Introduction
It is well established that diabetes mellitus (DM),
a common chronic metabolic disorder, has devastating effects on the
central nervous system. Cognitive dysfunction is considered to be
the most widespread complication of DM and is exacerbated via
increasing neuronal apoptosis (1).
In 2006, the term 'diabetes-associated cognitive deficits' (DACD)
was proposed as a novel concept to strengthen the recognition of
this disease (2). In addition, a
previous study revealed that the incidence of Alzheimer's disease,
which has the characteristics of cognitive deficits, among patients
with DM was double of that among patients with a normal glucose
metabolism (3), indicating that
drugs for alleviating DACD should act via improving glycemic
control.
The mitogen-activated protein kinases (MAPKs) are
conserved signal-transducing enzymes that have crucial roles in the
regulation of cellular function, including proliferation and
cellular apoptosis. There are at least three sub-groups of MAPKs:
Extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK) and p38 (4). ERK
activation improves cellular survival, while JNK and p38 MAPK
promote cell death. In fact, prior studies revealed that the
activated form of JNK, phosphorylated (p)-JNK, was markedly
augmented at the protein level in transgenic diabetic animals
(db/db mice) (5). Brain-derived
neurotrophic factor (BDNF) is an important member of the
neurotrophin family of growth factors. It was originally reported
to support several facets of central nervous system development,
including neuronal survival and the synapse formation (6). In addition, the glucose content has
been identified to be modulated by elevated generation of BDNF in
the pancreas of diabetic mice (7).
Furthermore, it was previously demonstrated that BDNF rapidly
enhanced insulin signaling in streptozotocin (STZ)-induced diabetic
mice (8), suggesting the
hypoglycaemic action of BDNF. These findings suggested that MAPK
cascades and BDNF provide potential therapeutic targets for the
treatment of DM-induced nerve injury.
Baicalin is an important natural product extracted
from the plant Scutellaria baicalensis Georgi and possesses
various pharmacological activities, including as anti-inflammatory
(9), anti-oxidative (10) and anti-apoptotic (11) properties. Waisundara et al
(12) found that baicalin
significantly diminished hyperglycemia-induced mitochondrial
membrane damage in Wistar rats. Li et al (13) also reported the anti-hyperglycemic
effects of baicalin on STZ-nicotinamide induced diabetic rats.
However, whether baicalin exerts protective effects against
cognitive deficits caused by diabetes has remained elusive. The
present study therefore investigated the hypothesis that baicalin
protects against DACD and that the neuroprotective effects are
associated with the modulation of MAPK signaling and BDNF. The
present study focused on the evaluation of the effects of baicalin
on DACD and explored the potential molecular mechanisms using an
STZ-induced rat model of diabetes mellitus.
Materials and methods
Experimental animals
Adult male Wistar rats (8-week-old; Beijing Animal
Center, Beijing, China) weighing 200–250 g were used in the present
study. They were kept in individual cages under a standard
environment (12:12 h light/dark cycle and 50–70% humidity; 37°C)
with free access to water and food. All surgical procedures were
performed in strict accordance with the guidelines established by
the Animal Care Committee of Central South University (Changsha,
China).
Establishment of diabetic rat model and
drug treatment
The rat model of diabetes was established by
intraperitoneal (i.p.) injection of a single dose of 65 mg/kg STZ
(Sigma-Aldrich, St. Louis, MO, USA), freshly dissolved in citrate
buffer (pH 4.4; 0.1 M). At 48 h post-STZ injection, blood samples
were acquired and plasma glucose levels were assessed using an
enzymatic glucose oxidase peroxidase diagnostic kit (Span
Diagnostic Chemicals, Surat, India). Animals with fasting plasma
glucose levels >250 mg/dl (14)
were deemed to be diabetic and selected for the subsequent
experiment. Animals were randomly divided into five groups each
consisting of eight rats: 1) The control group, which was injected
with citrate buffer only and received physiological saline
treatment (0.1 ml/100 g i.p.); 2) the vehicle group, in which
diabetes had been induced by STZ injection and which received
physiological saline treatment (0.1 ml/100 g i.p.); and the
baicalin groups, comprising diabetic rats which were administered
baicalin at doses of 3) 50, 4) 100 and 5) 200 mg/kg baicalin
(Sigma-Aldrich, St. Louis, MO, USA; purity, >95%; freshly
dissolved in physiological saline; i.p. injection), respectively.
Starting from the third day of the experiment until the seventh
week, the control and diabetic groups received vehicle or baicalin
treatment.
Upon finalization of the drug treatment period, the
animals from the different groups were subjected to the Morris
water maze test to evaluate their learning and memory function over
five consecutive days. Blood samples and hippocampal tissues were
then collected for subsequent biochemical analysis.
Morris water maze test
At seven weeks post-STZ injection, the cognitive
function of the rats was assessed by the Morris water maze test as
previously described (15) The
Morris water maze consisted of a circular water tank (inner
diameter, 90 cm; height, 50 cm), equipped with a digital pick-up
camera (V2.20; Nikon Corporation, Tokyo, Japan) 180 cm above the
water surface. The tank was filled with water, which was made
opaque by adding a white-colored dye (10 g/L; Shanghai Xinruan
Technology, Co., Ltd., Shanghai, China. The tank was divided into
four equal quadrants, which were labeled as North, South, East and
West. These cues were constant throughout the experiment. A round
escape platform was placed ~2 cm below the water surface. The
navigation test was performed over four consecutive days. In the
test, the escape latency (s) and path length (cm) to find the
platform were determined. Furthermore, the swimming speed was
calculated by dividing the path length by the time to find the
platform. On the fifth day, the escape platform was removed from
the tank and each animal was subjected to a spatial probe test. The
number of times the rat crossed the target quadrant (where the
platform was once hidden) and the time spent in the former platform
quadrant within 60 sec were measured.
