Introduction
The brain is an organ with a high metabolic
activity, and the normal functioning of neurons relies on a
constant energy supply to the brain. Neurons are highly dependent
on the mitochondrial oxidative system (OXPHOS) to produce adenosine
triphosphate (ATP) (1). The
structure, function and synapses of the neurons in the visual
cortex change depending on environmental factors, a phenomenon know
as experience-dependent plasticity. A previous study demonstrated
that mitochondrial dysfunction may be an important factor in the
inhibition of cortical neurons in visual disorders (2). In recent years, research on the
mechanisms underlying visual disorders, such as amblyopia, and on
the visual cortex have made significant progress. The mechanisms
underlying the signal transduction pathways of the visual cortex
are currently under investigation (3). Thus far, the signaling mechanisms
underlying the neuronal activity responsible for energy coupling
remains to be elucidated.
Nuclear respiratory factors (NRFs) are important
transcription factors that regulate mitochondrial oxidative
phosphorylation and regeneration (4). NRFs directly regulate mitochondrial
respiration via the transcriptional regulation of respiratory
enzyme chain subunit nuclear DNA (nDNA), and via the regulation of
mitochondrial transcription factors (mTF)A and B, and mitochondrial
DNA (mtDNA), thereby indirectly regulating the mitochondrial
respiratory enzyme chain subunit (5). A previous study demonstrated that
NRF-2 is involved in the mitochondrial energy coupling of visual
cortical neurons (6). The mRNA and
protein expression levels of NRF-2 were positively correlated with
neuronal excitability, and concordant with cytochrome coxidase
(COX) activity (6). In addition,
peroxisome proliferator-activated receptor γ coactivator 1α
(PGC-1α) is an important transcriptional coactivator, which is
involved in the mitochondrial synthesis of ATP. A previous study
demonstrated that PGC-1α is able to induce NRF gene expression,
thereby affecting OXPHOS expression (7). PGC-1α is able to promote the
transcription of NRFs genes, thereby changing mitochondrial
respiratory chain function (8).
However, the signaling mechanisms underlying the association
between PGC-1α and NRFs in the energy coupling process of visual
cortical neurons remain to be investigated.
Evidence from animal experiments and clinical
observations indicates that adenosine monophosphate-activated
protein kinase (AMPK) is an important effector in the regulation of
the energy metabolism of nervous system microenvironments (9,10).
AMPK is an important regulator of mitochondrial and cell energy
metabolism, and is associated with PGC-1α (11). In addition, AMPK may be an
important upstream signaling-activated molecule of the NRF
transduction pathway. However, the role of AMPK in visual cortical
neurons has yet to be elucidated. The present study hypothesized
that the AMPK signaling pathway may have an important role in the
energy coupling of visual cortical neurons.
The present study investigated the effects of
neuronal activity on AMPK and its association with PGC-1α, NRF-2α,
and mtTFA expression, as well as the role of mitochondria in a
primary neuron culture system. The present study aimed to determine
whether AMPK is involved in regulating the expression of NRF-2α and
PGC-1α in primary visual cortical nerouns.
Materials and methods
Primary culture of visual cortical
neurons
The present study was approved by the ethics
committee of the Chinese PLA General Hospital (Beijing, China; no.
2012045). One day-old Sprague-Dawley rat pups (Experimental Animal
Center, Chinese PLA General Hospital) were sacrificed by
decapitation under CO2 anesthesia (10% chloral hydrate;
Sigma-Aldrich, St. Louis, MO, USA; 0.3 ml/100 g; i.p.). The
meninges were subsequently removed. The primary visual cortex (V1,
area 17) was dissected under a Zeiss Axio Examiner microscope
(Zeiss, Oberkochen, Germany). The tissue samples were then
incubated for 10 min in trypsin/EDTA [0.05/0.02%, w/v in
phosphate-buffered saline (PBS)] (Sigma-Aldrich) at 37°C, and
triturated in order to cause the dissociation of individual cells.
The cells were plated onto 30-mm plastic dishes coated with
poly-L-lysine (Sigma-Aldrich) at a density of 5×105
cells/ml, in Dulbecco's modified Eagle's medium (Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (GE Healthcare Life Sciences, Logan, UT, USA) for 3 h. The
liquid medium was carefully discarded and replaced with a
neurobasal medium (Gibco Life Technologies) supplemented with B27
solution (Gibco Life Technologies) and 0.5 mM L-glutamine
(Sigma-Aldrich), and cultured for 24 h. A total of 2.5 μM
cytosine arabinoside (Sigma-Aldrich) was then added to the cultures
to inhibit the replication of non-neuronal cells. The cultures were
kept in cultivating medium at 37°C in an atmosphere containing 5%
CO2 for 5–6 days. The purity of the cultured neurons was
>95%.
Membrane depolarization was conducted as previously
described (12), and 25 mM KCl was
added to the visual cortical neurons at days 5–6 of culture.
PGC-1α, NRF-2α, mtTFA and AMPK detection was performed after 2, 4,
6, 8 and 12 h. To assess the effects of the various treatments, the
neuron cultures were pretreated with either 1 mM AMPK activator
5-aminoimidazole-4-carboxamide riboside (AICAR) (Sigma-Aldrich) or
50 μM resveratrol (Sigma-Aldrich) for 60 min. In order to
conduct the inhibitory experiments, the neurons were pretreated
with a final concentration of 10 μM AMPK antagonist compound
C (Sigma-Aldrich) for 30 min, prior to the depolarization
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the neurons using
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA,
USA), and DRR037S (Takara Biotechnology, Co., Ltd, Dalian, China)
was used for reverse transcription according to the manufacturer's
instructions. The RT-qPCR reaction was performed using a
Mastercycler Gradient PCR system (Eppendorf, Hamburg, Germany). The
PCR cycling conditions were as follows: Pre-denaturation at 94°C
for 3 min, followed by 30 cycles of denaturation at 95°C for 2 sec,
annealing at the appropriate temperature (NRF-2α, 57°C; PGC-1α,
58°C; mTFA, 56°C; 18S, 60°C) for 15 sec, extension at 72°C for 10
sec; and a final extension step at 72°C for 7 min.
