Introduction
Segmental peripheral nerve defects are a clinical
problem that may occur following trauma or tumor resection, and the
nerve gap is usually too large to allow primary end-to-end nerve
coaptation in the absence of tension. Under these conditions,
reconstruction of the peripheral nerve gap with grafts is
necessary, in order to avoid the complete loss of motor function
and sensation of the affected area. For the past 100 years,
reconstruction of the peripheral nerve gap has been achieved with
autografts, as these offered the highest chance of functional
recovery (1). However, limited
amounts of donor nerve, as well as deficits in the donor area
following nerve graft harvest restrict the clinical application of
nerve gap reconstruction using autografts (2). Therefore, current research has
focused on identifying an alternative to autogenous nerve
grafts.
Allografts have been suggested as an effective
alternative to autogenous nerve grafts in the repair of nerve gaps,
due to their similar physical, chemical, and mechanical properties.
A previous study reported that processed allograft has similar
effects on nerve function recovery, as compared with autografts in
certain animal studies (3).
However, the limited number of donors is unable to meet the
clinical requirements of the increasing number of recipients and
commercial allogeneic nerve grafts are not yet available.
Therefore, xenografts are now being considered as substitutes to
allografts and autografts, due to the large number of donors.
Xenografts have limitations when used as transplants
to repair nerve gaps, with the most significant limitation being
immune rejection caused by donor antigens (4). Immune rejection may impair xenograft
nerve regeneration, in a similar manner to allografts (5). Two primary methods are used to
achieve graft tolerance, one of which is the treatment of
recipients with immunosuppressive drugs, such as FK506 or
cyclosporin A (6); and the other
is processing of the nerve prior to grafting in order to render it
less immunogenic (7). The systemic
administration of immunosuppressive drugs is necessary for whole
organ transplants, but is not an efficient solution for peripheral
nerve transplants due to the side effects caused by the medication,
which may be more severe than the disease itself (8). Therefore, current research has
focused on decreasing the antigenicity of xenogeneic nerves with a
series of treatments prior to grafting, in order to inhibit the
immune response.
A previous study reported that chemically extracted
nerves were able to support axon growth in both laboratory and
clinical settings, due to the absence of cells and myelin in the
nerves, which act as major donor antigens (9). In addition, chemically extracted
nerves may support axon growth by maintaining the structural
integrity of the basal lamina, which has an important role in
guiding regenerating axons toward their targets (10). However, the majority of studies
regarding acellular nerve grafts have been conducted on
allotransplantation, and little data are available on acellular
nerve grafts in xenotransplantation. Furthermore, before xenograft
acellular nerves may be used in clinical settings, further research
is required in order to identify appropriate animal donor species,
which includes the selection and matching of a suitable combination
of donor grafts and host species.
In the present study, chemically extracted rabbit
facial acellular nerve grafts were used to repair 1 cm rat facial
nerve defects, and the outcome of axonal regeneration and
functional restoration following nerve gap reconstruction was
observed morphologically and electrophysiologically. The present
study hypothesized that acellular nerve xenografts may exhibit
comparable histological and functional outcomes as that of
acellular nerve allografts in repairing segmental nerve
defects.
Materials and methods
Donor nerve harvest
New Zealand white rabbits (weight, 2.0–2.4 kg;
Center of Animal Experiments, Chinese PLA General Hospital,
Beijing, China) were anesthetized by intravenous injection of 3%
pentobarbital sodium (Sangon Biotech Co., Ltd., Shanghai, China)
and Wistar rats (weight, 200–220 g; Center of Animal Experiments
Chinese PLA General Hospital were anesthetized by intraperitoneal
injection of 10% chloral hydrate (Sangon Biotech Co., Ltd.), a
postauricular incision was made, and the facial nerve was exposed.
A length of 4–5 cm of the rabbit facial nerves were removed as
xenograft nerves, and a length of 2 cm of rat facial nerves were
removed as allograft nerves. All of the tissue specimens were
washed in a distilled water bath and cryopreserved.
Facial nerve acellularization
The acellular nerves were prepared via a chemical
extraction process as described in our previous study (11). The facial nerve segments from both
the rats and rabbits were washed in distilled water for 12 h, prior
to being immersed in 3% Triton X-100 solution (Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd., Beijing, China) for 12 h. The
nerves were then immersed in a distilled water bath for 3 h, and
transferred for a 12 h period into a solution of 4% sodium
deoxycholate in distilled water. These steps were then repeated.