Assessment of acetylcholinesterase (AChE)
and choline acetylase (ChAT) activities
After baicalin treatment for seven weeks, the
activities of AChE and ChAT in the hippocampi of animals from
different groups were analyzed using respective commercial kits
(kit no. A023 for AChE and kit no. A079-1 for ChAT; Nanjing
Jiancheng Biotechnology Institute, Nanjing, China).
Western blot analysis
The rats from each group were sacrificed by
decapitation at seven weeks post-STZ injection. For western blot
analysis, the hippocampi were homogenized at a ratio of 1:5 (w/v)
in cold radioimmunoprecipitation assay lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 10% glycerol, 1% Nonidet P-40, 5 mM EDTA and
1 mM phenylmethylsulfonyl fluoride). After centrifugation at 13,200
× g for 20 min at 4°C, the supernatant was collected and divided
into aliquots, which were stored at −80°C.
Thirty micrograms of protein were separated by
SDS-PAGE and transferred onto nitrocellulose membranes (Millipore,
Billerica, MA, USA). After being blocked in 5% defatted milk for 1
h at room temperature, the membranes were probed respectively with
the following primary antibodies: Rabbit anti-ERK (1:300; cat. no.
sc-292838), mouse anti-phospho (p)-ERK (Tyr204) (1:200; cat. no.
sc-377400), goat anti-JNK (1:200; cat. no. sc-46006), mouse
anti-p-JNK (Thr183/Tyr185) (1:200; cat. no. sc-81502), rabbit
anti-p38 MAPK (1:200; cat. no. sc-535), rabbit anti-p-p38 MAPK
(1:200; cat. no. sc-101758), rabbit anti-BDNF (1:200; cat. no.
sc-20981), rabbit anti-Bax (1:200; cat. no. sc-526), rabbit
anti-Bcl-2 (1:200; cat. no. sc-492), rabbit anti-caspase-3 (1:300;
cat. no. sc-7148) and mouse anti-β-actin (1:2,000; cat. no.
sc-8432) (all from Santa Cruz Biotechnology, Inc, Dallas, TX, USA)
or mouse anti-GAPDH (1:2,000; cat. no. KC5G4; Kang Chen, China),
overnight at 4°C. The membranes were then washed three times with
PBS and incubated with horseradish peroxidase-conjugated goat
anti-rabbit antibody (1:5,000; cat. no. sc-2004), rabbit anti-goat
antibody (1:5,000; cat. no. sc-2768) or goat anti-mouse antibody
(1:5,000; cat. no. sc-2005) (all from Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Immunolabeled protein bands were
detected using an enhanced chemiluminescence (ECL) kit (cat. no.
32106; Pierce Biotechnology, Inc., Thermo Fisher Scientific,
Waltham, MA, USA). Films (Kodak, Rochester, NY, USA) were digitized
by a scanner (Fi-6130Z; Fujitsu, Hong Kong, China) and the relative
optical density of the bands was analyzed by Quantity One software
(v4.62; BioRad Laboratories, Inc., Hercules, CA, USA).
Detection of caspase-3 activity in the
hippocampus
Caspase-3 activity was evaluated by commercial kits
following the manufacturer's instructions (catalog no. BF3100;
R&D Systems, Minneapolis, MN, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation. Differences between groups were assessed by analysis of
variance. Statistically analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). A P<0.05 was considered
to indicate a statistically significant difference.
Results
Baicalin normalizes body weight and blood
glucose levels in STZ-induced diabetic rats
As shown in Table
I, the diabetic group exhibited an evident reduction in body
weight (P<0.01) to almost 50% of that in the control group.
However, treatment with various doses of baicalin (50, 100 and 200
mg/kg) almost completely prevented this diabetes-associated weight
loss (P<0.01). Furthermore, diabetic animals exhibited marked
elevation of plasma glucose levels (P<0.01), compared with the
control group, while Baicalin treatment dose-dependently reversed
the diabetes-associated increases in glucose (P<0.01).
 | Table IEffects of baicalin on body weight and
blood glucose levels in the control and streptozotocin-induced
diabetic rats (n=8) at the onset and at the end of the
experiment. |
Table I
Effects of baicalin on body weight and
blood glucose levels in the control and streptozotocin-induced
diabetic rats (n=8) at the onset and at the end of the
experiment.
| Treatment | Body weight (g)
| Plasma glucose
(mg/dl)
|
|---|
| Onset of study | End of study | Onset of study | End of study |
|---|
| Con | 215.68±3.25 | 266.68±3.32 | 105.68±3.35 | 107.23±2.11 |
| Vehicle | 221.57±4.26 | 126.66±2.88a | 107.46±3.15 | 596.36±3.28a |
| B50 | 226.37±5.48 | 252.36±3.37b | 109.11±3.87 | 226.54±2.98b |
| B100 | 227.47±4.66 | 259.58±4.32b | 106.46±2.12 | 189.57±3.08b |
| B200 | 230.53±4.87 | 261.67±6.01b | 108.93±3.57 | 179.98±3.65b |
Baicalin ameliorates learning and memory
deficits in STZ-induced diabetic rats
After seven weeks of baicalin or vehicle treatment,
mice performed the Morris water maze test in order to demonstrate
learning and memory function. As shown in Fig. 1A, no significant difference was
observed between any of the groups on the first day. From the
second day onwards, a longer escape latency was found in
STZ-treated rats (P<0.01) when compared to that in the control
group. Baicalin treatment significantly diminished
diabetes-associated increased in the escape latency in a
dose-dependent manner (P<0.01). Similarly, an obvious increase
in the mean path length over four consecutive days of training was
observed in the diabetic animals (P<0.01) as compared with that
in the control group (Fig. 1B),
while baicalin treatment dose-dependently reversed this phenomenon
(P<0.01). Furthermore, the time of the animals remaining in the
target quadrant and the number of times the animals crossed the
former platform location on day 5 were markedly decreased in
diabetic rats compared with those in the control group (P<0.01)
(Fig. 1C and D). However,
treatment with baicalin dose-dependently decreased these two
indices (P<0.01). All of these results indicated that
diabetes-associated decreases in learning and memory performance of
the animals were attenuated by treatment with baicalin. As to the
swimming speed, there was no statistical significance among all
groups during the four training days (Fig. 1E).
