clinical inflammatory factors in
patients with bacterial pneumonia. Mol Med Rep. 11:4345–4352.
2015.PubMed/NCBI
|
|
10
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar
|
|
11
|
Miao JH, Wang SQ, Zhang MH, Yu FB, Zhang
L, Yu ZX and Kuang Y: Knockdown of galectin-1 suppresses the growth
and invasion of osteosarcoma cells through inhibition of the
MAPK/ERK pathway. Oncol Rep. 32:1497–1504. 2014.PubMed/NCBI
|
|
12
|
Tsubaki M, Satou T, Itoh T, Imano M, Ogaki
M, Yanae M and Nishida S: Reduction of metastasis, cell invasion
and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced
blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol Appl
Pharmacol. 259:402–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diao CY, Guo HB, Ouyang YR, Zhang HC, Liu
LH, Bu J, Wang ZH and Xiao T: Screening for metastatic osteosarcoma
biomarkers with a DNA microarray. Asian Pac J Cancer Prev.
15:1817–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao P, Simpson JL, Zhang J and Gibson PG:
Galectin-3: Its role in asthma and potential as an
anti-inflammatory target. Respir Res. 14:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundblad V, Croci DO and Rabinovich GA:
Regulated expression of galectin-3, a multifunctional
glycan-binding protein, in haematopoietic and non-haematopoietic
tissues. Histol Histopathol. 26:247–265. 2011.
|
|
16
|
Funasaka T, Raz A and Nangia-Makker P:
Galectin-3 in angio-genesis and metastasis. Glycobiology.
24:886–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leal MF, Calcagno DQ, Chung J, de Freitas
VM, Demachki S, Assumpção PP, Chammas R, Burbano RR and Smith MC:
Deregulated expression of annexin-A2 and galectin-3 is associated
with metastasis in gastric cancer patients. Clin Exp Med. 2014.Epub
ahead of print.
|
|
18
|
Truran PP, Johnson SJ, Bliss RD, Lennard
TW and Aspinall SR: Parafibromin, galectin-3, PGP9.5, Ki67 and
cyclin D1: Using an immunohistochemical panel to aid in the
diagnosis of parathyroid cancer. World J Surg. 38:2845–2854. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirandola L, Nguyen DD, Rahman RL, Grizzi
F, Yuefei Y, Figueroa JA, Jenkins MR, Cobos E and Chiriva-Internati
M: Anti-Galectin-3 therapy: A new chance for multiple myeloma and
ovarian cancer? Int Rev Immunol. 33:417–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van den Brûle F, Califice S and Castronovo
V: Expression of galectins in cancer: A critical review. Glycoconj
J. 19:537–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng D, Hu Z, He F, Gao C, Xu L, Zou H,
Wu Z, Jiang X and Wang J: Downregulation of galectin-3 causes a
decrease in uPAR levels and inhibits the proliferation, migration
and invasion of hepatocellular carcinoma cells. Oncol Rep.
32:411–418. 2014.PubMed/NCBI
|
|
22
|
Huang CX, Zhao JN, Zou WH, Li JJ, Wang PC,
Liu CH and Wang YB: Reduction of galectin-3 expression reduces
pituitary tumor cell progression. Genet Mol Res. 13:6892–6898.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Balan V, Kho D, Hogan V,
Nangia-Makker P and Raz A: Galectin-3 regulates p21 stability in
human prostate cancer cells. Oncogene. 32:5058–5065. 2013.
View Article : Google Scholar
|
|
24
|
Kosacka M, Piesiak P, Kowal A, Golecki M
and Jankowska R: Galectin-3 and cyclin D1 expression in non-small
cell lung cancer. J Exp Clin Cancer Res. 30:1012011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D, Chen ZG, Liu SH, Dong ZQ, Dalin
M, Bao SS, Hu YW and Wei FC: Galectin-3 gene silencing inhibits
migration and invasion of human tongue cancer cells in vitro via
downregulating β-catenin. Acta Pharmacol Sin. 34:176–184. 2013.
View Article : Google Scholar
|
|
26
|
Song S, Ji B, Ramachandran V, Wang H,
Hafley M, Logsdon C and Bresalier RS: Overexpressed galectin-3 in
pancreatic cancer induces cell proliferation and invasion by
binding Ras and activating Ras signaling. PLoS One. 7:e426992012.
View Article : Google Scholar : PubMed/NCBI
|