Following a final wash in a distilled water bath for 3 h, the
extracted nerves were removed and placed in sterile
phosphate-buffered saline (pH 7.2), and stored at 4°C.
Experimental animals and study
design
A total of 18 Wistar rats (weight, 200–220 g) were
used. All animals were housed in a central animal care facility,
where temperatures ranged between 22 and 24°C and humidity levels
ranged between 50 and 60%, with 12-hour light-dark cycles and food
and water ad libitum. The rats were divided into three
groups (n=6): An acellular nerve allograft group, an acellular
nerve xenograft group and an autologous nerve graft group.
Surgical procedure
Following intraperitoneal injection of 10% chloral
hydrate (0.4 ml/100 mg; Sangon Biotech Co., Ltd.), a postauricular
incision was made on the right side of the face, and the parotid
gland and facial nerve were exposed. A segment of the buccal facial
nerve branch was resected leaving a 1 cm gap. In the allograft
group, the facial nerve gap was reconstructed using an acellular
facial nerve graft from another rat. In the xenograft group, the
facial nerve gap was reconstructed using an acellular facial nerve
graft from a rabbit. In the auto-graft group, an incision parallel
to the femur was made, and the peroneal nerve was exposed via a
gluteal muscle-splitting approach. Subsequently, the facial nerve
gap was reconstructed using an autogeneic peroneal nerve graft. The
donor nerves were trimmed to match the diameter and length of the
facial nerve of recipients prior to grafting. The nerve grafts were
anastomosed to the proximal and distal nerve stumps under an OPMI
6-SD microscope (Carl Zeiss, Oberkochen, Germany) using 9-0 nylon
sutures (Shandong Sinorgmed Co., Ltd., Heze, China). The incision
was sutured and disinfected using povidone iodine. All experimental
procedures were approved by the Ethics Committee of the Chinese PLA
general hospital.
Functional evaluation of nerve
regeneration
Electrophysiological examination was conducted on
the rats prior to sacrifice using an NDI-200P+ electroneurogram
device (Shanghai Haishen Medical Electronic Instrument Co., Ltd.,
Shanghai, China), in order to detect the extent of facial nerve
functional recovery. The rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (0.4 ml/100 mg), and the buccal
branches of the right facial nerves and corresponding
orbicularisoris muscles were exposed. Excitation electrodes were
placed on the proximal end of the nerve, and recording electrodes
were placed on the distal end of the graft. The proximal end was
then stimulated with square waves, the action potential caused by
the stimulus was recorded, and the nerve conduction velocity was
calculated.
Histological and morphological evaluation
of nerve regeneration
Histological staining
Hematoxylin and Eosin (HE; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) staining was performed to observe the
constitution of acellular nerves. Twelve weeks following the
surgical procedure, six animals in each group were anesthetized by
intraperitoneal injection of 10% chloral hydrate (0.4 ml/100 mg),
and the anastomosis site of the graft was exposed to allow
observation of nerve growth. The nerve graft was then harvested and
the distal part of the anatomosis site (2 mm) was fixed in 4%
paraformaldehyde for 24 h. Following dehydration using graded
ethanol baths, the tissue sections were fixed in paraffin, and
selected for histological and morphological analysis under random
high power fields. Masson's trichrome staining (Shanghai Seebio
Biotech Co., Ltd., Shanghai, China) was performed to observe nerve
fiber regeneration and cell growth. Semi-thin (2 μm)
sections were then cut from the tissue sections prior to staining
with toluidine blue (Shanghai Rongbo Biotech Co., Ltd., Shanghai,
China) in order to measure regenerating axon count, myelinated axon
count, fiber diameter, and myelin thickness. The images were
captured using a BX51 light microscope (Olympus Corporation, Tokyo,
Japan).
Immunohistochemical staining
Immunohistochemistry was performed on 5 μm
sections to observe the distribution of Schwann cells in the
regenerated nerve fibers. The slides containing the tissue sections
were blocked with goat serum for 30 min (Beijing Zhongshan Jinqiao
Biotech Co., Ltd.) at room temperature. The tissue sections were
subsequently incubated with anti-S100 rabbit monoclonal primary
antibody (1:500; cat. no. ZA-0225; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) overnight at 4°C. Following three washes
in distilled water, the sections were incubated with biotin-labeled
anti-rabbit immunoglobulin G secondary antibody (cat. no.