The following primers were used: PGC-1α forward,
5′-AGTGTGCTGCTCTGGTTGGTG-3′, and reverse, 5′-GGA
GGGTCATCGTTTGTGGTC-3′; NRF-2α forward, 5′-AGG TGACGAGATGGGCTGC-3′,
and reverse, 5′-CGTTGTCCC CATTTTTGCG-3′; mtTFA forward,
5′-GAAAGCACAAAT CAAGAGGAG-3′, and reverse, 5′-CTGCTTTTCATCATG
AGACAG-3′; AMPK forward, 5′-ATGCAGCTTCGCAATC GAT-3′, and reverse,
5′-GACAGCCAGCCACTCTGGTT-3′; and acetyl-coenzyme A carboxylase (ACC)
forward, 5′-AGGAGGGAAGGGAATCAGAA-3′, and reverse,
5′-TGTGCTGCAGGAAGATTGAC-3′. The RT-qPCR reaction used 18S rRNA to
normalize the data (forward, 5′-CGGCTACCACATCCAAGGAA-3′, and
reverse, 5′-GCTGGAATTACCGCGGCT-3′). The PCR products were then
separated by 1% agarose gel electrophoresis, at 100 V for 30 min,
and the results were analyzed using a Gel Imaging system (Media
Cybernetics, Inc., Rockville, MD, USA). The values were normalized
to 18S rRNA.
AMPK detection
The following conditions were used at various time
points prior to KCl depolarization: 1 h prior to experimentation,
the neurons were treated with a final concentration of 1 mM AMPK
activator AICAR or 50 μM resveratrol, and 0.5 h prior to KCl
depolarization, a final concentration of 10 μM AMPK
inhibitor compound C was added to the cultured cells, and the
AMPK-induced changes in the visual cortical neurons were observed
in vitro by RT-qPCR and luminometer.
Measurement of mitochondrial ATP
content
The cultured cells were washed with cold PBS (pH
7.4), incubated in somatic cell ATP-releasing reagent
(Sigma-Aldrich) for 5 min, and harvested using a cell scraper. ATP
was used at a 1:100 dilution in order to conduct the ATP detection
of working fluid. A total of 100 μl ATP detection reagent
(Sigma-Aldrich) was added to the harvested cells, and light
emission was determined using a BioSpectrometer luminometer
(Eppendorf, Hamburg, Germany). The data were compared using an ATP
standard curve to calculate the total ATP concentration of each