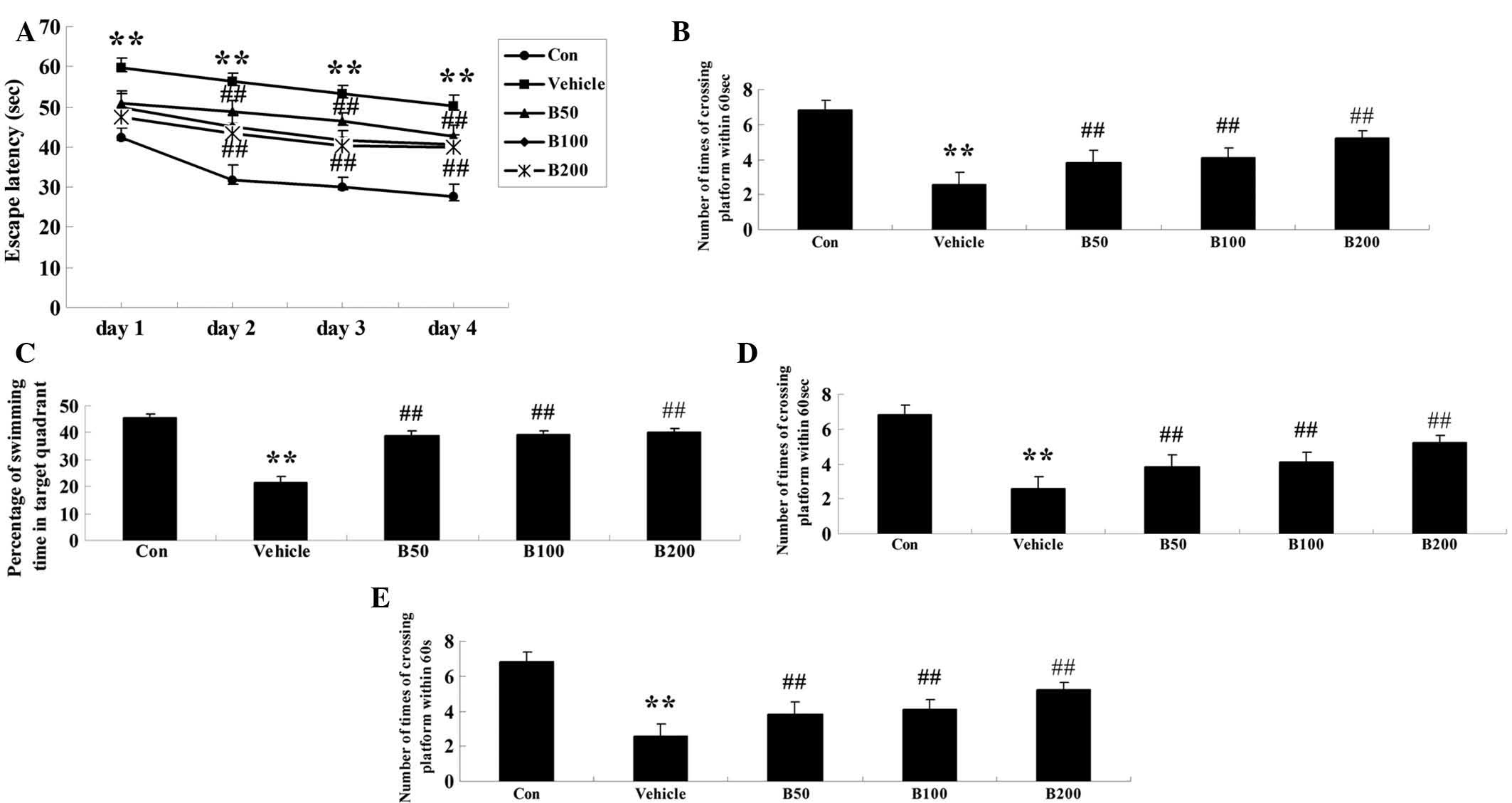 | Figure 1Assessment of the effects of baicalin
on the cognitive performance of rats in the water maze test.