SP-9000-D; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 2
h at room temperature, and 3,3′-diaminobenzidine substrate
(Sigma-Aldrich, St. Louis, MO, USA) was used to develop colored
stains. The images were captured using an Olympus DP50 camera
(Olympus Corporation) connected to a TS100 fluorescence microscope
(Nikon Corporation, Tokyo, Japan). The data were analyzed using an
MPLAS 500 color pathology picture analysis system (Wuhan Qingping
Imaging Technology Co., Ltd., Wuhan, China).
Transmission electron microscopy
(TEM)
Uranyl acetate and lead citrate double staining
(Qingdao Jieshi Kang Biotech Co., Ltd., Qingdao, China) were
performed on ultra-thin nerve tissue sections following fixation
with 3% glutaraldehyde and embedding with resin, in order to detect
the ultrastructure of the regenerated nerve fibers under a Hitachi
H-7000 TEM (Hitachi Co., Tokyo, Japan).
Statistical analysis
To compare the statistically significant differences
among the various groups, the data were analyzed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to analyze the data. All experimental results
were expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Acellular nerve structure
HE staining indicated that cells and myelin were
absent in both the transverse and longitudinal sections of the
acellular nerves; however, the basal membrane remained intact. TEM
further demonstrated that only collagen fibers, which are the
principle component of the extracellular matrix (ECM), were
preserved following chemical extraction decellularization, and
formed a regular structure of basal lamina tubes (Fig. 1).
General observations
All of the nerve transplants exhibited adequate
continuity between the proximal and distal stumps of the recipient
nerves, and did not present edema. No significant inflammation was
present surrounding the grafts, and only minor adherence and scar
tissue formation were observed between the transplants and nearby
tissues, allowing the nerve to be easily separated.
Histological observations of nerve
regeneration
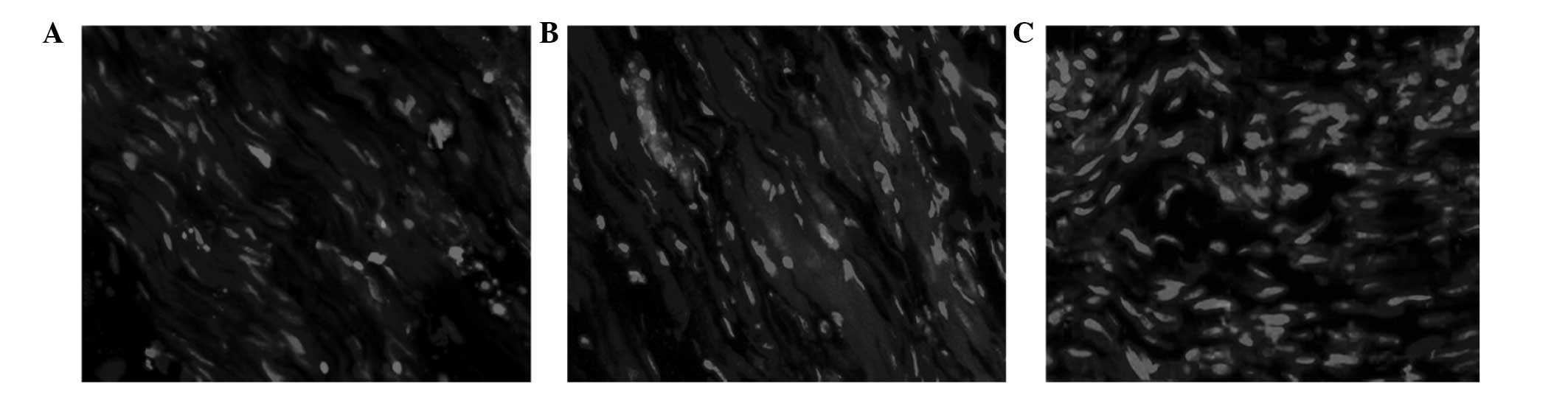
Masson's trichrome staining was performed 12 weeks
following the surgical procedure, and indicated that in a similar
manner to autografts, abundant regenerative nerve fiber bundles
invaded the center of the transplants in both the allografts and
xenografts. Neovascularization and a small amount of fibrous tissue
hyperplasia were also observed (Fig.