sample.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean from three independent experiments, each repeated in
duplicate. Statistically significant differences between the
experimental and control groups were deter mined using unpaired
Student's t-test, and one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
mRNA expression levels of PGC-1α, NRF-2α
and mtTFA, and the ATP content in mitochondria
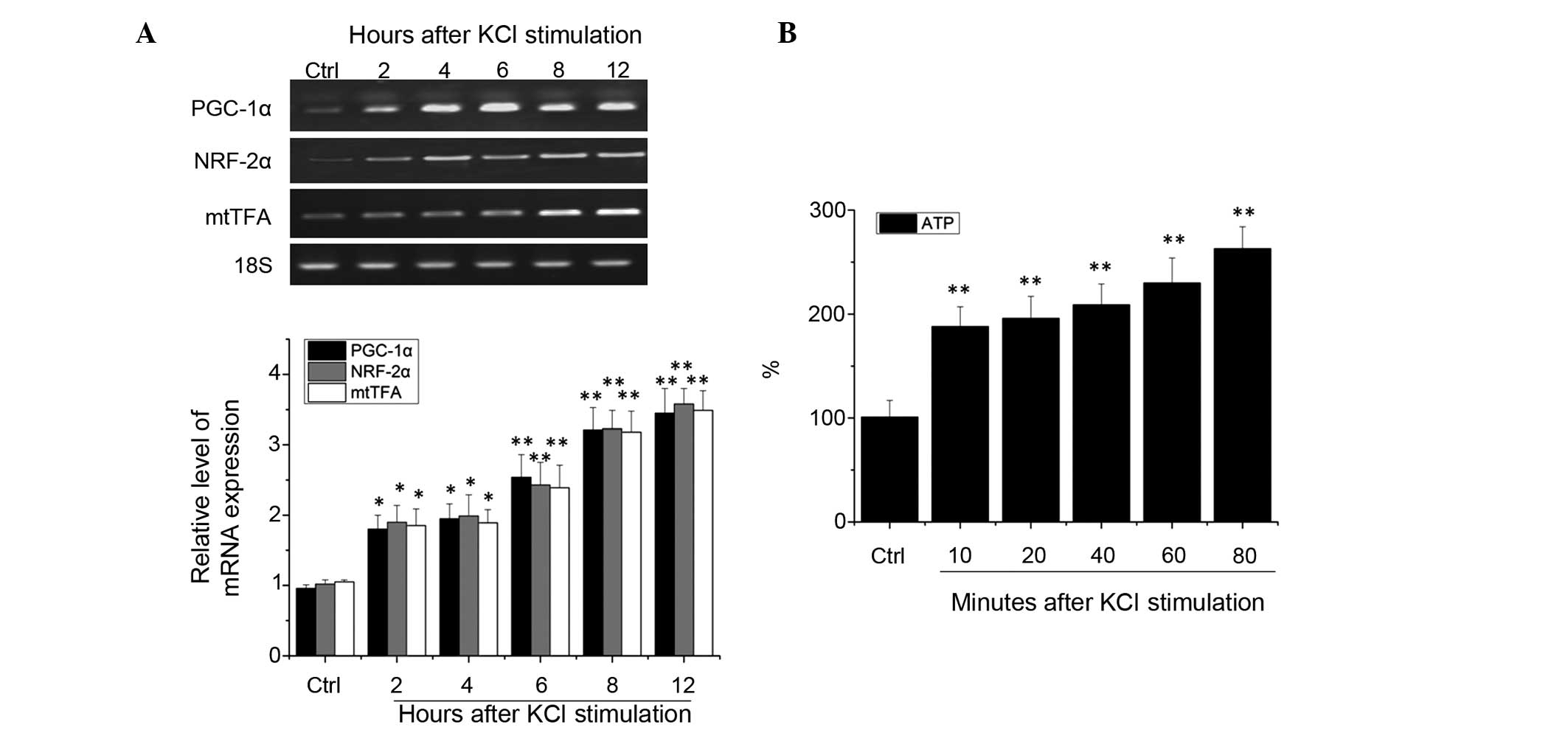
RT-qPCR analysis demonstrated that, as compared with
the control group, 2 h after depolarization, the mRNA expression
levels of PGC-1α, NRF-2α and mtTFA increased significantly in the
rat cortical neurons (P<0.05). After 4 h, the expression levels
remained elevated in a time-dependent manner (P<0.01), with a
peak after 12 h (P<0.01; Fig.
1A).
These experiments suggest that neuronal excitability
of the visual cortex upregulates mitochondrial transcription
factors PGC-1α and NRF-2α mRNA. To directly observe the association
between the neurons in the visual cortex in the excited and
metabolic states, the intracellular levels of ATP content in
excited visual cortical neurons were measured. Following
depolarization for 10 min, the levels of ATP in the cultured
neurons increased significantly (P<0.01). After 20–60 min, the
levels of ATP in the cells continued to rise, and after 80 min of
stimulation the elevated ATP levels were maintained, as compared
with the control group (P<0.01; Fig. 1B). These results suggest that
neuronal excitability in the visual cortex is able to increase the
intracellular levels of ATP, and may regulate energy coupling.
mRNA expression levels of AMPK and
ACC
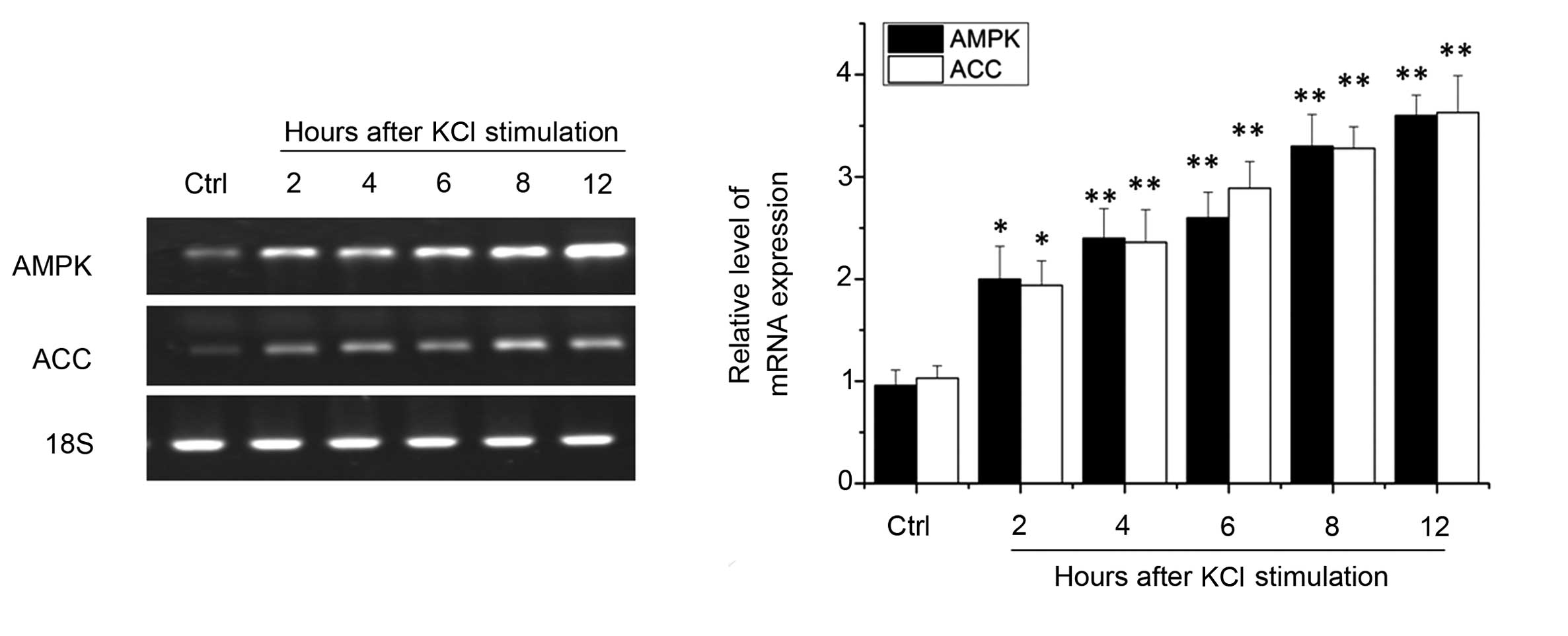
Due to the important role of AMPK in neuronal energy
metabolism, the present study investigated the mRNA expression
levels of AMPK and ACC in the various treatment groups. RT-qPCR
analysis revealed that, following 2 h of depolarization, the mRNA
expression levels of AMPK were significantly elevated (P<0.05).
