Introduction
Osteosarcoma (OS) is one of the most common types of
malignancy with a poor prognosis (1). The altered expression of oncogenes or
tumor suppressors has an important role in the progression,
invasion and metastasis of OS (1).
Therefore, gene therapy is a promising strategy for the treatment
of OS (2).
Melanoma differentiation-associated gene 7
(mda7)/interleukin (IL)-24 belongs to the IL-10 cytokine family and
induces apoptosis in various types of cancer by binding to two
heterodimeric receptors, IL-20R1 and IL-22R1 (3). The expression of IL-20R1 and IL-22R1
in cancer cells ensures the fact that IL-24 may be used as an
effective anti-tumor agent in future clinical cancer therapy
(4). In fact, IL-24 has been
demonstrated to exert anti-tumor activity in certain types of
cancer, including hepatocellular carcinoma (5) and lung cancer (6). Notably, adenovirus-mediated IL-24
delivery has been demonstrated to suppress the growth of OS
(7). However, the efficacy of
IL-24 is relatively limited in OS, since the expression level of
IL-24 cannot meet clinical demands.
Unlike the current nonreplicative adenoviral vector,
oncolytic adenoviruses are able to proliferate selectively in
cancer cells, due to the action of their tumor-specific promoter.
It is worth noting that oncolytic adenoviruses have no significant
cytotoxicity to normal tissues. The effect of these oncolytic
adenoviruses has been verified in various types of cancer in
vitro and in vivo (8).
Of note, oncolytic adenoviruses can also be used for gene therapy
by delivering a tumor suppressor into cancer cells. During the
replication of vectors, the copies of carried genes are also
increased, allowing a higher expression level of inserted
therapeutic genes.
In the present study, a recombinant oncolytic
adenovirus for inducing a high expression of IL-24 (OA-IL-24) was
generated. The inhibitory effect of OA-IL-24 on the growth of
osteosarcoma was investigated in the current study, in addition to
its influence on the sensitivity of OS cells to doxorubicin.
Materials and methods
Cell lines and culture
The human OS cell line, U2OS, was purchased from the
American Type Culture Collection (Manassas, VA, USA). The human
embryonic kidney (HEK) cell line HEK-293 was obtained from Microbix
Biosystems, Inc. (Toronto, Canada). The cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL, Gaithersburg, MD, USA) and 4 mM
L-glutamine at 37°C in a humidified incubator in an atmosphere of
5% CO2.
Primary culture
In the present study, primary OS cells were used to
assess the efficiency of adenoviral vectors, Ad-IL-24 and OA-IL-24.
Primary OS cells were obtained with written informed consent from
patients following the previously described procedures (9) approved by the Ethics Committee of
Jilin University (Changchun, China). Briefly, fresh OS samples were
obtained from OS patients during surgery and then were directly
placed in media with 20% FBS, minced with sterile scissors and
digested for 1 h. Subsequently, the separated cells were
centrifuged at 320 × g for 10 min and then were suspended in media
containing 20% FBS according to routine procedures (9).
Adenoviruses, chemicals and plasmids
Two replication-deficient adenoviruses, Ad-enhanced
green fluorescent protein (EGFP) and Ad-IL24, were kindly provided
by Dr Jing Xu (The First People's Hospital of Yunnan Province,
Kunming, China). OA-EGFP and OA-IL-24 were produced as described
below. A plasmid containing human telomerase reverse transcriptase
promoter-regulated mutant E1A and a cytomegalovirus
promoter-regulated expression cassette was also generously provided
by Dr. Xu. EGFP and IL-24 genes were inserted into the cassette at
NotI and XhoI sites. The two recombinant plasmids
were then transduced into the BJ5183 E. coli strain together
with pAd Easy-1, which encodes adenoviral genomic sequences, for
subsequent homogenous recombination. The recombinant vectors were
then transfected into HEK-293 cells following PacI
digestion. The produced adenoviruses were termed OA-EGFP and
OA-IL-24. The construction of adenoviral vectors used in the
present study is depicted in Fig.
1A. These adenoviruses were then purified using a CsCl gradient
ultracentrifugation method. The titers of the adenoviruses were
determined using a tissue culture infectious dose 50 assay.
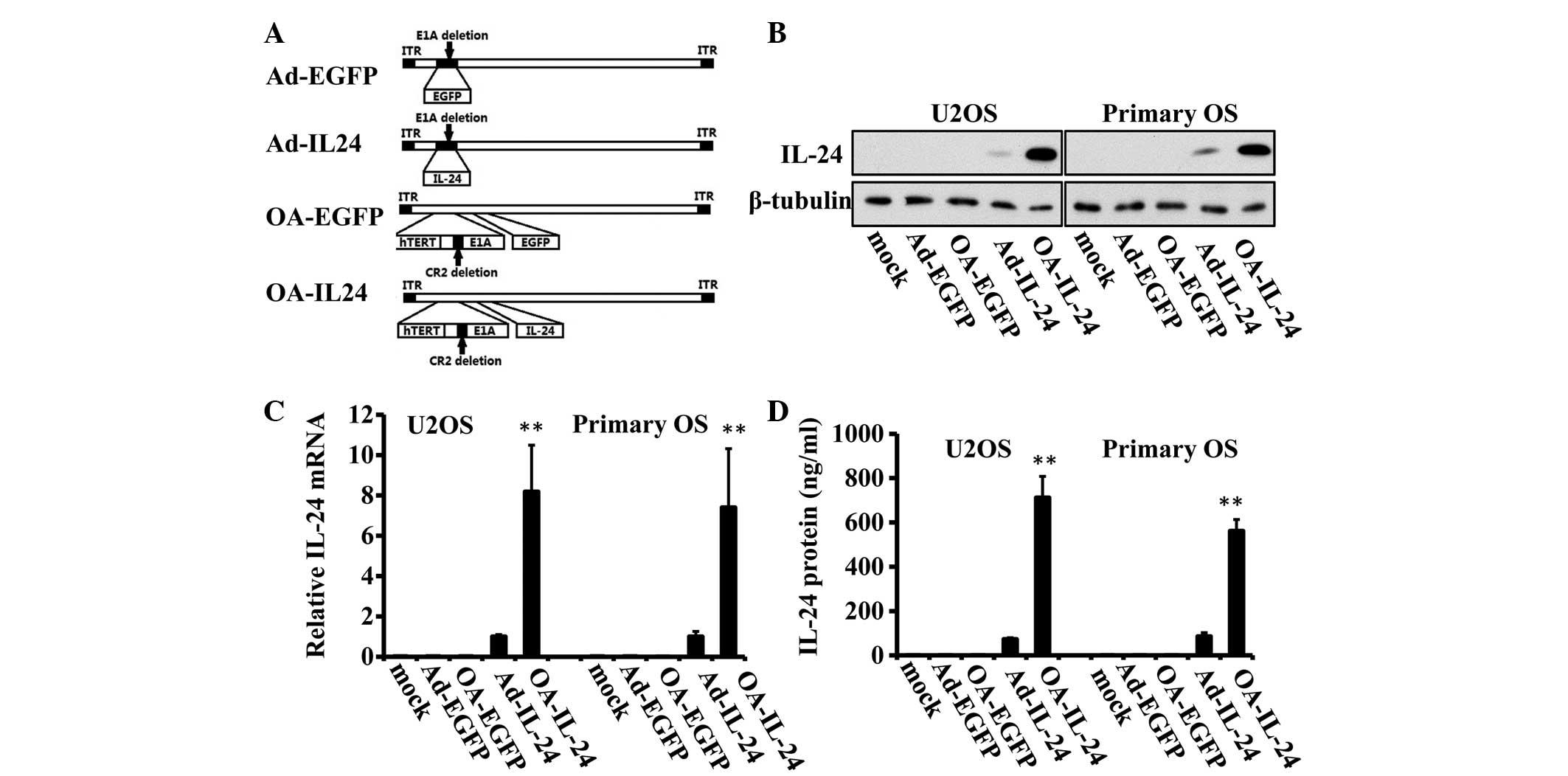 | Figure 1OA-IL-24 expresses IL-24 at a higher
level than Ad-IL-24 in OS cells. (A) Schematic structures of
OA-IL-24 and Ad-IL-24 as well as two control adenoviruses, OA-EGFP