Effects of baicalin on (A) the escape latency, (B) mean path
length, (C) mean percentage of time spent in the target quadrant,
(D) the number of times of crossing platform and (E) swimming speed
in streptozotocin-induced diabetic rats. Values are expressed as
the mean ± standard deviation (n=8). **P<0.01,
compared with Con group; ##P<0.01, compared with
vehicle group. Groups: Con, control; vehicle, diabetes; B50,
baicalin (50 mg/kg)-treated; B50, baicalin (100 mg/kg)-treated;
B200, baicalin (200 mg/kg)-treated. |
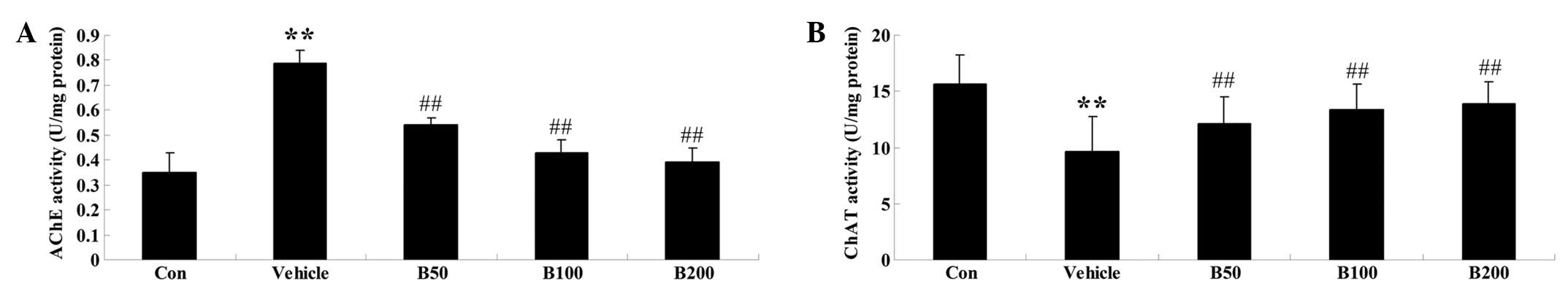
Baicalin treatment normalizes the
activities of AChE and ChAT in STZ-induced diabetic rats
As shown in Fig.
2A, the activity of AChE was found to be significantly elevated
in the hippocampi of diabetic rats (P<0.01) compared with that
in the control animals. However, administration of various doses of
baicalin (50, 100 and 200 mg/kg) obviously prevented these
increases (P<0.01). Furthermore, the hippocampi of diabetic rats
exhibited decreased activity of ChAT, while baicalin treatment
reversed this phenomenon in a dose-dependent manner (P<0.01)
(Fig. 2B).
Baicalin attenuates diabetes-induced
changes in the activation of MAPK proteins and BDNF expression
The activation of the MAPK proteins ERK, JNK and p38
were determined in the hippocampi of mice of different groups by
western blot analysis (Fig. 3).
Quantitative analysis revealed an evident decrease of p-ERK, the
activated form of ERK, in the hippocampi of diabetic rats
(P<0.01) (Fig. 3A and B), while
a marked elevation of p-JNK and p-p38 was observed (P<0.01)
(Fig. 3C–F). Baicalin treatment of
the diabetes-induced rats evidently augmented the expression levels
of p-ERK protein (P<0.01) and diminished the protein levels of
p-JNK (P<0.01) and p-p38 (P<0.01), compared with those in the
vehicle-treated diabetic group. Furthermore, the protein levels of
BDNF were significantly decreased in diabetic animals compared with
those in the control group (P<0.01; Fig. 3G and H). However, treatment of the
diabetes-induced rats with baicalin (50, 100 and 200 mg/kg)
evidently increased the protein levels of BDNF (P<0.01).
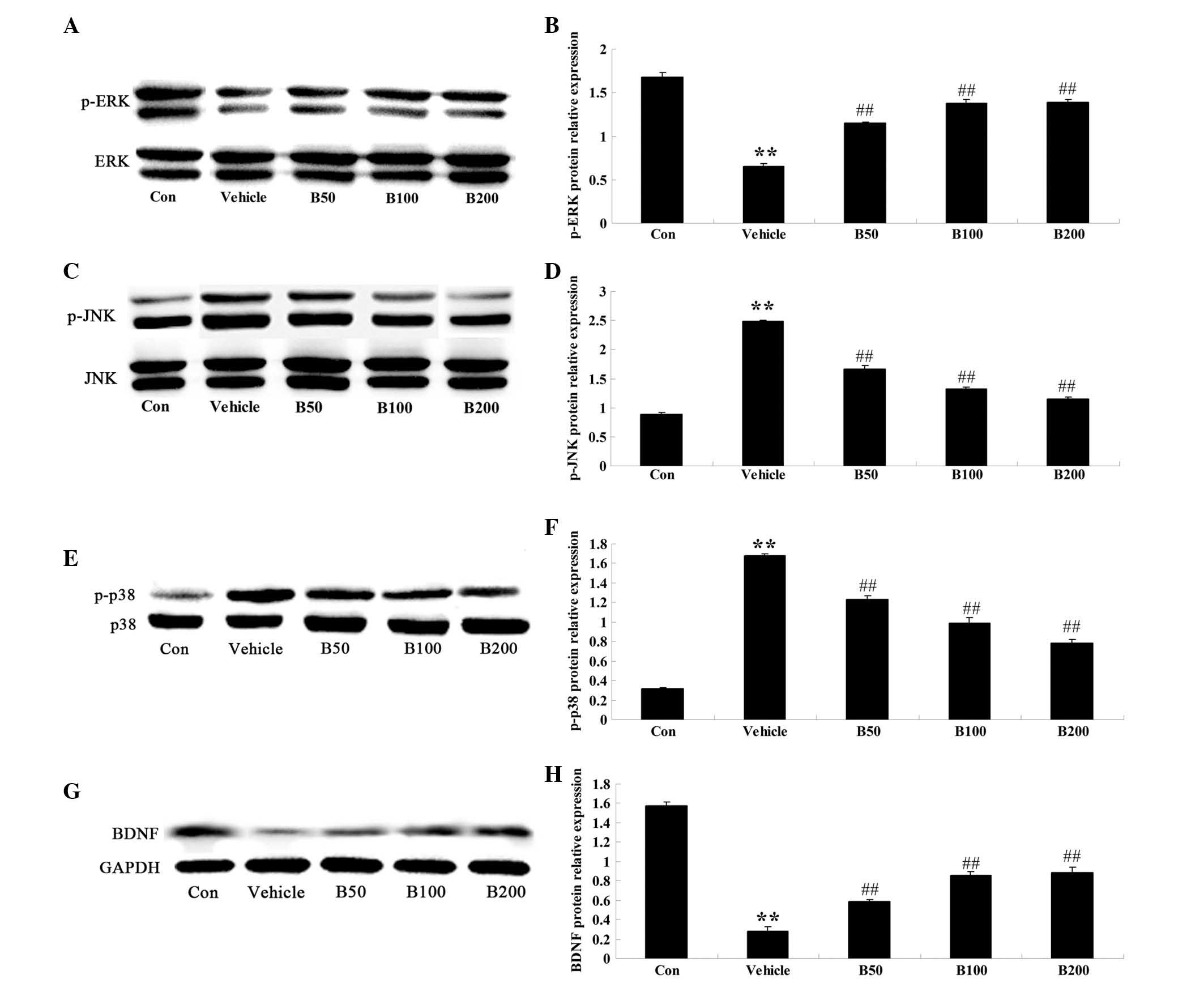 | Figure 3Effects of baicalin on the MAPK
cascades and BDNF protein expression in the hippocampi of
streptozotocin-induced diabetic rats. (A, C, E and G)
Representative immunoblot images of p-ERK, ERK, p-JNK, JNK, p-p38
MAPK, p38 MAPK, BDNF, β-actin and GAPDH, respectively, in
hippocampi of rats from different groups. p-ERK: 44 and 42 kDa;
ERK: 44 and 42 kDa; p-JNK: 54 and 46 kDa; JNK: 54 and 46 kDa;
p-p38: 38 kDa; p38: 38 kDa; β-actin: 43 kDa; GAPDH: 36 kDa. (B, D,