2A-C). These results suggest that the regenerative axons are
able to pass through the 1 cm acellular nerve transplants,
regardless of graft type.
Toluidine blue staining
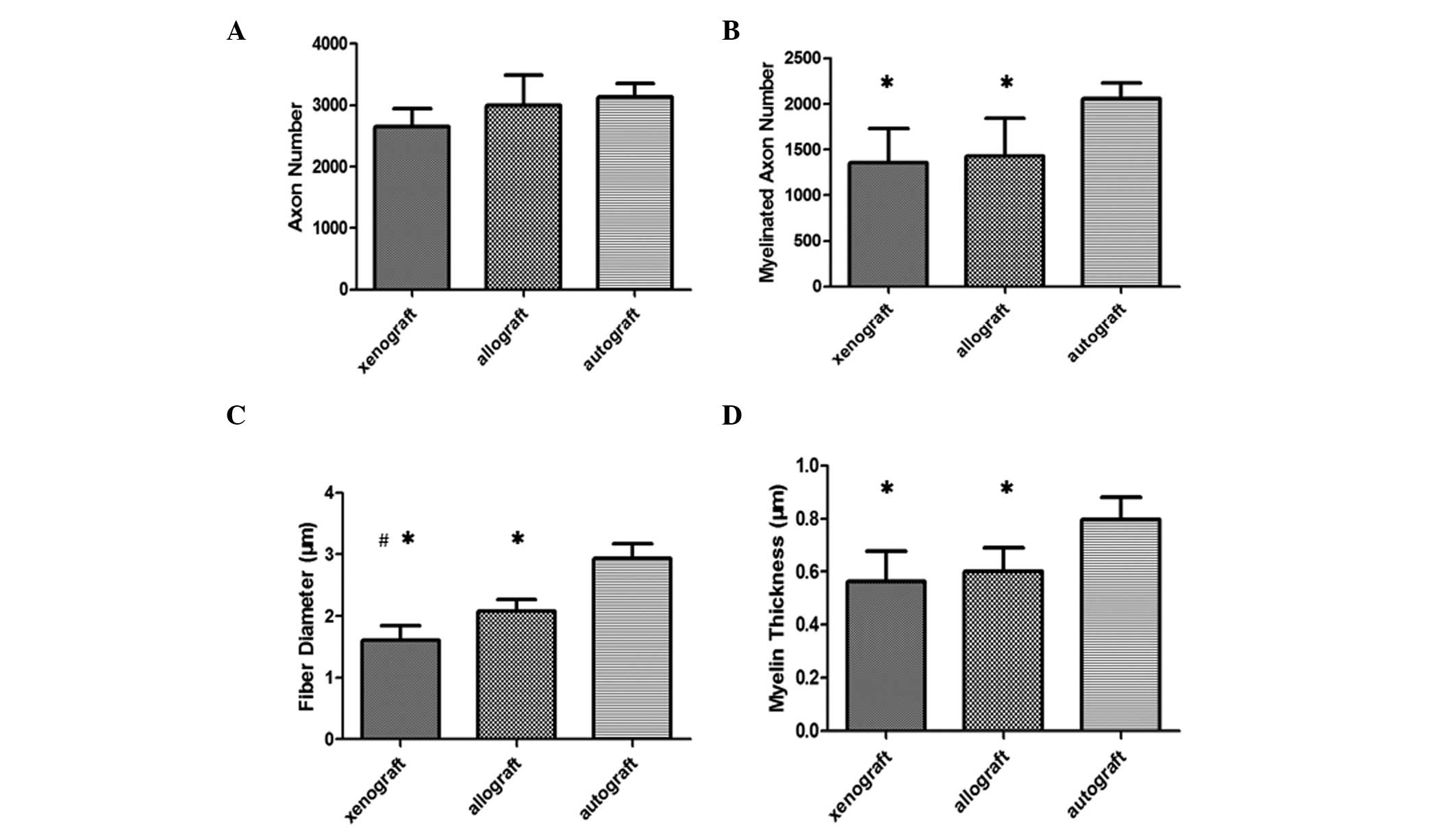
Nerve regeneration was quantitatively evaluated
using histomorphometric measurements following toluidine blue
staining. Both myelinated nerve fibers and non-myelinated nerve
fibers were located in the cross-sections of the regenerating
nerves of the three groups. However, the axons appeared more
uniform in the autograft group, as compared with those of either
acellular nerve graft groups (Fig.