Similar results were observed in the mRNA expression levels of AMPK
kinase downstream component ACC (P<0.05), suggesting that
neurons are able to rapidly activate AMPK protein kinase activity.
After 4–8 h, the mRNA expression levels of AMPK and ACC continued
to increase (P<0.01), and after 12 h this increase in expression
was maintained (P<0.01) (Fig.
2). These results suggest that visual cortical neurons are also
able to activate AMPK kinase by excitation.
mRNA expression levels of PGC-1α, NRF-2α
and mtTFA, and ATP content in mitochondria following pretreatment
with compound C
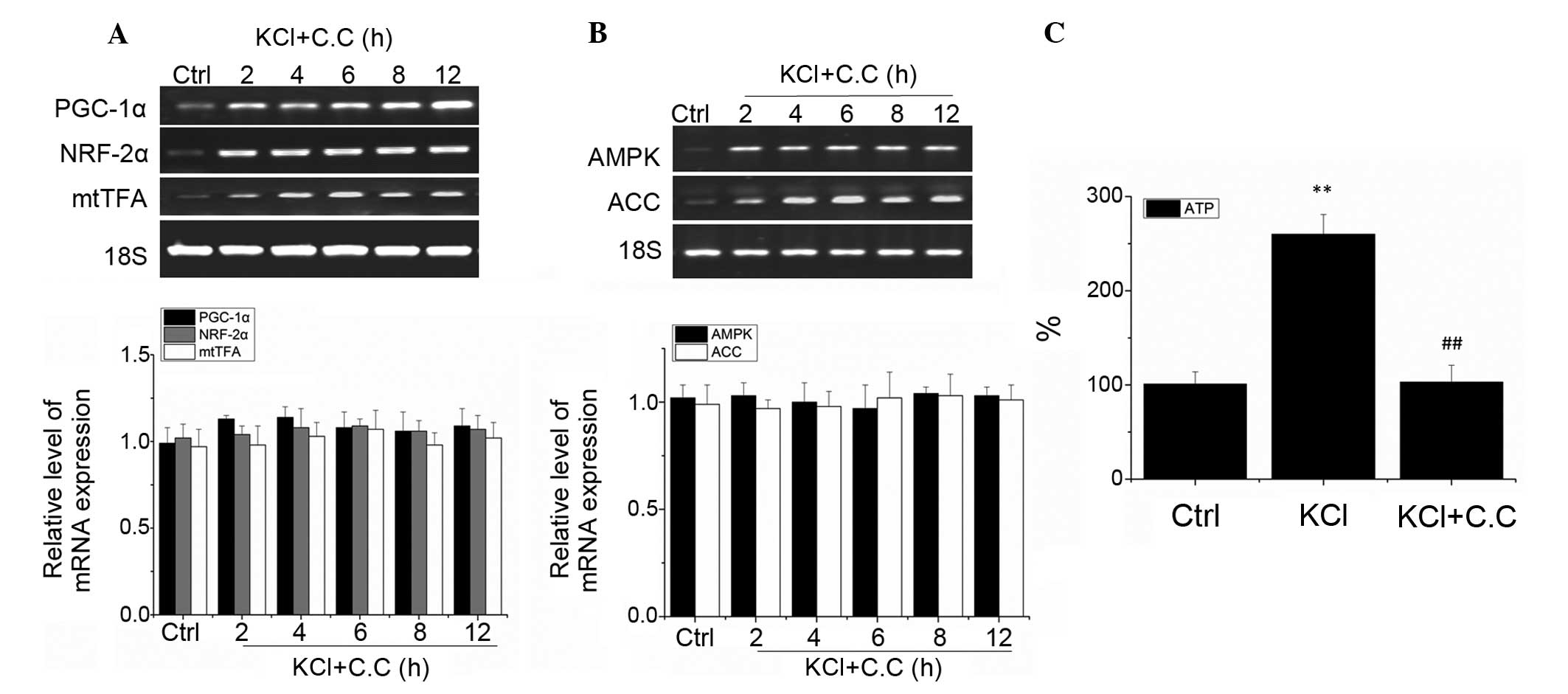
To investigate whether AMPK is involved in PGC-1α,
NRF-2α and mtTFA excitation-dependent regulation in visual cortical
neurons, the neurons were pretreated for 30 min with the AMPK
antagonist compound C (10 μM), prior to being treated with
25 mM KCl in order to induce depolarization. RT-qPCR analysis
demonstrated that no significant changes in the mRNA expression
levels of PGC-1α, NRF-2α and mtTFA occurred following KCl treatment
for 2, 4, 6, 8 and 12 h, as compared with the control group
(P>0.05; Fig. 3A). These
results suggest that the AMPK antagonist compound C inhibited the
depolarization-induced upregulation of PGC-1α, NRF-2α, and mtTFA
mRNA. Furthermore, no significant changes in the mRNA expression
levels of AMPK and ACC were observed (P>0.05; Fig. 3B). These results suggest that in
visual cortical neurons, the upregulation of PGC-1α, NRF-2α and
mtTFA are regulated by AMPK-induced excitation.