and Ad-EGFP are shown. The E1A gene was deleted in Ad-IL-24 and
Ad-EGFP vectors, while in OA-IL-24 and OA-EGFP, the E1A promoter
was placed with a hTERT promoter. In addition, a 24 bp region was
removed from the E1A genes. (B) U2OS and a primary OS cell were
infected with Ad-EGFP, OA-EGFP, Ad-IL-24 or OA-IL-24 (10 MOI) for
48 h. Immunoblot assays were performed to detect the expression
level of IL-24. β-tubulin was used as an endogenous reference. (C)
Quantitative polymerase chain reaction assays were used to detect
the mRNA level of IL-24. (D) ELISA assays were employed to evaluate
the quantity of secreted IL-24 protein. **P<0.01, vs.
Ad-IL-24. OS, osteosarcoma; ITR, inverse terminal repeats; IL,
interleukin; hTERT, human telomerase reverse transcriptase; MOI,
multiplicity of infection; EGFP, enhanced green fluorescent
protein; OA, oncolytic adenovirus. |
Doxorubicin and chloroquine were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The plasmids used in the
present study, pcDNA3.1-EGFP, pcDNA3.1-Pgp and pcDNA3.1-BCRP1, were
all provided by Dr Jing Xu (The First People's Hospital of Yunnan
Province).
mRNA detection
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen Life Technologies, Grand Island, NY, USA)
according to the manufacturer's instructions. Reverse transcription
quantitative polymerase chain reaction (RT-qPCR) was performed
according to the manufacturer's instructions of the RevertAid™
First Strand cDNA Synthesis kit (Fermentas, Pittsburgh, PA, USA) to
generate cDNA. Subsequently, qPCR was performed with SYBR premix Ex
Taq (RR420A; Takara Bio Inc., Otsu, Japan) on an Applied Biosystems
7300 Real-Time PCR System supplied with analytical software
(AutoCaller™ Real-time PCR Analysis Software, version 1.0; Applied
Biosystems, Pittsburgh, PA, USA). GAPDH was used as an endogenous
reference. The primer sequences used were as follows: IL-24,
forward 5′-TCTGCAGGGACAGAGCATT-3′; and reverse
5′-CGAGTGTGGGTGGGAAATG-3′; GAPDH, forward 5′-TCAGTGGTGGACCTGA-3′
and reverse 5′-TGCTGTAGCCAAATTC-3′.
ELISA
A two-antibody sandwich ELISA was used to detect
human IL-24 in the present study. The following antibodies were
used: Mouse monoclonal anti-human IL-24 antibody (1:200; MAB1965;
R&D Systems, Minneapolis, MN, USA), goat polyclonal anti-human
IL-24 antibody (1:500; AF1965; R&D Systems) and polyclonal
peroxidase-conjugated rabbit anti-goat IgG (1:500; HAF017; H&L;
R&D Systems). The absorbance was read at a wavelength of 450 nm
using a 550 Microplate Reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The concentration of IL-24 was determined
according to a standard curve.
Cell proliferation assay
U2OS (5×103 per well) and primary OS
cells were plated in 96-well plates. Overnight, the indicated
adenoviruses or doxorubicin were added into each well. At the
indicated time points,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
1 mg/ml) was added to the cultures. After 4 h incubation, the media
were removed and dimethyl sulfoxide was added into each well. The
absorption values of each well were measured on a microplate reader
(Infiniate 200 PRO; Tecan, Männedorf, Switzerland). The
proliferation rate was calculated according to the following
formula: Proliferation rate (folds) = (absorption values of tested
well - absorption values of empty well) / (absorption values of
control well - absorption values of empty well).
Detection of cell apoptosis
The rates of apoptotic cells were determined by flow
cytometric analysis of the sub-G0/G1
population. Cells were fixed in cold 70% ethanol for 1 h and then
incubated with 10 mg/ml RNase A at 37°C for another 1 h. The cells
were stained with propidium iodide (PI; 200 mg/ml), followed by
flow cytometric analysis of the sub-G0/G1
population (Aria II; BD Biosciences, San Jose, CA, USA). A total of
1×105 cells were counted for each sample.
Immunoblot assay
The protein was extracted from cells with
M-PER® Mammalian Protein Extraction Reagent (Thermo
Fisher Scientific, Rockford, IL, USA). Immunoblot analysis was
performed according to routine protocols (10). Briefly, the isolated proteins were
separated by polyacrylamide gel electrophoresis and then
transferred onto 0.45 µm nitrocellulose membranes. The
membranes were blocked with 5% fat-free dry milk in
phosphate-buffered saline (PBS) and incubated with primary
antibodies. Overnight, the membrane was incubated with
corresponding secondary antibodies and visualized with SuperSignal
West Dura Extended Duration Substrate (Thermo Fisher Scientific).