2D–F). No significant difference in axon number was observed
between the various groups (P>0.05; Fig. 3A), whereas the number of myelinated
axons was higher in the autograft group, as compared with the
xenograft and allograft groups (Fig.
3B), and a higher number of unmyelinated axons were located in
the acellular xenograft and allograft nerve groups, as compared
with the autograft group. Fiber diameter and myelin sheath
thickness in the autograft group increased significantly, as
compared with in the allograft and xenograft groups (P<0.05;
Fig. 3C and D). With the exception
of fiber diameter, the differences in nerve regeneration values
between the allograft and the xenograft group were not
statistically significant (P>0.05; Fig. 3).
TEM observations
In addition to myelinated and non-myelinated nerve
fibers, Schwann cells were also located in the cross-sections of
regenerating nerve fibers, as determined by TEM (Fig. 4A–C). Schwann cells from the host
were able to migrate into the chemically processed acellular nerve
grafts, in order to form myelin sheaths enclosing the regenerative
axons. In addition, the autografts exhibited significantly higher
myelinated axon number, myelin sheath thickness, and fiber diameter
(P<0.05), as compared with both allografts and xenografts.
Immunohistochemical staining
Immunohistochemical staining indicated that
S-100-positive cells arranged along the new nerve fibers in each of
the three groups, forming a long shuttle. A higher number of
positive cells were present in the autograft group, as compared
with the xenograft and allograft nerve groups (Fig. 5).
Nerve conduction velocity
examination
Electrophysiological analysis determined that nerve
conduction velocity in the autograft group was significantly
higher, as compared with the xenograft and allograft acellular
nerve graft groups, 12 weeks following transplant (P<0.05).
However, no significant differences were observed between the
allograft and xenograft groups (Fig.
6).
Discussion
The structure of the nerve graft is able to
influence nerve regeneration capacity, with acellular nerve grafts
containing natural tubular structures that support axon ingrowth,
and therefore may be used as a biomaterial to bridge peripheral
nerve gaps (12–14). In addition, acellular nerve grafts
contain components of the ECM, and are predominantly composed of
laminin, heparin sulfate proteoglycan, fibronectin, and collagen
type IV, which release growth factors to guide axonal elongation
and promote Schwann cell migration (15–17).
Due to these advantages, acellular nerve grafts have been
considered an ideal alternative to autografts. Previous studies
have focused on the use of acellular allografts for peripheral
nerve gap reconstruction (3,12–14),
whereas the use of acellular xenografts has rarely been reported.
However, xenografts are available in greater quantities, which
would provide grafts for an increasing number of potential
recipients. In the present study, HE staining and TEM demonstrated
that acellular nerves derived from rabbits presented native
three-dimensional basal lamina tube microstructures, and the
collagen fibers of the conduit wall were arranged regularly. Both
the xenografts and allografts had similar numbers of regenerative
axons as the autografts, indicating that the donor ECM structure
mimics the native nerve and may provide a favorable local
environment for axon regeneration.
Schwann cells, endothelial cells, and macrophages
from donor nerves are the principle antigens responsible for
immunological rejection, which can impair nerve regeneration in
xenografts (18–20). Therefore, removal of cellular
components prior to grafting is essential for successful nerve
regeneration. In the present study, a chemical extraction method
was used to eliminate cellular antigens, and no cellular components
were detected following acellular histomorphometric examination of
the HE-stained nerves, as determined by TEM. As a result, no
immunological rejection or inflammation was observed 12 weeks
following surgical graft transplantation, demonstrating that
chemically extracted nerves could result in immune tolerance to
both allografts and xenografts.