The measurement of the levels of ATP after 5 h of
KCl treatment revealed that the intracellular ATP levels in visual
cortical neurons are significantly upregulated, as compared with
the control group (P<0.01). Furthermore, pretreatment with AMPK
antagonist compound C was able to completely block the upregulation
of ATP in rat visual cortical neurons (P>0.05 as compared with
the control group, and P<0.01 as compared with the KCl group;
Fig. 3C). These results suggest
that AMPK is an important regulator of energy excitation coupling
in visual cortical neurons.
mRNA expression levels of PGC-1α, NRF-2α
and mtTFA, and the levels of ATP mitochondria treated with AICAR
and resveratrol
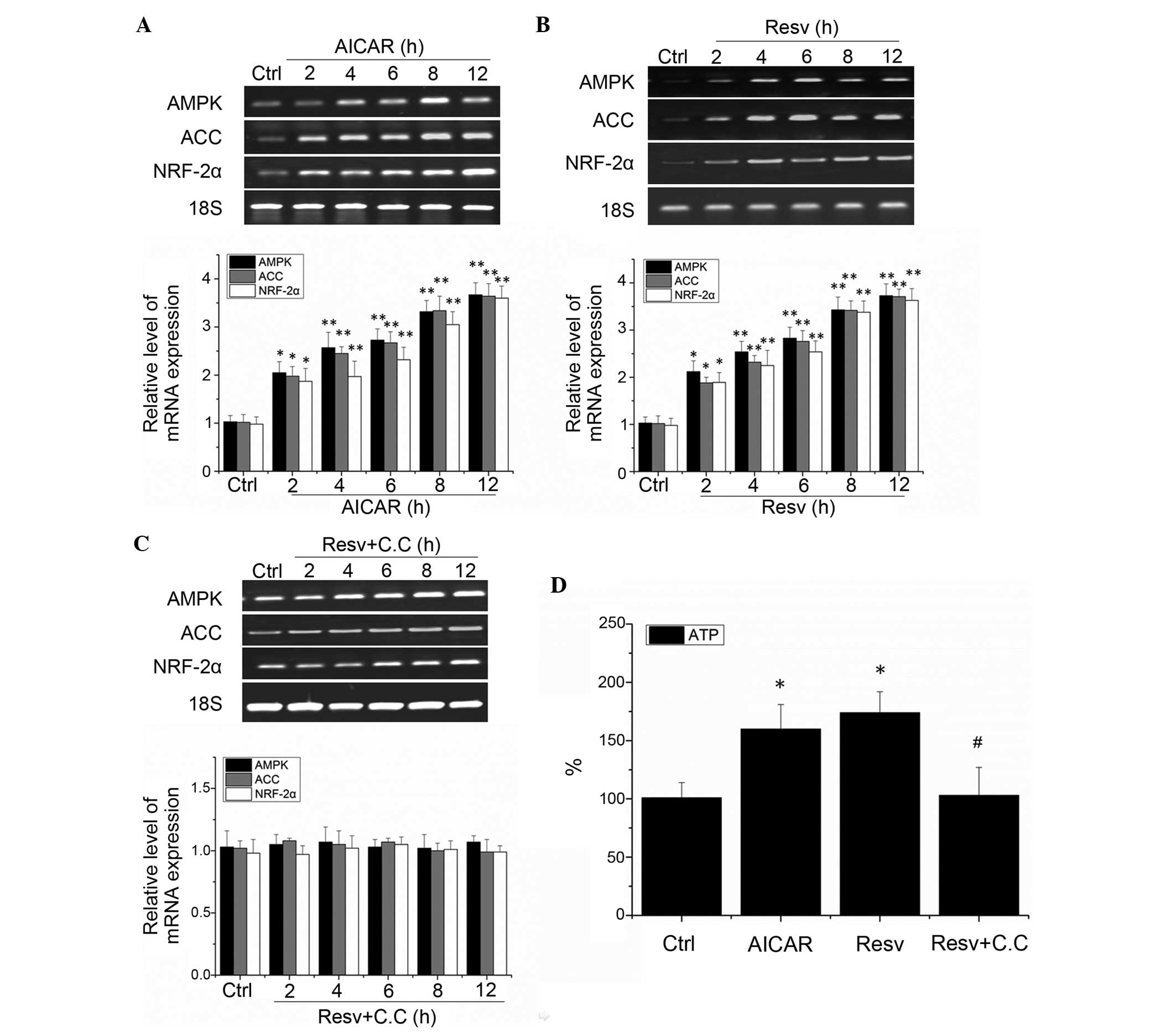
The results of the present study demonstrated that
AMPK is an important regulator of visual cortical neuron excitation
energy coupling, and may mediate visual cortical neuron NRF-2α
excitation-dependent regulation. To further investigate the
association between AMPK and NRF-2α, the effects of the AMPK
agonist AICAR and resveratrol on the expression levels of NRF-2α in
visual cortical neurons were observed. As compared with the control
group, treatment with 1 mM AICAR for 2 h (Fig. 4A) or 20 μM resveratrol for 2
h (Fig. 4B) significantly
increased the mRNA expression levels of AMPK, ACC and NRF-2α in the
neurons (P<0.01). These results suggest that the activity levels
of AMPK are regulated by AICAR and resveratrol. Furthermore, the
neuronal excitation induced by resveratrol was inhibited by the
AMPK antagonist compound C (10 μM), and the activation of
NRF-2α mRNA expression was also inhibited (Fig. 4C). These results suggest that the
regulatory effects of resveratrol on the activation of NRF-2α in
neurons is dependent on AMPK activation. Therefore, activation of
AMPK is able to effectively regulate the NRF-2α expression in
cortical neurons.
To investigate the effects of AMPK on visual
cortical neurons, the present study examined neuronal ATP levels.
Treatment with 1 mM AICAR and 20 μM resveratrol
significantly increased neuronal ATP levels (P<0.01).