All primary antibodies (excluding anti-IL-24) were purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA). The Cell
Signaling Technology antibodies (1:1,000) used were as follows:
Monoclonal rabbit anti-human cleaved poly (ADP-ribose) polymerase
(#5625), monoclonal rabbit anti-human cleaved caspase-3 (#9664);
polyclonal rabbit anti-human β-tubulin (#2146); monoclonal rabbit
anti-human LC3B (#3868); monoclonal mouse anti-human Pgp (#5640);
polyclonal rabbit anti-human BCRP1 (#4477); monoclonal rabbit
anti-human MRP1 (#14685). The signal intensity was measured using a
chemiluminescent detection system (Pierce Biotechnology Inc.).
Animal experiment
The procedures for animal experiments were approved
by the Committee on the Use and Care of Animals (Jilin University,
Changchun, China). U2OS tumor xenografts were established by
subcutaneously injecting 5×106 cells into the left
flanks of 4–6-week-old BALB/c nude mice (n=30; Shanghai Institutes
for Biological Sciences, Shanghai, China). When tumors reached
between 7 and 10 mm in diameter, 30 mice were randomly divided into
five groups (PBS; n=6; Ad-EGFP, n=6; Ad-IL-24, n=6; OA-EGFP, n=6;
OA-IL-24, n=6). The established tumors were intratumorally
administered with 100 µl PBS alone or with 1×109
pfu of Ad-EGFP, Ad-IL-24, OA-EGFP and OA-IL-24. The diameters of
tumors were measured with calipers and the tumor volume was
calculated using the following formula: Tumor volume
(mm3) = maximal length (mm) × (perpendicular width
(mm))2 / 2. During the experiments, no mice died from
tumor loading.
Histological staining and apoptosis
detection in tumor xenografts
One animal was randomly selected from each group for
histological staining of IL-24 or apoptosis detection assays, 7
days after administration. Their tumors were harvested and fixed
with formalin. Histological staining was performed on
formalin-fixed, paraffin-embedded tissue sections using the
streptavidin biotin peroxidase complex method. Goat polyclonal
anti-human IL-24 antibody (1:100; sc-8704; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used to stain IL-24
protein in tumors. The sections were counterstained with
hematoxylin. Apoptosis detection was performed using a TUNEL
Apoptosis Detection kit (Millipore, Boston, MA, USA) according to
the manufacturer's instructions.
Statistical analysis
All statistical tests in the present study were
two-tailed Student's test. SPSS software, version 19.0 (IBM SPSS,
Armonk, NY, USA) was used for analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
OA-IL-24 leads to a higher expression of
IL-24 compared with Ad-IL-24 in OS cells
A recombinant oncolytic adenovirus expressing IL-24
(OA-IL-24) was generated and a recombinant proliferation-deficient
vector containing IL-24, Ad-IL-24, as well as OA-EGFP and Ad-IL-24,
were used as controls (Fig.
1A).
Initially, the expression level of IL-24 was
compared in U2OS OS cell lines and primary OS cells between
different adenoviral vectors. The immunoblot assay indicated that
OA-IL-24 expressed higher levels of IL-24 in these two cells
compared with Ad-IL-24 (Fig. 1B).
The analysis of mRNA expression levels also confirmed the advanced
expression capacity of oncolytic adenovirus for IL-24 expression
(Fig. 1C). Consistently, ELISA
assays revealed that the quantity of IL-24 secreted into media was
significantly higher in OA-IL-24-infected cells, compared with
Ad-IL-24 (6.55–9.71-fold; Fig.
1D).
The above data suggested that the oncolytic
adenovirus induces a higher expression of IL-24 compared with the
replication-deficient adenovirus.
OA-IL-24 suppresses the proliferation and
induces apoptosis to a greater extent compared with Ad-IL-24
Given that OA-IL-24 expresses a higher level of
IL-24 than Ad-IL-24, the ability to suppress cell proliferation and
induce apoptosis was subsequently compared between the two
adenoviral vectors. MTT assays revealed that Ad-IL-24 reduced the
proliferation of U2OS and primary OS cells by 40–50%, while
OA-IL-24 was able to suppress their proliferation by ~90% (Fig. 2A). Furthermore, flow cytometric
analysis of the sub-G0/G1 population
indicated that OA-IL-24 infection led to a higher percentage of
apoptotic cells in U2OS and primary OS cells compared with Ad-IL-24
(Fig. 2B). This effect was also
confirmed by immunoblot assays, which demonstrated that the
expression level of cleaved caspase-3 and poly (ADP-ribose)
polymerase was higher in OA-IL-24-treated cells than
Ad-IL-24-treated ones (Fig.
2C).