Chemically-treated grafts exhibited an absence of
Schwann cells, as compared with autografts, which are essential for
nerve regeneration and maturation (21). During nerve regeneration, and
following axon growth into the graft, Schwann cells enter the graft
and reoccupy the basal lamina, resulting in the formation of
myelin, which contributes to the recovery of neural function
(22,23). In the present study the amount of
regenerative axons was similar between the acellular nerve graft
groups and the autograft group; however, the number of myelinated
axons, the thickness of the myelin sheath, and the fiber diameter,
which represent the maturity of regenerative axons, were markedly
reduced in the acellular nerve graft groups, as compared with the
autograft group. The higher number of unmyelinated axons in the
acellular nerve grafts may be due to the fact that the leading
front of new axons in regenerating nerves are composed of
non-myelinated axons (24), which
remain immature and may become myelinated as Schwann cells invade
the graft. Immunohistochemical staining and TEM analysis
demonstrated that Schwann cells were able to migrate into
regenerative axons to form myelin sheaths in both the xenograft and
allograft acellular nerve groups, but a smaller number of Schwann
cells were available in these two groups, as compared with the
autograft group, which could reduce the speed of axonal growth and
maturation. These data suggested that acellular nerves are able to
support nerve fiber ingrowth within the autogenous nerve. Autograft
nerves exhibited advantages, due to the fact that autogenous nerve
grafts were the only grafts to contain living Schwann cells, which
could increase the speed of myelin formation.
Previous studies have suggested that a deficiency in
Schwann cells in the regenerated nerves of xenograft and allograft
groups may partly be due to the extensive duration of the
extraction process, which may reduce the number of factors that are
able to aid Schwann cell migration (22,25).
Therefore, further studies are required in order to shorten the
time taken to carry out the extraction process, thus allowing the
retention of growth factors released from the ECM. In previous
studies, Schwann cells (26,27),
and neurotrophic factors (28,29)
have been placed into grafts in order to improve nerve
regeneration. A previous study demonstrated that grafts containing
Schwann cells were able to provide a better environment for nerve
regeneration (30); conversely,
other studies demonstrated that acellular nerves had the same
ability to support axon regeneration (31,32).
In addition to histological examination, autografts
also exhibited the best nerve regeneration results following
electrophysiological analysis. The data indicated that nerve
conduction velocity in the autograft group was markedly higher, as
compared with those in the acellular nerve graft groups, however no
significant differences were present between the allograft and
xenograft groups, results that were concordant with those of
previous histological findings. A previous study reported that
there is close correlation between histomorphometric and
electrophysiological analysis results (16), and increased nerve regeneration is
indicative of increased functional recovery (33). Rats receiving xenografts displayed
poorer functional recovery, as compared with those receiving
autografts shortly following grafting, however over a longer period
of time, functional recovery was similar between the two groups
(9). These results suggested that
xenografts and autografts lead to similar functional recovery over
a suitable period of time; however, autografts are able to induce
rapid functional recovery, as compared with acellular xenografts,
due to the presence of better cellular support. In the present
study, the data were measured 12 weeks following grafting, but in
order to confirm whether Schwann cells are able to induce
functional nerve recovery in autografts at earlier time points,
further long-term studies are required.
The results of the present study demonstrated that
chemically-treated allografts and xenografts had similar effects on
nerve regeneration, including regenerative axon number, myelinated
axon number, and thickness of myelin sheath, leaving only fiber
diameter which was statistically different. These results are
concordant with those of a previous study (34), and indicate that chemically
extracted nerves have similar axon regeneration abilities when
repairing a short nerve defect. However, another study reported
that allografts perform better, as compared with xenografts, when
repairing a 14 mm rat sciatic nerve defects (35). A possible cause for these
differences in regeneration potential in the latter study was the
use of human-derived processed nerves, whereas the present study
used rabbit-derived processed nerves as xenografts to repair rat
nerve defects. Animal experiments have confirmed that in order to
bridge a nerve defect, xenografts from certain host species induce
better nerve regeneration than others (36). However, it remains unclear why and
how the xenografts from these various host species affect nerve
regeneration. Therefore, when comparing these two studies, genetic
differences that may affect nerve regeneration must be taken into
account. Furthermore, the method of selection and matching of a
suitable combination of donor grafts and host species requires
further study in order to improve axonal outgrowth.
The present study had limitations insofar as the
experimental model focused on a small gap defect alone. However, to
repair a 1 cm nerve gap, chemically extracted acellular xenografts
may be used as substitutes to allografts. In the future, wider gaps
should be reconstructed in order to examine whether allografts and
xenografts have similar effects on nerve regeneration.
The present study demonstrated that chemically
extracted acellular facial nerves no longer contained cellular
material, and maintained the structural integrity of the basal
lamina, which mimicked the native nerves and supported facial nerve
regeneration, resulting in functional recovery both in allografts
and in xenografts. However, autografts exhibited superior
functional recovery and nerve regeneration, as compared with
allografts and xenografts. Allografts and xenografts exhibited a
similar ability to induce nerve regeneration, with the exception of
fiber diameter. In conclusion, chemically extracted acellular
allografts and xenografts have comparable effects on reconstruction
of short facial nerve defects.