Furthermore, pretreatment with the AMPK antagonist compound C was
able to inhibit resveratrol-induced upregulation of ATP compared
(P>0.005, as compared with the control group, and P<0.01, as
compared with the resveratrol group; Fig. 4D). These results indicate that
activation of AMPK is able to adjust the energy levels in visual
cortical neurons.
Discussion
Energy metabolism is an important source of life,
and the central nervous system consumes a large amount of energy.
The brain accounts for only 2% of the total body weight, but
consumes 20% of its total glucose (13). Little energy is stored within the
central nervous system, and a balance between energy supply and
consumption is maintained. The appropriate energy balance
regulation of such a high energy consuming and low energy storage
system is essential (14).
The role of visual cortical neurons is largely
dependent on the energy provided by the ATP produced by the
oxidation of glucose. The high energy demand of the central nervous
system derives from two processes: The basic process of nurturing
(such as the maintenance of cellular membrane integrity and protein
synthesis), and physiological function (such as the electrical
activity of neurons). The majority of the visual cortical neuron
energy expenditure is utilized to maintain visual stimulation.
Plasticity of the neuronal energy metabolism and
neuronal excitability are tightly coupled processes. Neurons are
highly dependent on OXPHOS to produce ATP (15). For visual cortical neurons, the
neuronal excitation energy coupling mechanism is particularly
important. Due to the fact that visual cortex plasticity depends on
visual experience, the role of normal visual stimulus-induced
neuronal excitability in maintaining neuronal physiological
function in the visual cortex is of great significance (16). Research conducted in the primate
animal model, and clinical observations of amblyopia demonstrated
that mitochondrial biogenesis and dysfunction are important factors
in optic atrophy, amblyopia, and other visual pathway diseases.
These results suggested that mitochondrial dysfunction is an
important factor in visual deprivation and visual functional
changes in cortical neurons, and the maintenance the mitochondrial
energy balance may be important for the correct functioning of
cortical neurons (17).
Visual stimulation and experience are important
factors in the development of visual cortical neurons. The
composition, function, and synaptic transmission and distribution
of neurons in the visual cortex vary according to visual
environment and experience, a process termed experience-dependent
plasticity of visual development (18). Inhibition of the input of visual
information negatively affects the development of the visual
cortex. In addition, energy metabolism may regulate visual cortical
neuron activity levels (19), with
altered gene expression resulting in changes in neuronal
plasticity. These neuronal plasticity changes, including
mitochondrial dysfunction, in visual cortical neurons are a central
mechanism underlying visual deprivation-induced amblyopia (20).
OXPHOS takes place in the mitochondria (21). The process of OXPHOS is highly
dependent on NRFs (22). The NRF
family includes NRF-1 and NRF-2. The main function of NRFs is to
regulate the expression of respiratory chain and mitochondrial RNA
endonuclease and mtTFA. NRF-2 consists of two subunits, NRF-2α and
NRF-2β. The primary function of NRF-2α is the regulation of genes
associated with the mitochondrial energy metabolism. The functional
binding sites of NRF-2α include a COX subunit promoter, ATP
synthase β, mtTFA, and mtTFB (23). NRF-2α regulates the activity levels
of the COX gene in the brain, when the energy demand of the cells
change (6). Notably, NRF-2α has a
role in the regulation of cellular respiratory function (24).
mtTFA and mtTFB, encoded by nDNA, are transcription
factors that regulate the transcription and replication of mtDNA
(25). mtTFA regulates
transcriptional activity, which also depends on the synergistic
effects of NRF-2α. COX is the rate limiting enzyme in the
production of ATP, and is the only molecule able to transfer
electrons to cytochrome c (26). The formation of COX is dependent on
the coordinated function of mtDNA and nDNA. NRF-2α is involved in
this important and complex process. The expression of COX is
directly regulated by NRF-2α. In addition, NRF-2α is able to
activate mtTFA and mtTFB in an indirect manner, in order to
regulate transcription.
PGC-1α is an important coregulator of mitochondrial
hyperplasia. One of the primary functions of PGC-1α is to promote
the transcription of NRF-1 and NRF-2, and to coordinate the
function of the mitochondrial respiratory chain in order to meet
the requirements of the various cellular energy states (27). These effects may be further
promoted by the expression of OXPHOS components, such as mtTFA,
mtTFB, and COX II and IV (11).
The results of the present study demonstrated that
in response to KCl depolarization, visual cortical neurons
upregulate PGC-1α, NRF-2α and mtTFA expression. This in turn
enhanced the ability of mitochondria to produce ATP. Additionally,
the results suggested that the PGC-1α, NRF-2α and mtTFA signaling
pathways are associated with neuronal activity in rat visual
cortical neurons.
Compared with a previous study (12), the expression levels of NRF-2α
increased later than those of NRF-1. This may suggest that in the
visual cortex, NRFs have a different pattern of response.
AMPK is a widely distributed serine/threonine
protein kinase in eukaryotic cells. It is activated when cellular
energy is insufficient, promotes the oxidation of fatty acids and
glucose transport, and increases intracellular ATP levels. In
addition, AMPK inhibits the synthesis of glycogen, fat, and
cholesterol and reduces the consumption of ATP (28).
AMPK has a multi-level role in neuronal energy
metabolism. Changes in the activity levels of AMPK in hypothalamic
neurons may regulate individual appetite and feeding behavior, in
order to control the energy balance via intake and consumption. An
AMPKα2 knockout mouse model demonstrated that sympathetic nervous
system AMPK is involved in the regulation of insulin sensitivity in
peripheral tissues (29).