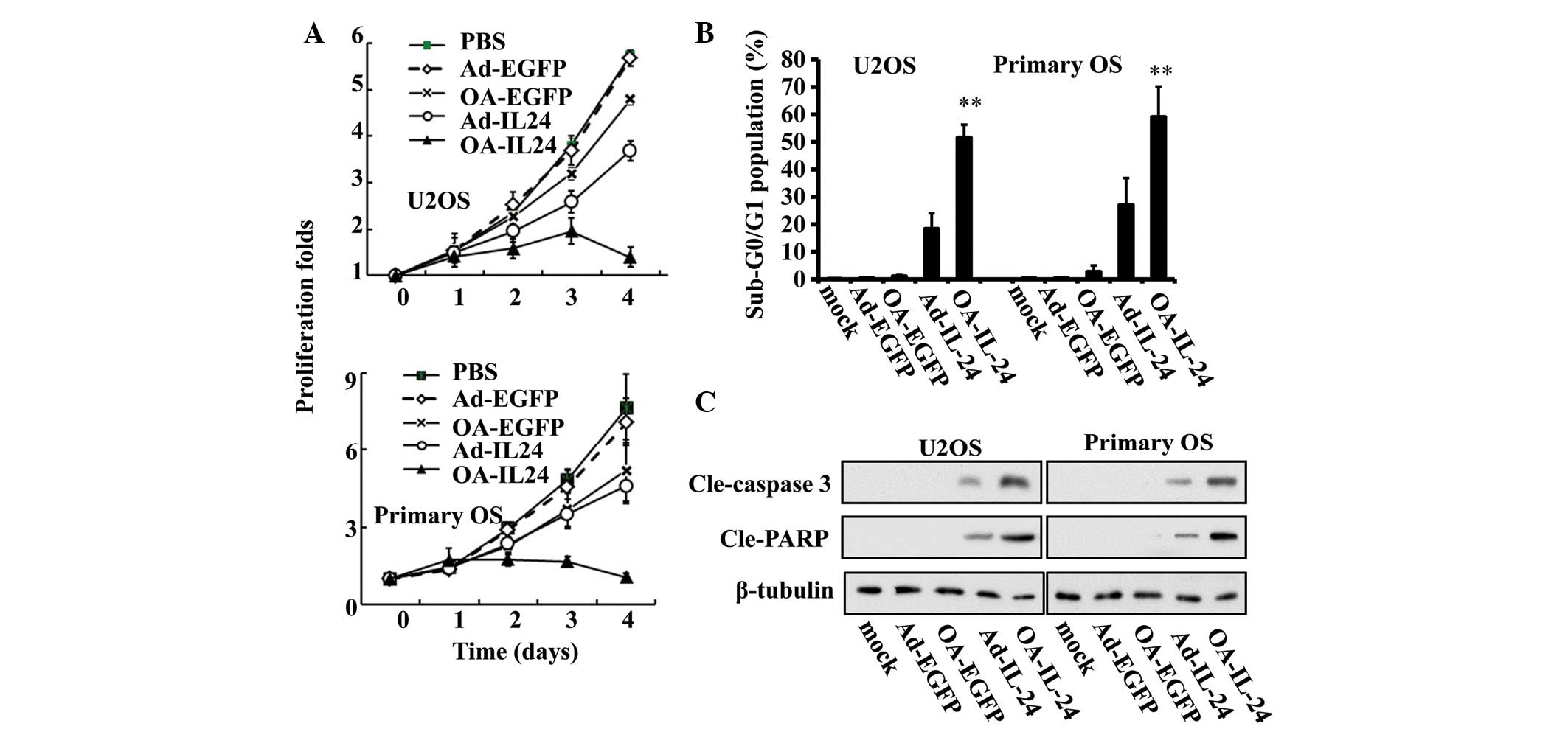 | Figure 2OA-IL-24 suppresses proliferation and
induces apoptosis to a greater extent than Ad-IL-24. (A) U2OS and
primary OS cells were infected with Ad-EGFP, OA-EGFP, Ad-IL-24 or
OA-IL-24 (10 MOI) for the indicated time periods. MTT assays were
used to measure the proliferation rates of the two cells. (B) After
48 h treatment with the above adenoviruses, the percentage of cells
in the sub-G0/G1 population were determined
by flow cytometric analysis. **P<0.01, vs. Ad-IL-24.
(C) The expression levels of cleaved caspase 3 and PARP
(cle-caspase 3 and cle-PARP) were also detected by immunoblot
assays. OS, osteosarcoma; IL, interleukin; EGFP, enhanced green
fluorescent protein; MOI, multiplicity of infection; PARP, poly
(ADP-ribose) polymerase; PBS, phosphate-buffered saline; OA,
oncolytic adenovirus. |
The above data demonstrated that OA-IL-24 exhibited
a higher ability to suppress proliferation and induce apoptosis in
OS cells than Ad-IL-24.
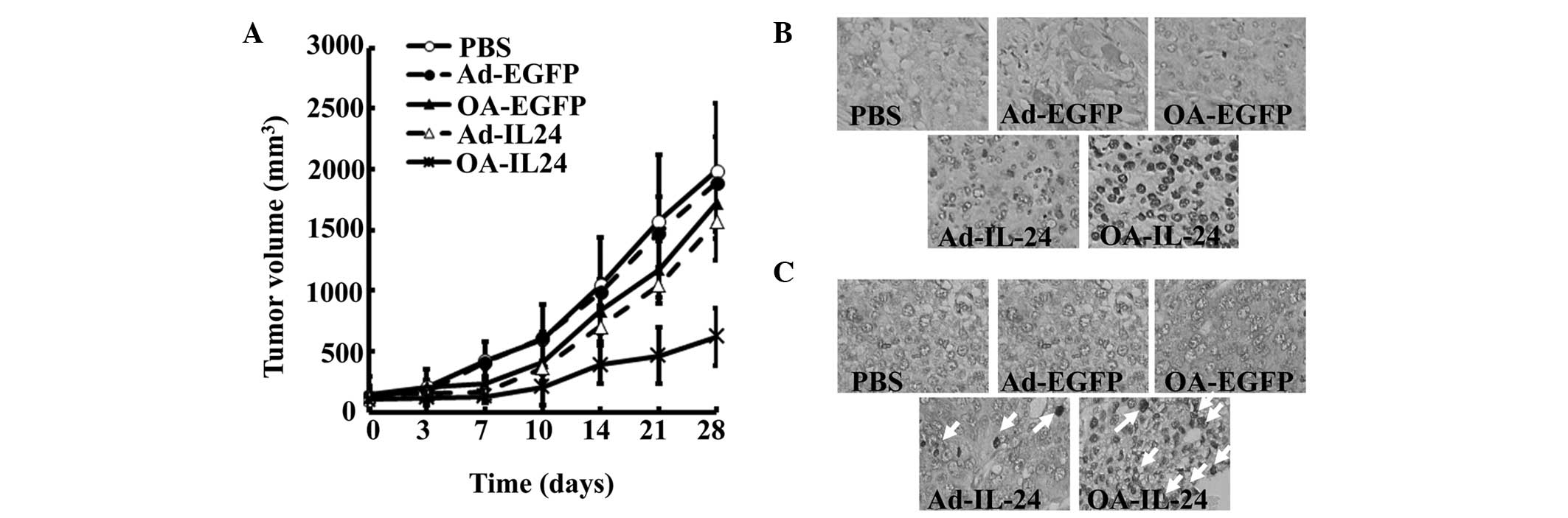
OA-IL-24 inhibits the growth of human OS
xenografts in mice to a greater extent than Ad-IL-24
Since OA-IL-24 suppressed the proliferation and
induced apoptosis in a more efficient manner compared with
Ad-IL-24, the present study aimed to compare the ability of
OA-IL-24 and Ad-IL-24 to reduce OS growth in vivo. A mouse
model bearing OS xenografts was established by subcutaneously
injecting U2OS cells. Subsequently, Ad-EGFP, OA-EGFP, Ad-IL-24 and
OA-IL-24 were directly injected into the tumors. The tumor volume
curve demonstrated that OA-IL-24 significantly reduced the growth
of U2OS xenografts by ~75%, while OA-EGFP and Ad-IL-24 partially
suppressed their growth (Fig. 3A).