F and H) Quantified expression levels obtained from the blots by
densitometric analysis normalized to the non-phosphorylated species
or GAPDH, respectively. Values are expressed as the mean ± standard
deviation (n=8). **P<0.01, compared with Con group;
##P<0.01, compared with vehicle group. Groups: Con,
control; vehicle, diabetes; B50, baicalin (50 mg/kg)-treated; B50,
baicalin (100 mg/kg)-treated; B200, baicalin (200 mg/kg)-treated.
MAPK, mitogen-activated protein kinase; BDFN, brain-derived
neurotrophic factor; p-ERK, phosphorylated extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase. |
Baicalin inhibits apoptosis-associated
signaling in STZ-induced diabetic rats
In order to investigate whether baicalin affects the
expression levels of regulator proteins of apoptosis in the
hippocampi of rats with STZ-induced diabetes, western blot analysis
was performed to detect the protein levels of caspase-3, Bax and
Bcl-2 in the hippocampal samples from various experimental groups.
As shown in Fig. 4A and B,
caspase-3 protein levels in diabetic rats were significantly
augmented compared with those in the control group (P<0.01),
while baicalin treatment of the diabetic rats resulted in an
obvious reduction of caspase-3 to almost basal levels (P<0.01).
Furthermore, the diabetic rats exhibited elevated expression levels
of Bax and decreased expression levels of Bcl-2 compared with those
in the vehicle-treated group (P<0.01) (Fig. 4C and D), which was significantly
attenuated by treatment of the diabetic animals with baicalin
(P<0.01).
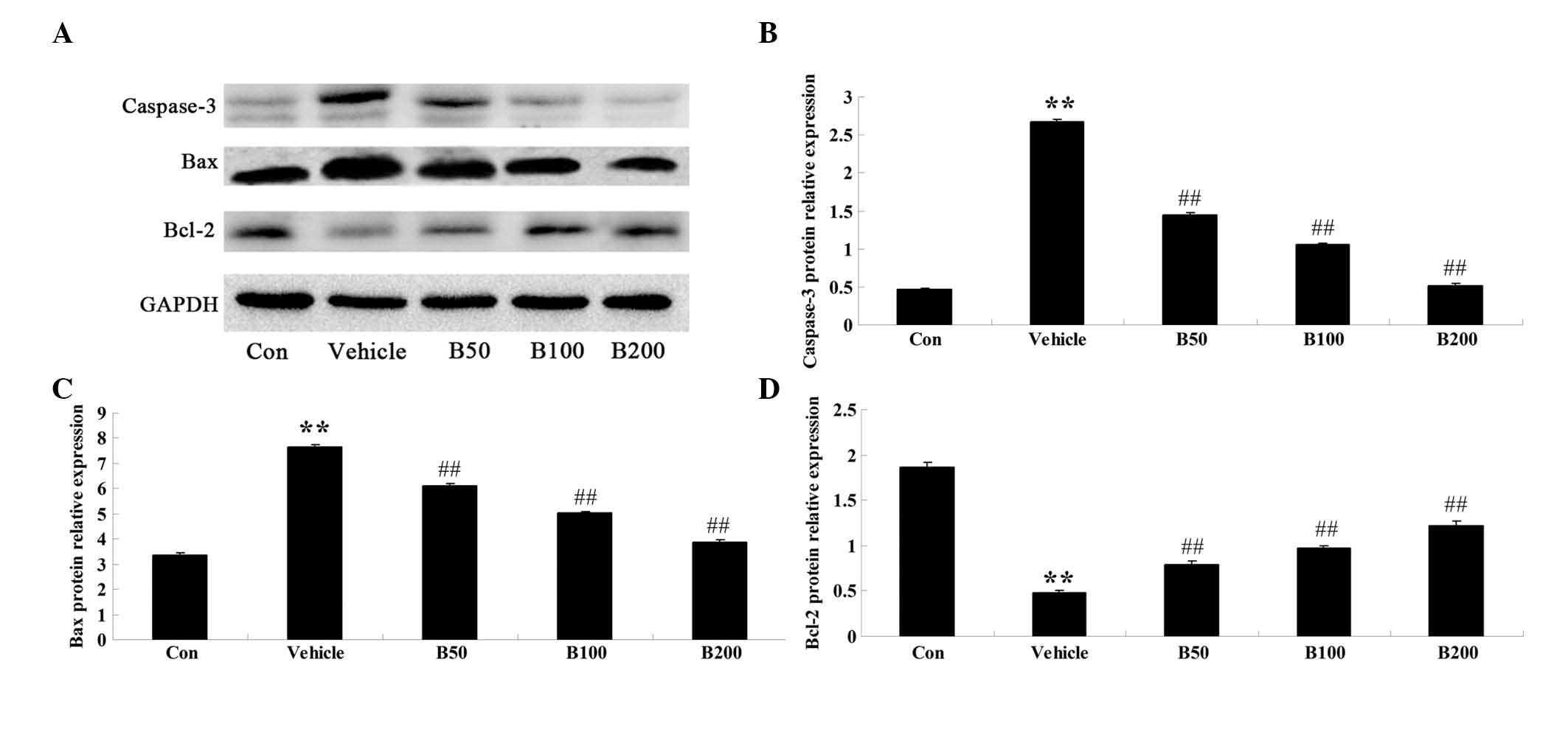 | Figure 4Effects of baicalin on the expressions
of regulatory proteins of apoptosis in the hippocampi of
streptozotocin-induced diabetic rats. (A) Representative immunoblot
images of caspase-3, Bax, Bcl-2 and β-actin in rat hippocampi from
different groups. Caspase-3: 20 kDa; Bax: 23 kDa; Bcl-2: 26 kDa;
β-actin: 43 kDa. (B–C) Quantitative expression levels of (B)
caspase-3 (C) Bax and (D) Bcl-2. Values are expressed as the mean ±
standard deviation (n=8). **P<0.01, compared with Con
group; ##P<0.01, compared with vehicle group. Groups:
Con, control; vehicle, diabetes; B50, baicalin (50 mg/kg)-treated;
B50, baicalin (100 mg/kg)-treated; B200, baicalin (200
mg/kg)-treated. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
Baicalin inhibits caspase-3 activity in
STZ-induced diabetic rats
In order to verify the anti-apoptotic effects of
baicalin in the hippocampi of rats with diabetes, the activity of
caspase-3 was further analyzed using a colorimetric assay. As shown
in Fig. 5, caspase-3 was obviously
activated in STZ-induced diabetic rats compared with that in the