Acknowledgments
This study was funded by a grant from the National
Natural Science Foundation of China (grant. no. 30872898) and a
grant from Beijing Municipal National Science Foundation (grant.
no. 7132173).
References
|
1
|
Millesi H: Techniques for nerve grafting.
Hand Clin. 16:73–91. 2000.PubMed/NCBI
|
|
2
|
Terzis JK, Sun DD and Thanos PK:
Historical and basic science review: Past, present, and future of
nerve repair. J Reconstr Microsurg. 13:215–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong H, Chen B, Lu S, Zhao M, Guo Y and
Hou S: Nerve regeneration and functional recovery after a sciatic
nerve gap is repaired by an acellular nerve allograft made through
chemical extraction in canines. J Reconstr Microsurg. 23:479–487.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu LJ, Sun JB, Liu ZG, Gong X, Cui JL and
Sun XG: Immune responses following mouse peripheral nerve
xenotransplantation in rats. J Biomed Biotechnol. 2009:4125982009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platt JL: A perspective on xenograft
rejection and accommodation. Immunol Rev. 141:127–149. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udina E, Voda J, Gold BG and Navarro X:
Comparative dose-dependence study of FK506 on transected mouse
sciatic nerve repaired by allograft or xenograft. J Peripher Nerv
Syst. 8:145–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans PJ, Midha R and Mackinnon SE: The
peripheral nerve allograft: A comprehensive review of regeneration
and neuroimmunology. Prog Neurobiol. 43:187–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scherer MN, Banas B, Mantouvalou K,
Schnitzbauer A, Obed A, Krämer BK and Schlitt HJ: Current concepts
and perspectives of immunosuppression in organ transplantation.
Langenbecks Arch Surg. 392:511–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Accioli De Vaconcellos ZA, Duchossoy Y,
Kassar-Duchossoy L and Mira JC: Experimental median nerve repair by
fresh or frozen nerve autografts and xenografts. Ann Chir Main Memb
Super. 18:74–84. 1999. View Article : Google Scholar
|
|
10
|
Hudson TW, Liu SY and Schmidt CE:
Engineering an improved acellular nerve graft via optimized
chemical processing. Tissue Eng. 10:1346–1358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu M, Zhang L, Niu Y, Xiao H, Tang P and
Wang Y: Repair of whole rabbit facial nerve defects using facial
nerve allografts. J Oral Maxillofac Surg. 68:2196–2206. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karabekmez FE, Duymaz A and Moran SL:
Early clinical outcomes with the use of decellularized nerve
allograft for repair of sensory defects within the hand. Hand (NY).
4:245–249. 2009. View Article : Google Scholar
|
|
13
|
Brooks DN, Weber RV, Chao JD, Rinker BD,
Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE,
Wisotsky SM, et al: Processed nerve allografts for peripheral nerve
reconstruction: A multicenter study of utilization and outcomes in
sensory, mixed, and motor nerve reconstructions. Microsurgery.
32:1–14. 2012. View Article : Google Scholar
|
|
14
|
Cho MS, Rinker BD, Weber RV, Chao JD,
Ingari JV, Brooks D and Buncke GM: Functional outcome following
nerve repair in the upper extremity using processed nerve
allograft. J Hand Surg Am. 37:2340–2349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rovak JM, Bishop DK, Boxer LK, Wood SC,
Mungara AK and Cederna PS: Peripheral nerve transplantation: The
role of chemical acellularization in eliminating allograft
antigenicity. J Reconstr Microsurg. 21:207–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore AM, MacEwan M, Santosa KB, Chenard
KE, Ray WZ, Hunter DA, Mackinnon SE and Johnson PJ: Acellular nerve
allografts in peripheral nerve regeneration: A comparative study.
Muscle Nerve. 44:221–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salonen V, Aho H, Röyttä M and Peltonen J:
Quantitation of Schwann cells and endoneurial fibroblast-like cells
after experimental nerve trauma. Acta Neuropathol. 75:331–336.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hudson TW, Zawko S, Deister C, Lundy S, Hu
CY, Lee K and Schmidt CE: Optimized acellular nerve graft is
immunologically tolerated and supports regeneration. Tissue Eng.