Histological staining revealed that OA-IL-24 led to a higher
expression of human IL-24 in tumor xenografts in mice, than
Ad-IL-24 (Fig. 3B). Accordingly,
apoptotic rates were found to be significantly increased in tumors
injected with OA-IL-24, compared with Ad-IL-24 (Fig. 3C).
The above data demonstrated that OA-IL-24 was able
to express higher levels of IL-24 and induce apoptosis to a greater
extent than Ad-IL-24 in vivo. These advantages allowed
OA-IL-24 to suppress tumor growth more potently.
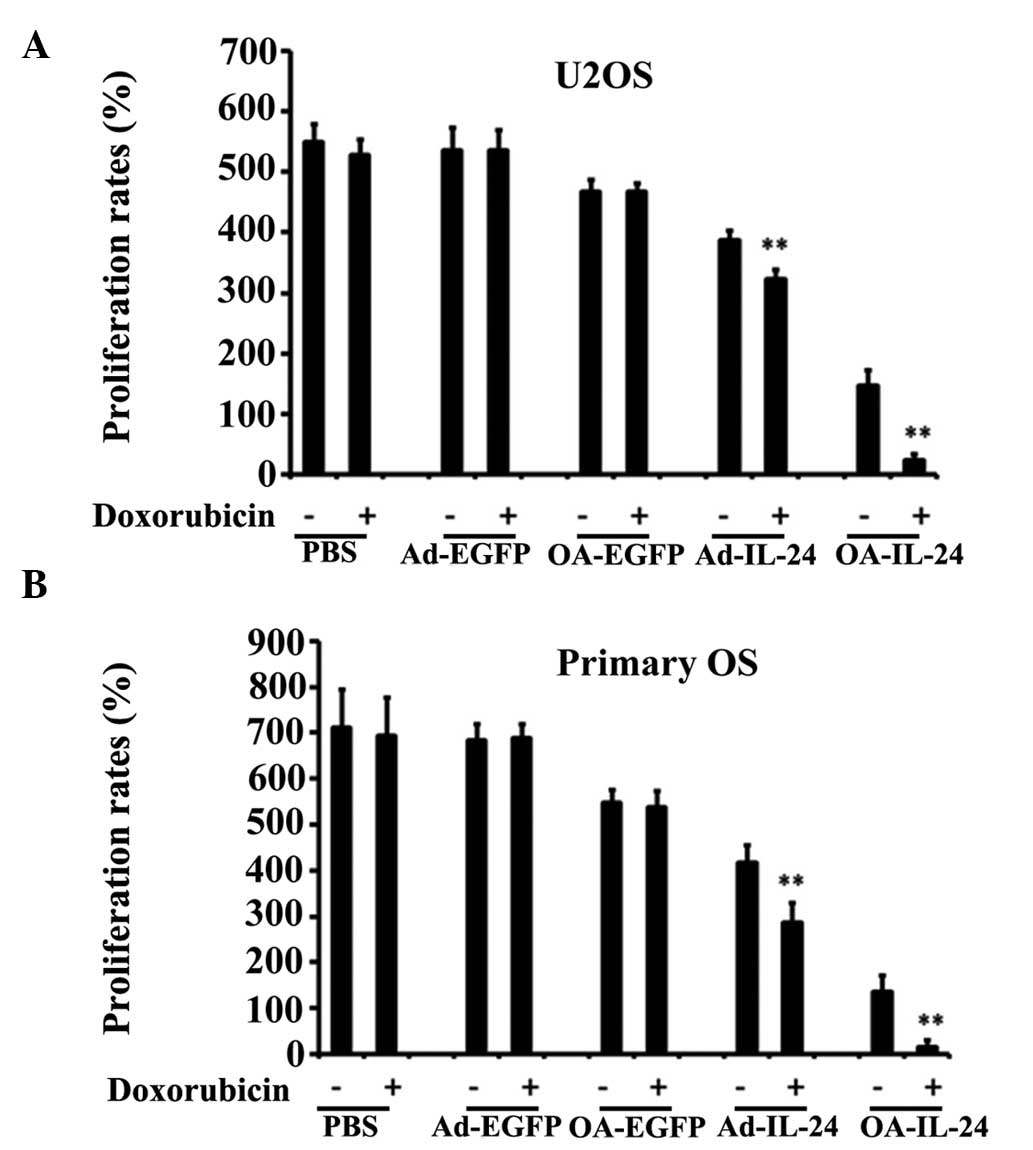
OA-IL-24 reverses multiple drug
resistance (MDR) of OS cells more efficiently than Ad-IL-24
IL-24 was reported to reverse multiple drug
resistance (MDR) of colon cancer cells (11), thus the present study aimed to
determine whether IL-24 can also affect the MDR of OS cells. MTT
assays were used to detect the viability of cells treated with
doxorubicin and indicated adenoviral vectors. The results revealed
that the proliferation rates of U2OS and primary OS cells, which
were infected with OA-IL-24 or Ad-IL-24, were further reduced when
doxorubicin was added (Fig. 4A and
B), indicating that IL-24 was able to increase the sensitivity
of U2OS and primary OS cells to doxorubicin.
OA-IL-24 and Ad-IL-24 suppresses the
expression of Pgp and BCRP1 and autophagy in OS cells
Subsequently, the present study aimed to examine the
mechanism by which OA-IL-24 and Ad-IL-24 enhanced the bioactivity
of doxorubicin. Pgp, BCRP1 and multidrug resistance protein 1
(MRP1) are well-known MDR-associated proteins. Therefore, their
expression levels in OS cells infected with OA-IL-24 or Ad-IL-24
were investigated. The data indicated that IL-24 reduced the
expression of Pgp and BCRP1, but not MRP1, in U2OS and primary OS
cells (Fig. 5A). The inhibitory
effect of OA-IL-24 on the expression of these two proteins appeared
to be higher than Ad-IL-24 (Fig.
5A).