control (P<0.01). However, treatment with baicalin significantly
attenuated this diabetes-induced caspase activation in a
dose-dependent manner (P<0.01).
Discussion
The present study revealed a novel mechanism by
which baicalin exerts protective effects on the brains of diabetic
rats against cognitive deficits via altering MAPK cascades and
suppressing BDNF protein levels. Body weight and plasma glucose
levels of diabetic rats were normalized by baicalin treatment,
suggesting a hypoglycemic effect of baicalin. Diabetic rats showed
decreased learning and memory function in the water maze test,
which was improved by treatment with baicalin. Furthermore,
baicalin inhibited diabetes-induced increases in AChE activity and
decreases in ChAT activity in rat hippocampi. In addition, baicalin
attenuated diabetes-induced decreases of p-ERK and BDNF protein
levels as well as increases of p-JNK and p-p38 protein levels.
These findings indicated that the neuroprotective mechanisms of
baicalin likely involved the modulation of MAPK cascades and
inhibition of BDNF. Western blot analysis further revealed that
baicalin treatment attenuated diabetes-associated upregulation of
apoptosis-associated signaling, by diminishing increases in
caspase-3 and Bax as well as decreases in Bcl-2. In line with these
results, colorimetric analysis revealed a reduction of caspase-3
activity in baicalin-treated diabetic rats.
Chronic hyperglycemia was reported to be closely
associated with the pathogenesis of cognitive dysfunction (16). Indeed, patients with type 1
diabetes showed cognitive impairment according to neurological
cognitive tests, including visuospatial deficits (17) as well as impairment of learning and
memory (18). These findings
suggested that glycemic control is essential to ameliorate DACD.
Previous studies demonstrated the protective effects of several
medicinal herbs against cognitive decline in diabetic animals on
the basis of their hypoglycemic properties (19,20).
The present study illustrated that baicalin significantly decreased
blood glucose levels and had neuroprotective effects against DACD
in rats. Another previous study reported the anti-hyperglycemic
effects of baicalin in a STZ-nicotinamide induced rat model of
diabetes, which was in part consistent with the results of the
present study (13). Furthermore,
Waisundara et al (12) also
reported that baicalin decreased hyperglycemia-induced
mitochondrial damage in diabetic rats, which further evidenced the
hypoglycemic properties of baicalin. In conclusion, the present and
previous studies indicated that the hypoglycemic properties of
baicalin were in parallel with its amelioration of cognitive
impairment in diabetes.