Activation of AMPK in the brain protects against neuronal injury
induced by energy deprivation (30) and AMPK mutations may cause a broad
spectrum of neurodegenerative changes at the cellular level
(31). AMPK regulation is
sensitive to the ratio of intracellular adenosine mono-phosphate
and ATP; when the intracellular levels of ATP decrease, AMPK
rapidly activates the regulation of the intracellular energy
metabolism. Changes in intracellular Ca2+ concentration
may also activate AMPK. AMPK is an important factor in the
regulation of the energy balance of the nervous system. A previous
study demonstrated that AMPK is able to activate NRFs and change
the levels of excitation in mitochondrial synthesis (32).
KCl-induced membrane depolarization significantly
stimulated neuronal AMPK, and the expression of its downstream
kinase, ACC. These results suggest that AMPK is activated in
neurons by neuronal activity. Furthermore, the depolarization of
visual cortical neurons resulted in significant increases in
neuronal ATP levels. The association between neuronal excitability
and energy response was inhibited by the AMPK inhibitor compound C.
These results suggested that AMPK has an important role in visual
cortical neuron energy coupling. Using the AMPK agonist AICAR to
activate AMPK resulted in significant upregulation of PGC-1α and
NRF-2α expression in visual cortical neurons. In addition, previous
studies have demonstrated that resveratrol is an effective
activator of AMPK (33–35). Similarly, the results of the
present study indicated that resveratrol is able to activate AMPK
kinase activity in visual cortical neurons, and the upregulation of
neuronal PGC-1α by resveratrol suggests a dependence on NRF-2α
expression levels. These effects were suppressed by the AMPK
inhibitor compound C. These results demonstrate that resveratrol
significantly increased the expression levels of PGC-1α and NRF-2α
in visual cortical neurons via the AMPK signaling pathway. The
activation of AMPK led to the upregulation of PGC-1α and NRF-2α
expression, and eventually to a neuronal energy response and a
significant increase in the intracellular levels of ATP.
In conclusion, the results of the present study
demonstrate that AMPK participates in the energy metabolism of
visual cortical neurons. In addition, AMPK is an important
transcriptional regulator of the neuronal energy excitation
response, which is mediated by the PGC-1α and NRF-2α signaling
pathway.
Acknowledgments
No financial support was received for the present
study.
References
|
1
|
Kann O and Kovács R: Mitochondria and
neuronal activity. Am J Physiol Cell Physiol. 292:C641–C657. 2007.
View Article : Google Scholar
|
|
2
|
Leruez S, Amati-Bonneau P, Verny C,
Reynier P, Procaccio V, Bonneau D and Milea D: Mitochondrial
dysfunction affecting visual pathways. Rev Neurol (Paris).
170:344–354. 2014. View Article : Google Scholar
|
|
3
|
Nisoli E, Clementi E, Moncada S and
Carruba MO: Mitochondrial biogenesis as a cellular signaling
framework. Biochem Pharmacol. 67:1–15. 2004. View Article : Google Scholar
|
|
4
|
Kuo JJ, Chang HH, Tsai TH and Lee TY:
Curcumin ameliorates mitochondrial dysfunction associated with
inhibition of gluconeogenesis in free fatty acid-mediated hepatic
lipoapoptosis. Int J Mol Med. 30:643–649. 2012.PubMed/NCBI
|
|
5
|
Shi Y, Dierckx A, Wanrooij PH, Wanrooij S,
Larsson NG, Wilhelmsson LM, Falkenberg M and Gustafsson CM:
Mammalian transcription factor A is a core component of the
mitochondrial transcription machinery. Proc Natl Acad Sci USA.
109:16510–16515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ongwijitwat S, Liang HL, Graboyes EM and
Wong-Riley MT: Nuclear respiratory factor 2 senses changing
cellular energy demands and its silencing down-regulates cytochrome
oxidase and other target gene mRNAs. Gene. 374:39–49. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li WY, Yao CX, Zhang SF, Wang SL, Wang TQ,
Xiong CJ, Li YB and Zang MX: Improvement of myocardial lipid
accumulation and prevention of PGC-1alpha induction by fenofibrate.
Mol Med Rep. 5:1396–1400. 2012.PubMed/NCBI
|
|
8
|
Hossain MB, Ji P, Anish R, Jacobson RH and
Takada S: Poly(ADP-ribose) polymerase 1 interacts with nuclear
respiratory factor 1 (NRF-1) and plays a role in NRF-1
transcriptional regulation. J Biol Chem. 284:8621–8632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manwani B and McCullough LD: Function of
the master energy regulator adenosine monophosphate-activated
protein kinase in stroke. J Neurosci Res. 91:1018–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rousset CI, Leiper FC, Kichev A, Gressens
P, Carling D, Hagberg H and Thornton C: A dual role for
AMP-activated protein kinase (AMPK) during neonatal
hypoxic-ischaemic brain injury in mice. J Neurochem. 133:242–252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scarpulla RC: Metabolic control of
mitochondrial biogenesis through the PGC-1 family regulatory
network. Biochim Biophys Acta. 1813:1269–1278. 2011. View Article : Google Scholar :
|
|
12
|
Yang SJ, Liang HL and Wong-Riley MT:
Activity-dependent transcriptional regulation of nuclear
respiratory factor-1 in cultured rat visual cortical neurons.
Neuroscience. 141:1181–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Q, Wang S, Li Y, Wang P, Li S, Guo Y
and Yao R: The role of the mitochondrial calcium uniporter in
cerebral ischemia/reperfusion injury in rats involves regulation of
mitochondrial energy metabolism. Mol Med Rep. 7:1073–1080.