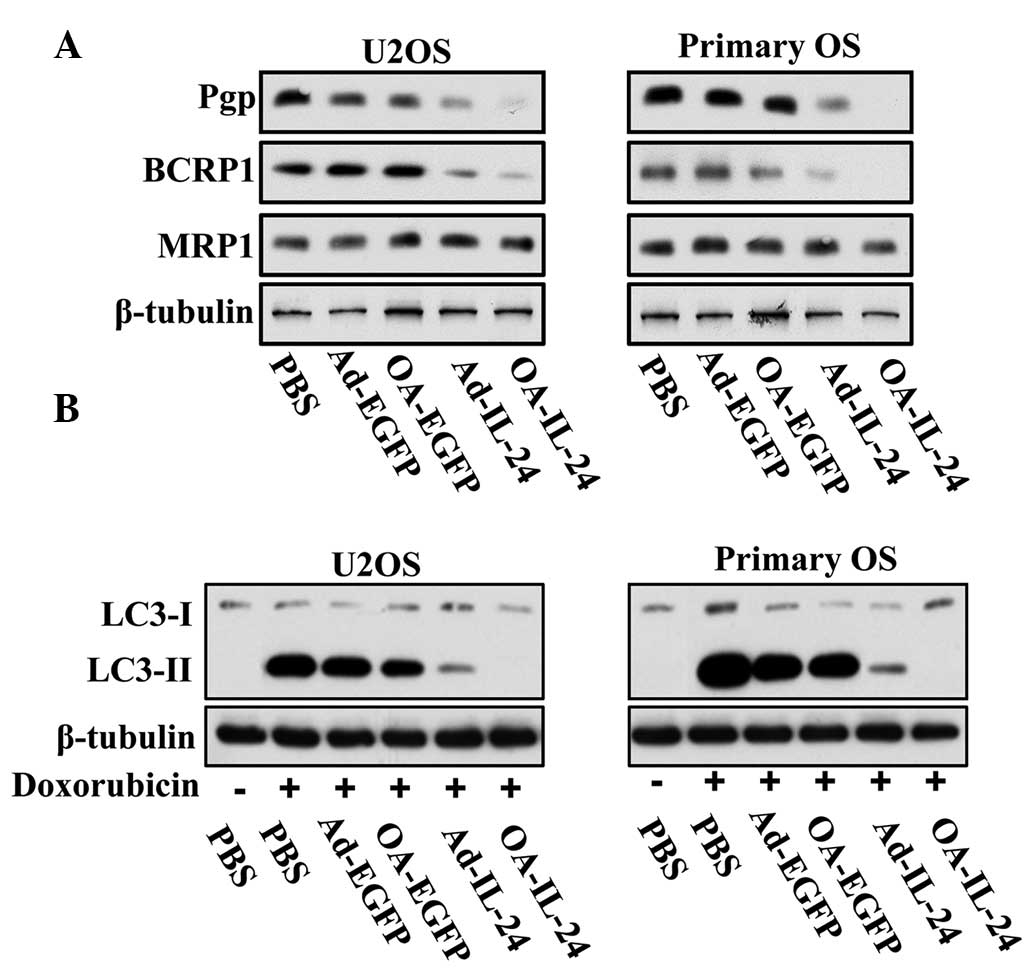 | Figure 5IL-24 suppresses MDR-related proteins
and autophagy in OS cells. (A) U2OS and primary OS cells were
infected with Ad-EGFP, OA-EGFP, Ad-IL-24 or OA-IL-24 (10 MOI) for
48 h. Immunoblot assays were performed to detect the expression
level of Pgp, BCRP1 and MRP1. (B) These two cell lines were
infected with Ad-EGFP, OA-EGFP, Ad-IL-24 or OA-IL-24 (10 MOI) with
or without doxorubicin (100 nM) for 48 h. Immunoblot assays were
performed to detect the expression level of LC3-I and LC3-II. OS,
osteosarcoma; MOI, multiplicity of infection; LC3,
microtubule-associated protein 1A/1B-light chain 3; EGFP, enhanced
green fluorescent protein; MDR, multidrug resistance; OA, oncolytic
adenovirus; PBS, phosphate-buffered saline; MRP1, multidrug
resistance protein 1. |
By contrast, autophagy has been reported to
contribute to the resistance of OS cells to doxorubicin (12). Thus, immunoblot assays were
employed to detect the expression levels of micro-tubule-associated
protein 1A/1B-light chain 3 form 2 (LC3-II), which indicated the
onset of autophagy, in order to evaluate the effect of IL-24
expression on doxorubicin-induced autophagy. The results
demonstrated that IL-24 was able to suppress the expression of
LC3-II induced by doxorubicin (Fig.
5B) in the two cell lines. Consistent with their effects on
MDR-related proteins, OA-IL-24 was able to suppress autophagy more
potently than Ad-IL-24.
The above data demonstrated that OA-IL-24 can reduce
the expression of Pgp and BCRP1, as well as the induction of
autophagy, more potently than Ad-IL-24.
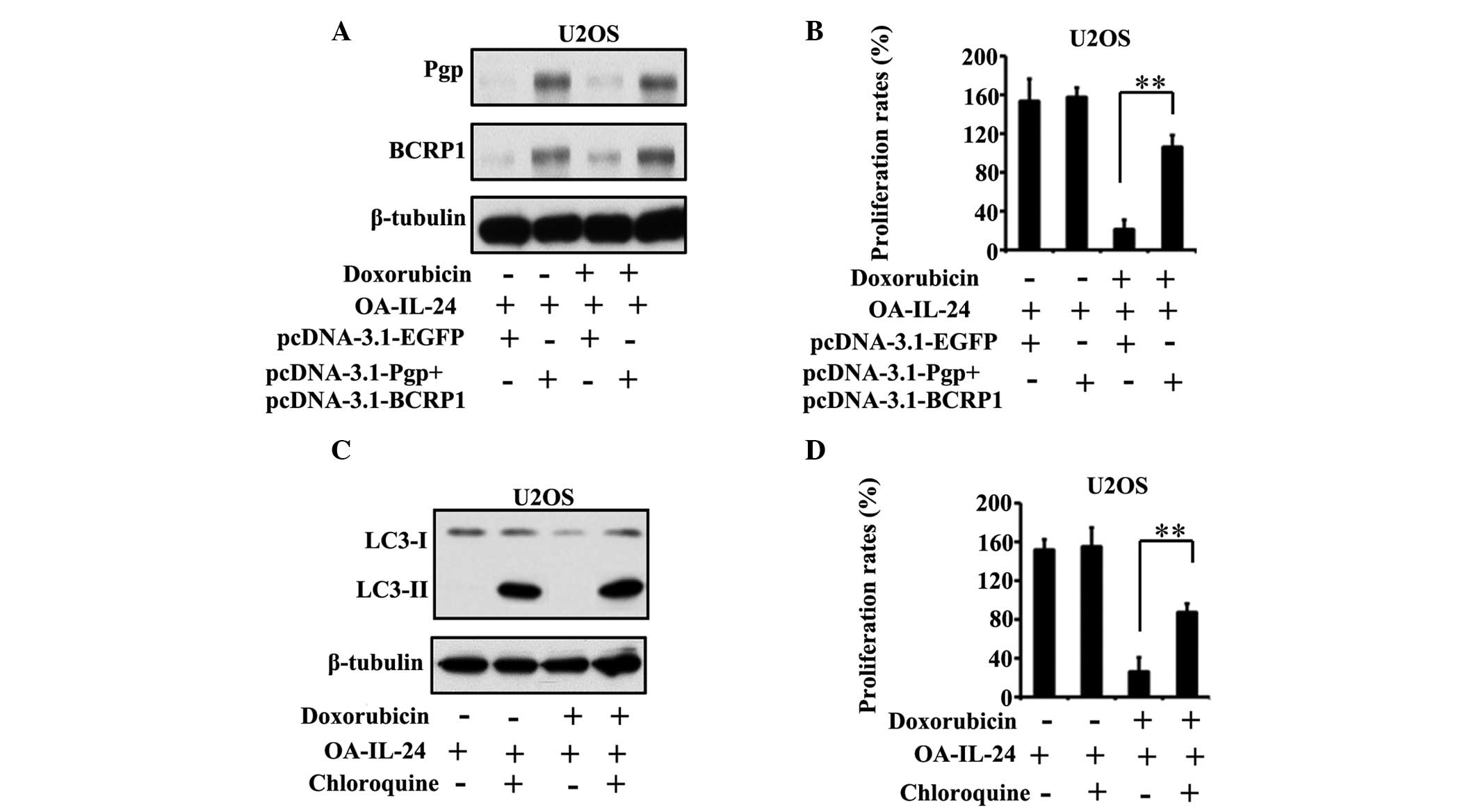
IL-24 suppression of MDR-associated
proteins and autophagy is required for its effect on the
cytotoxicity of doxorubicin
To confirm the role of Pgp and BCRP1 as well as
autophagy on the effects of IL-24 on the cytotoxicity of
doxorubicin, the expression of these two proteins and autophagy in
U2OS cells were restored. Since the aim of the present study was to
examine the importance of Pgp, BCRP1 and autophagy on the effect of
IL-24 on the anti-tumor activity of doxorubicin, OA-IL-24 was used
to overexpress IL-24 in U2OS cells. pcDNA-3.1-Pgp and
pcDNA-3.1-BCRP1 were transfected into U2OS cells at the same time,
and the expression of Pgp and BCRP1 was found to be highly elevated
in the absence and presence of doxorubicin, compared with
pDNA-3.1-EGFP-transfected groups (Fig.
6A). The restoration of Pgp and BCRP1 was able to partially
prevent the effect of IL-24 on the cytotoxicity of doxorubicin to
U2OS cells (Fig. 6B).
In addition, chloroquine was used to stimulate
autophagy in U2OS cells (Fig. 6C).
The results demonstrated that chloroquine-induced autophagy was
able to partially eradicate the effect of IL-24 on the cytotoxicity
of doxorubicin (Fig. 6D).
These data suggested that the suppression of Pgp and
BCRP1 as well as autophagy accounted for IL-24-dependent
sensitization of OS cells to doxorubicin.
Discussion
IL-24 has been well documented to exhibit anti-tumor
activity in various types of cancer (13), including OS (7). The most promising feature of IL-24 is