AChE and ChAT are considered as two specific markers
of cholinergic dysfunction, which has a critical role in the
pathogenesis of cognitive deficits induced by diabetes (21). Under normal circumstances,
acetylcholine, an index positively correlated with memory function,
is decomposed by AChE and synthesized by ChAT in the hippocampus
and cerebral cortex (21). In
fact, the selective AChE inhibitors donepezil and huperzine A have
been shown to improve the learning and memory performance in a
gerbil model of ischemia (22) and
a rat model of diabetes (23). The
results of the present study demonstrated that treatment with
baicalin markedly attenuated diabetes-associated increases in AChE
activity and decreases in ChAT activity. Furthermore, preliminary
experiments by our group identified synergic effects of the AChE
inhibitor donepezil and baicalin on the reduction of plasma glucose
levels, suggesting that baicalin exerts its antihyperglycemic
effects in an AChE-dependent manner.
It is known that neuronal apoptosis is involved in
the occurrence of diabetes-induced learning and memory dysfunction
(24). The present study revealed
an evident attenuation of diabetes-associated increases of
caspase-3 (an executioner molecule in the apoptotic cascades) and
Bax (a pro-apoptotic molecule) as well as decreases of Bcl-2 (a
cell survival molecule) in the hippocampi of rats by administration
of baicalin. These findings suggested that baicalin exerted its
neuroprotective effects against cognitive deficits in
diabetes-induced rats via suppressing the apoptotic signaling
pathway.
Emerging evidence supports that MAPK is a key
regulator of long-term synaptic plasticity and long-term memory
(25,26). Furthermore, the ERK cascade has
been reported to contribute to synaptic plasticity that underlies
learning and memory (27).
Activation of ERK by MAPK/ERK kinase resulted in the
phosphorylation of cyclic adenosine monophosphate response
element-binding protein and subsequently improved long-term memory
in young rats (27). In addition,
Xuan et al (28) found that
the inhibition of p-p38 by fenofibrate facilitated the functional
recovery of memory deficits in rat hippocampi following global
cerebral ischemia. In fact, suppression of p38 and JNK
phosphorylation were shown to prevent high-glucose-induced
neurotoxicity in PC12 cells (29).
The present study demonstrated that baicalin attenuated
DACD-associated decreases in ERK1/2 phosphorylation and increases
in p-JNK and p-p38 phosphorylation, which was partly consistent
with the results of previous studies (28). This also implied that the
modulation of the MAPK signaling pathway is involved in the
neuroprotective effects of baicalin against DACD.
BDNF is the major component of the neurotrophin
family and reduces the risk of mild cognitive impairments in
patients (30). It was also
reported that decreased levels of BDNF in hippocampi led to serious
cognitive dysfunction in a STZ-induced rat model of diabetes
(21). Consistent with these
findings, the present study revealed that baicalin rescued
diabetes-associated decreases of BDNF in the hippocampi of rats,
suggesting that baicalin protects against DACD via elevating the
BDNF content in the hippocampus.
In conclusion, the present study illustrated that
baicalin has normalizes blood glucose levels, improves learning and
memory function and reduces neuronal damage in diabetic rats.
Modulation of MAPK cascades and elevation of BDNF expression may be
linked with the neuroprotective effects of baicalin in diabetic
rats. According to the results of the present study, baicalin may
serve as a useful drug for the treatment of patients with DACD;
however, it is essential to further explore whether other
modulators are involved in neuroprotective effects of baicalin
against DACD.
Acknowledgments
This work was financially supported by the National
Natural Science Foundation of China (gran no. 81302750) and the
China Postdoctoral Science Foundation (gran no. 2014M552168).
References
|
1
|
Wrighten SA, Piroli GG, Grillo CA and
Reagan LP: A look inside the diabetic brain: Contributors to
diabetes-induced brain aging. Biochim Biophys Acta. 1792:444–453.
2009. View Article : Google Scholar
|
|
2
|
Mijnhout GS, Scheltens P, Diamant M,
Biessels GJ, Wessels AM, Simsek S, Snoek FJ and Heine RJ: Diabetic
encephalopathy: A concept in need of a definition. Diabetologia.
49:1447–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwon KJ, Lee EJ, Kim MK, Kim SY, Kim JN,
Kim JO, Kim HJ, Kim HY, Han JS, Shin CY, et al: Diabetes augments
cognitive dysfunction in chronic cerebral hypoperfusion by
increasing neuronal cell death: Implication of cilostazol for
diabetes mellitus-induced dementia. Neurobiol Dis. 73:12–23. 2015.
View Article : Google Scholar
|
|
4
|
Dai J, Chen L, Qiu YM, Li SQ, Xiong WH,
Yin YH, Jia F and Jiang JY: Activations of GABAergic signaling,
HSP70 and MAPK cascades are involved in baicalin's neuroprotection
against gerbil global ischemia/reperfusion injury. Brain Res Bull.
90:1–9. 2013. View Article : Google Scholar
|
|
5
|
Park CH, Yokozawa T and Noh JS: Oligonol,
a low-molecular-weight polyphenol derived from lychee fruit,
attenuates diabetes-induced renal damage through the advanced
glycation end product-related pathway in db/db mice. J Nutr.
144:1150–1157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bath KG and Lee FS: Variant BDNF
(Val66Met) impact on brain structure and function. Cogn Affect
Behav Neurosci. 6:79–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa T, Tsuchida A, Itakura Y,
Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M and
Noguchi H: Brain-derived neurotrophic factor regulates glucose
metabolism by modulating energy balance in diabetic mice. Diabetes.