10:1641–1651. 2004. View Article : Google Scholar
|
|
19
|
Gulati AK and Cole GP: Nerve graft
immunogenicity as a factor determining axonal regeneration in the
rat. J Neurosurg. 72:114–122. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gulati AK: Immune response and
neurotrophic factor interactions in peripheral nerve transplants.
Acta Haematol. 99:171–174. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whitlock EL, Tuffaha SH, Luciano JP, Yan
Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE and
Borschel GH: Processed allografts and type I collagen conduits for
repair of peripheral nerve gaps. Muscle Nerve. 39:787–799. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sondell M, Lundborg G and Kanje M:
Regeneration of the rat sciatic nerve into allografts made
acellular through chemical extraction. Brain Res. 795:44–54. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi A, Moradzadeh A, Tong A, Wei C,
Tuffaha SH, Hunter DA, Tung TH, Parsadanian A, Mackinnon SE and
Myckatyn TM: Treatment modality affects allograft-derived Schwann
cell phenotype and myelinating capacity. Exp Neurol. 212:324–336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu SY and Gordon T: The cellular and
molecular basis of peripheral nerve regeneration. Mol Neurobiol.
14:67–116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roomi MW, Ishaque A, Khan NR and Eylar EH:
The PO protein. The major glycoprotein of peripheral nerve myelin.
Biochim Biophys Acta. 536:112–121. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fox IK, Schwetye KE, Keune JD, Brenner MJ,
Yu JW, Hunter DA, Wood PM and Mackinnon SE: Schwann-cell injection
of cold-preserved nerve allografts. Microsurgery. 25:502–507. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hess JR, Brenner MJ, Fox IK, Nichols CM,
Myckatyn TM, Hunter DA, Rickman SR and Mackinnon SE: Use of
cold-preserved allografts seeded with autologous Schwann cells in
the treatment of a long-gap peripheral nerve injury. Plast Reconstr
Surg. 119:246–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee AC, Yu VM, Lowe JB III, Brenner MJ,
Hunter DA, Mackinnon SE and Sakiyama-Elbert SE: Controlled release
of nerve growth factor enhances sciatic nerve regeneration. Exp
Neurol. 184:295–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otto D, Unsicker K and Grothe C:
Pharmacological effects of nerve growth factor and fibroblast
growth factor applied to the transectioned sciatic nerve on neuron
death in adult rat dorsal root ganglia. Neurosci Lett. 83:156–160.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Luo H, Zhang Z, Lu Y, Huang X,
Yang L, Xu J, Yang W, Fan X, Du B, et al: A nerve graft constructed
with xenogeneic acellular nerve matrix and autologous
adipose-derived mesenchymal stem cells. Biomaterials. 31:5312–5324.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Accioli-De-Vaconcellos ZA,
Kassar-Duchossoy L and Mira JC: Long term evaluation of
experimental median nerve repair by frozen and fresh nerve
autografts, allografts and allografts repopulated by autologous
Schwann cells. Restor Neurol Neurosci. 15:17–24. 1999.
|
|
32
|
Frerichs O, Fansa H, Schicht C, Wolf G,
Schneider W and Keilhoff G: Reconstruction of peripheral nerves
using acellular nerve grafts with implanted cultured Schwann cells.
Microsurgery. 22:311–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fraher J and Dockery P: A strong myelin
thickness-axon size correlation emerges in developing nerves
despite independent growth of both parameters. J Anat. 193:195–201.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia H, Wang Y, Tong XJ, Liu GB, Li Q,
Zhang LX and Sun XH: Sciatic nerve repair by acellular nerve
xenografts implanted with BMSCs in rats xenograft combined with
BMSCs. Synapse. 66:256–269. 2012. View Article : Google Scholar
|
|
35
|
Wood MD, Kemp SW, Liu EH, Szynkaruk M,
Gordon T and Borschel GH: Rat-derived processed nerve allografts
support more axon regeneration in rat than human-derived processed
nerve xenografts. J Biomed Mater Res A. 102:1085–1091. 2014.
View Article : Google Scholar
|
|
36
|
Kvist M, Sondell M, Kanje M and Dahlin LB:
Regeneration in, and properties of, extracted peripheral nerve
allografts and xenografts. J Plast Surg Hand Surg. 45:122–128.
2011. View Article : Google Scholar : PubMed/NCBI
|