its selective cytotoxicity to cancer cells. IL-24 has no
significant effect on the viability of normal cells, although it
has been reported to promote the proliferation of dermal cells,
which may lead to psoriasis (14).
The outstanding tumor specificity permits IL-24 to be further
assessed for its clinical application.
Han et al verified that adenoviral
vector-mediated IL-24 expression suppresses the growth of MG-63 OS
cells (7). However, the expression
levels of IL-24 by this replication-deficient adenovirus are
limited, since the vectors cannot proliferate in tumor cells and
thus the copies of IL-24-coding fragments cannot be increased. In
order to solve this issue, researchers have developed oncolytic
adenoviruses for increasing the expression of the gene of interest.
Oncolytic adenoviruses possess the ability to replicate in cancer
cells, rather than normal cells, due to the use of a tumor-specific
promoter for regulating E1A expression. In fact, oncolytic
adenoviruses have been verified to be efficient at expressing a
high level of IL-24 in various types of cancer (15). The present study further
demonstrated that oncolytic adenoviruses can also be used for IL-24
expression in OS cells.
MDR compromises the efficacy of chemotherapy in
clinical treatment, and therefore, eliminating MDR of cancer cells
is urgently required for improving outcomes. IL-24 has been
demonstrated to reverse the MDR phenotype of colon cancer (16), gastric carcinoma (17) and hepatoma (18), possibly by decreasing the
expression of Pgp (16). Oncolytic
adenovirus-mediated IL-24 expression also suppressed the resistance
of colon cancer cells to doxorubicin and 5-fluorouracil (11). The present study further
demonstrated that IL-24 was able to reverse the MDR of OS cells by
downregulating Pgp and BCRP1.
In addition to MDR-related proteins, autophagy can
also reduce the cytotoxicity of doxorubicin. Doxorubicin treatment
induces the onset of autophagy in U2OS and Saos-2 OS cells. In
turn, autophagy minimizes apoptosis in OS cells. Interfering with
autophagy by using siRNA or 3-MA was able to eradicate the
inhibitory effect of autophagy on apoptosis in these cells
(12). The present study also
confirmed that doxorubicin induces autophagy in U2OS cells.
Notably, IL-24 was found to inhibit adaptive autophagy and
therefore augment the anti-tumor activity of doxorubicin. In
accordance with our results, other studies have also observed the
inhibitory effect of IL-24 on autophagy in certain types of cancer,
including leukemia (19) and
prostate carcinoma (20).
Despite its relatively selective cytotoxicity to
tumor cells, IL-24 has also been found to affect the behavior of
normal cells. For instance, IL-24 overexpression can promote
unlimited proliferation of dermal cells in mice, which may lead to
psoriasis (14). The activation of
TNF receptor signaling in keratinocytes is responsible for
IL-24-dependent psoriasis-like skin inflammation (21). A TNF antagonist, Etanercept, can
relieve psoriasis by downregulating IL-24 expression (22). Therefore, novel strategies are
required to minimize the effect of IL-24 on normal dermal cells.