49:436–444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuchida A, Nakagawa T, Itakura Y,
Ichihara J, Ogawa W, Kasuga M, Taiji M and Noguchi H: The effects
of brain-derived neurotrophic factor on insulin signal transduction
in the liver of diabetic mice. Diabetologia. 44:555–566. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Ping J, Xu HD, Fu HJ and Zhou ZH:
Synergistic effect of a novel oxymatrine-baicalin combination
against hepatitis B virus replication, alpha smooth muscle actin
expression and type I collagen synthesis in vitro. World J
Gastroenterol. 12:5153–5159. 2006.PubMed/NCBI
|
|
10
|
Hwang JM, Wang CJ, Chou FP, Tseng TH,
Hsieh YS, Hsu JD and Chu CY: Protective effect of baicalin on
tert-butyl hydro-peroxide-induced rat hepatotoxicity. Arch Toxicol.
79:102–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung SH, Kang KD, Ji D, Fawcett RJ, Safa
R, Kamalden TA and Osborne NN: The flavonoid baicalin counteracts
ischemic and oxidative insults to retinal cells and lipid
peroxidation to brain membranes. Neurochem Int. 53:325–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waisundara VY, Hsu A, Tan BK and Huang D:
Baicalin reduces mitochondrial damage in streptozotocin-induced
diabetic Wistar rats. Diabetes Metab Res Rev. 25:671–677. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li HT, Wu XD, Davey AK and Wang J:
Antihyperglycemic effects of baicalin on streptozotocin -
nicotinamide induced diabetic rats. Phytother Res. 25:189–194.
2011.
|
|
14
|
Kuhad A and Chopra K: Effect of sesamol on
diabetes-associated cognitive decline in rats. Exp Brain Res.
185:411–420. 2008. View Article : Google Scholar
|
|
15
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryan CM, Geckle MO and Orchard TJ:
Cognitive efficiency declines over time in adults with Type 1
diabetes: Effects of micro- and macrovascular complications.
Diabetologia. 46:940–948. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sachon C, Grimaldi A, Digy JP, Pillon B,
Dubois B and Thervet F: Cognitive function, insulin-dependent
diabetes and hypoglycaemia. J Intern Med. 231:471–475. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wredling R, Levander S, Adamson U and Lins
PE: Permanent neuropsychological impairment after recurrent
episodes of severe hypoglycaemia in man. Diabetologia. 33:152–157.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu YW, Zhu X, Li W, Lu Q, Wang JY, Wei YQ
and Yin XX: Ginsenoside Re attenuates diabetes-associated cognitive
deficits in rats. Pharmacol Biochem Behav. 101:93–98. 2012.
View Article : Google Scholar
|
|
20
|
Kuhad A and Chopra K: Curcumin attenuates
diabetic encephalopathy in rats: Behavioral and biochemical
evidences. Eur J Pharmacol. 576:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Feng L, Ma D, Zhang M, Gu J, Wang
S, Fu Q, Song Y, Lan Z, Qu R, et al: Neuroprotective effect of
paeonol on cognition deficits of diabetic encephalopathy in
streptozotocin-induced diabetic rat. Neurosci Lett. 549:63–68.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang CY, Zheng W, Wang T, Xie JW, Wang SL,
Zhao BL, Teng WP and Wang ZY: Huperzine A activates Wnt/β-catenin
signaling and enhances the nonamyloidogenic pathway in an Alzheimer
transgenic mouse model. Neuropsychopharmacology. 36:1073–1089.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S,
Xie N, Wang L, Chen T, Shaw C, et al: Donepezil attenuates
hippocampal neuronal damage and cognitive deficits after global
cerebral ischemia in gerbils. Neurosci Lett. 510:29–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim DS, Kim JY and Han Y: Curcuminoids in
neurodegenerative diseases. Recent Patents CNS Drug Discov.
7:184–204. 2012. View Article : Google Scholar
|
|
25
|
Sharma SK, Sherff CM, Shobe J, Bagnall MW,
Sutton MA and Carew TJ: Differential role of mitogen-activated
protein kinase in three distinct phases of memory for sensitization
in Aplysia. J Neurosci. 23:3899–3907. 2003.PubMed/NCBI
|
|
26
|
Adams JP and Sweatt JD: Molecular
psychology: Roles for the ERK MAP kinase cascade in memory. Annu
Rev Pharmacol Toxicol. 42:135–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang JJ, Okutani F, Inoue S and Kaba H:
Activation of the mitogen-activated protein kinase/extracellular
signal-regulated kinase signaling pathway leading to cyclic AMP
response element-binding protein phosphorylation is required for
the long-term facilitation process of aversive olfactory learning
in young rats. Neuroscience. 121:9–16. 2003. View Article : Google Scholar
|
|
28
|
Xuan AG, Chen Y, Long DH, Zhang M, Ji WD,
Zhang WJ, Liu JH, Hong LP, He XS and Chen WL: PPARα Agonist
Fenofibrate Ameliorates Learning and Memory Deficits in Rats
Following Global Cerebral Ischemia. Mol Neurobiol. 2014.
|
|
29
|
Aminzadeh A, Dehpour AR, Safa M,
Mirzamohammadi S and Sharifi AM: Investigating the protective
effect of lithium against high glucose-induced neurotoxicity in
PC12 cells: Involvements of ROS, JNK and P38 MAPKs, and apoptotic
mitochondria pathway. Cell Mol Neurobiol. 34:1143–1150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Forlenza OV, Diniz BS, Teixeira AL, Ojopi
EB, Talib LL, Mendonça VA, Izzo G and Gattaz WF: Effect of
brain-derived neurotrophic factor Val66Met polymorphism and serum
levels on the progression of mild cognitive impairment. World J
Biol Psychiatry. 11:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|