2013.PubMed/NCBI
|
|
14
|
Rodriguez-Rodriguez P, Almeida A and
Bolaños JP: Brain energy metabolism in glutamate-receptor
activation and excitotoxicity: role for APC/C-Cdh1 in the balance
glycolysis/pentose phosphate pathway. Neurochem Int. 62:750–756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Breuer ME, Koopman WJ, Koene S, Nooteboom
M, Rodenburg RJ, Willems PH and Smeitink JA: The role of
mitochondrial OXPHOS dysfunction in the development of neurologic
diseases. Neurobiol Dis. 51:27–34. 2013. View Article : Google Scholar
|
|
16
|
Patterson CA, Duijnhouwer J, Wissig SC,
Krekelberg B and Kohn A: Similar adaptation effects in primary
visual cortex and area MT of the macaque monkey under matched
stimulus conditions. J Neurophysiol. 111:1203–1213. 2014.
View Article : Google Scholar :
|
|
17
|
Yu Wai Man CY, Chinnery PF and Griffiths
PG: Optic neuropathies - importance of spatial distribution of
mitochondria as well as function. Med Hypotheses. 65:1038–1042.
2005. View Article : Google Scholar
|
|
18
|
Sur M, Nagakura I, Chen N and Sugihara H:
Mechanisms of plasticity in the developing and adult visual cortex.
Prog Brain Res. 207:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chevrollier A, Guillet V, Loiseau D,
Gueguen N, de Crescenzo MA, Verny C, Ferre M, Dollfus H, Odent S,
Milea D, et al: Hereditary optic neuropathies share a common
mitochondrial coupling defect. Ann Neurol. 63:794–798. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nie F and Wong-Riley M: Nuclear
respiratory factor-2 subunit protein: Correlation with cytochrome
oxidase and regulation by functional activity in the monkey primary
visual cortex. J Comp Neurol. 404:310–320. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tao M, You CP, Zhao RR, Liu SJ, Zhang ZH,
Zhang C and Liu Y: Animal mitochondria: Evolution, function, and
disease. Curr Mol Med. 14:115–124. 2014. View Article : Google Scholar
|
|
22
|
Wong-Riley MT: Bigenomic regulation of
cytochrome c oxidase in neurons and the tight coupling between
neuronal activity and energy metabolism. Adv Exp Med Biol.
748:283–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escrivá H, Rodríguez-Peña A and Vallejo
CG: Expression of mitochondrial genes and of the transcription
factors involved in the biogenesis of mitochondria Tfam, NRF-1 and
NRF-2, in rat liver, testis and brain. Biochimie. 81:965–971. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Souza D, Lai RY, Suen M and Hood DA:
mRNA stability as a function of striated muscle oxidative capacity.
Am J Physiol Regul Integr Comp Physiol. 303:R408–R417. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girodet PO, Ozier A, Bara I, Tunon de Lara
JM, Marthan R and Berger P: Airway remodeling in asthma: New
mechanisms and potential for pharmacological intervention.
Pharmacol Ther. 130:325–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayall TP, Bjarnason I, Khoo UY, Peters TJ
and Macpherson AJ: Mitochondrial gene expression in small
intestinal epithelial cells. Biochem J. 308:665–671. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Venditti P, Bari A, Di Stefano L, Cardone
A, Della Ragione F, D'Esposito M and Di Meo S: Involvement of
PGC-1, NRF-1, and NRF-2 in metabolic response by rat liver to
hormonal and environmental signals. Mol Cell Endocrinol. 305:22–29.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams T, Courchet J, Viollet B, Brenman
JE and Polleux F: AMP-activated protein kinase (AMPK) activity is
not required for neuronal development but regulates axogenesis
during metabolic stress. Proc Natl Acad Sci USA. 108:5849–5854.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang P, Zhang RY, Song J, Guan YF, Xu TY,
Du H, Viollet B and Miao CY: Loss of AMP-activated protein
kinase-α2 impairs the insulin-sensitizing effect of calorie
restriction in skeletal muscle. Diabetes. 61:1051–1061. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klip A, Schertzer JD, Bilan PJ, Thong F
and Antonescu C: Regulation of glucose transporter 4 traffic by
energy deprivation from mitochondrial compromise. Acta Physiol
(Oxf). 196:27–35. 2009. View Article : Google Scholar
|
|
31
|
Garcia-Roves PM, Osler ME, Holmström MH
and Zierath JR: Gain-of-function R225Q mutation in AMP-activated
protein kinase gamma3 subunit increases mitochondrial biogenesis in
glycolytic skeletal muscle. J Biol Chem. 283:35724–35734. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan W, Zhang H, Liu P, Wang H, Liu J, Gao
C, Liu Y, Lian K, Yang L, Sun L, et al: Impaired mitochondrial
biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling
contributing to increased vulnerability in diabetic heart. Basic
Res Cardiol. 108:3292013. View Article : Google Scholar
|
|
33
|
Choi YJ, Suh HR, Yoon Y, Lee KJ, Kim DG,
Kim S and Lee BH: Protective effect of resveratrol derivatives on
high-fat diet induced fatty liver by activating AMP-activated
protein kinase. Arch Pharm Res. 37:1169–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang J, Chen LL, Xiao FX, Sun H, Ding HC
and Xiao H: Resveratrol improves non-alcoholic fatty liver disease
by activating AMP-activated protein kinase. Acta Pharmacol Sin.
29:698–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vingtdeux V, Giliberto L, Zhao H,
Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies
P and Marambaud P: AMP-activated protein kinase signaling
activation by resveratrol modulates amyloid-beta peptide
metabolism. J Biol Chem. 285:9100–9113. 2010. View Article : Google Scholar : PubMed/NCBI
|