For example, miRNAs that are enriched in dermal cells can be
employed to regulate the expression of IL-24 mediated by adenoviral
vectors. A similar strategy has been used for TRAIL expression
delivered by recombinant adenoviruses in order to prevent its
cytotoxicity to hepatic tissues (23).
In conclusion, an oncolytic adenovirus was generated
to express IL-24 in OS cells and this strategy resulted in a high
expression of IL-24. MDR of OS can be reversed by IL-24 and,
therefore, OA-IL-24 is worth evaluating further for clinical
application to overcome cancer MDR.
References
|
1
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar
|
|
2
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villani F, Galimberti M and Comazzi R:
Early cardiac toxicity of 4′-iodo-4′-deoxydoxorubicin. Eur J
Cancer. 27:1601–1604. 1991. View Article : Google Scholar
|
|
4
|
Dash R, Bhoopathi P, Das SK, Sarkar S,
Emdad L, Dasgupta S, Sarkar D and Fisher PB: Novel mechanism of
MDA-7/IL-24 cancer-specific apoptosis through SARI induction.
Cancer Res. 74:563–574. 2014. View Article : Google Scholar :
|
|
5
|
Luo J, Xia Q, Zhang R, Lv C, Zhang W, Wang
Y, Cui Q, Liu L, Cai R and Qian C: Treatment of cancer with a novel
dual-targeted conditionally replicative adenovirus armed with
mda-7/IL-24 gene. Clin Cancer Res. 14:2450–2457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Lv H, Xie Y, Sheng W, Xiang J and
Yang J: Enhanced tumor suppression by an ING4/IL-24 bicistronic
adenovirus-mediated gene cotransfer in human non-small cell lung
cancer cells. Cancer Gene Ther. 18:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han Y, Miao J, Sheng W, Wang X, Jing Y,
Shan Y, Liu T, Bao W and Yang J: Interleukin 24 inhibits growth and
induces apoptosis of osteosarcoma cells MG-63 in vitro and in vivo.
Sheng Wu Gong Cheng Xue Bao. 25:1538–1545. 2009.In Chinese.
|
|
8
|
Pesonen S, Kangasniemi L and Hemminki A:
Oncolytic adenoviruses for the treatment of human cancer: Focus on
translational and clinical data. Mol Pharm. 8:12–28. 2011.
View Article : Google Scholar
|
|
9
|
Sun X, Geng X, Zhang J, Zhao H and Liu Y:
miR-155 promotes the growth of osteosarcoma in a HBP1-dependent
mechanism. Mol Cell Biochem. 403:139–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma L, Liu J, Liu L, Duan G, Wang Q, Xu Y,
Xia F, Shan J, Shen J, Yang Z, et al: Overexpression of the
transcription factor MEF2D in hepatocellular carcinoma sustains
malignant character by suppressing G2-M transition genes. Cancer
Res. 74:1452–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Mo Y, Wang X, Liu J, Zhang X, Wang
J, Hu L, Yang C, Chen L and Wang Y: Conditionally replicative
adenovirus-based mda-7/IL-24 expression enhances sensitivity of
colon cancer cells to 5-fluorouracil and doxorubicin. J
Gastroenterol. 48:203–213. 2013. View Article : Google Scholar
|
|
12
|
Zhao D, Yuan H, Yi F, Meng C and Zhu Q:
Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma.
Mol Med Rep. 9:1975–1981. 2014.PubMed/NCBI
|
|
13
|
Whitaker EL, Filippov VA and
Duerksen-Hughes PJ: Interleukin 24: Mechanisms and therapeutic
potential of an anti-cancer gene. Cytokine Growth Factor Rev.
23:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan JR, Blumenschein W, Murphy E, Diveu
C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S,
Kimball AB, et al: IL-23 stimulates epidermal hyperplasia via TNF
and IL-20R2-dependent mechanisms with implications for psoriasis
pathogenesis. J Exp Med. 203:2577–2587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu XY and Gu JF: Targeting
gene-virotherapy of cancer. Cell Res. 16:25–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Emdad L, Lebedeva IV, Su ZZ, Sarkar D,
Dent P, Curiel DT and Fisher PB: Melanoma differentiation
associated gene-7/interleukin-24 reverses multidrug resistance in
human colorectal cancer cells. Mol Cancer Ther. 6:2985–2994. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Z, Bian G, Sheng W, He S, Yang J and
Dong X: Adenovirus-mediated IL-24 expression enhances the
chemo-sensitivity of multidrug-resistant gastric cancer cells to
cisplatin. Oncol Rep. 30:2288–2296. 2013.PubMed/NCBI
|
|
18
|
Fang P, Zhang X, Gao Y, Ding CR, Cui F and
Jiao SC: Reversal effect of melanoma differentiation associated
gene-7/interleukin-24 on multidrug resistance in human
hepatocellular carcinoma cells. Anat Rec (Hoboken). 295:1639–1646.
2012. View
Article : Google Scholar
|
|
19
|
Yang C, Tong Y, Ni W, Liu J, Xu W, Li L,
Liu X, Meng H and Qian W: Inhibition of autophagy induced by
overexpression of mda-7/interleukin-24 strongly augments the
antileukemia activity in vitro and in vivo. Cancer Gene Ther.
17:109–119. 2010. View Article : Google Scholar
|
|
20
|
Bhutia SK, Dash R, Das SK, Azab B, Su ZZ,
Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, et al: Mechanism of
autophagy to apoptosis switch triggered in prostate cancer cells by
antitumor cytokine melanoma differentiation-associated gene
7/interleukin-24. Cancer Res. 70:3667–3676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumari S, Bonnet MC, Ulvmar MH, Wolk K,
Karagianni N, Witte E, Uthoff-Hachenberg C, Renauld JC, Kollias G,
Toftgard R, et al: Tumor necrosis factor receptor signaling in
keratinocytes triggers interleukin-24-dependent psoriasis-like skin
inflammation in mice. Immunity. 39:899–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Smith N, Maier L, Xia W,
Hammerberg C, Chubb H, Chen C, Riblett M, Johnston A, Gudjonsson
JE, et al: Etanercept suppresses regenerative hyperplasia in
psoriasis by acutely downregulating epidermal expression of
interleukin (IL)-19, IL-20 and IL-24. Br J Dermatol. 167:92–102.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Ma L, Li C, Zhang Z, Yang G and
Zhang W: Tumor-targeting TRAIL expression mediated by miRNA
response elements suppressed growth of uveal melanoma cells. Mol
Oncol. 7:1043–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|