Introduction
Osteoporosis is a common disease, which is
characterized by low bone mass and micro-architectural
deterioration of bone tissue, leading to increased bone fragility
and an increased risk of fracture (1). Postmenopausal osteoporosis (PO) is
one of four types of osteoporosis and is suggested to directly
result from a lack of endogenous oestrogen in menopausal females
(2). This disease affects millions
of females >50 years of age worldwide and treatment of PO is
placing an increasing economic burden on society.
The etiology of PO is multifactorial. In addition to
the effects of estrogen, calcium and other environmental factors on
bone structure and fracture, there is a marked genetic effect on
osteoporosis risk in postmenopausal women (3). Mullin et al (4) concluded that genetic variation in
ARHGEF3 served a role in the determination of bone density in
Caucasian females, and proposed that the RhoGTPase-RhoGEF pathway
is associated with PO. A previous study identified that
chondroadherin is a novel regulator of bone metabolism, which
suppresses pre-osteoclast motility and bone resorption, with a
potential effect for the treatment of PO (5).
A previous study described the transcriptional
alterations in 84 trans-iliac bone biopsies associated with bone
mineral density (BMD) variations in postmenopausal women (6). This previous study identified that
sclerostin and dickkopf homolog 1, both involved in the Wnt
signaling pathway, exhibited a clear correlation and was involved
in bone metabolism. Jemtland et al (7) analyzed these data to identify
osteoporosis candidate genes and identified that the transcription
factor (TF), SOX4, and the bone matrix proteins, MMP13 and MEPE,
were all downregulated in osteoporosis. However, the authors
focused on the gene expression levels associated with the BMD
variation, rather than an intensive analysis of the gene regulation
changes and interactions in PO, of which the underlying mechanisms
remain to be elucidated.
Therefore, the data mentioned above was obtained and
the differentially expressed genes (DEGs) between PO samples and
healthy controls were assessed by genome-wide microarray analysis,
in addition to performing a more comprehensive analysis, to achieve
an improved understanding of the mechanisms of this disease.
Various bioinformatic methods were applied to identify the
potential modulators, including TFs and microRNAs of the DEGs, and
the significant pathways associated with the DEGs, in addition to
identification of the functional modules in the interaction network
of the DEGs involved in PO.
Materials and methods
Data acquisition and preprocessing
The expression data, numbered E-MEXP-1618 (6), were downloaded from the ArrayExpress
database (8) provided by the
European Bioinformatics Institute (Saffron Walden, UK). All 66
trans-iliac bone biopsy samples were obtained from postmenopausal
females, including 27 osteoporosis patients (mean age, 69.6 years,
range, 51.6–86.1 years) and 39 healthy controls (mean age, 61.7
years, range, 49.7–80.9 years).
The primary data was standardized and transformed
into expression values using the Robust Multi-array Average
algorithm (www.bioconductor.org) (9) in R language (version 2.4.1), based on
the microarray platform Affymetrix GeneChip Human Genome U133 plus
2.0 (Affymetrix, Inc., Santa Clara, CA, USA).
DEG screening
The DEGs were screened out by significance analysis
using the Empirical Bayes methods within Limma package (10) in R language. The adjusted P-value
represents the P-value adjusted using the Benjamini-Hochberg method
(11), following Student's t-test,
with <0.1 as a cut-off criterion.
Pathway enrichment of DEGs
Pathway enrichment analysis of the DEGs was
performed using the Database for Annotation, Visualization and
Integrated Discovery (12) online
tools version 6.7 based on the Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway databases (13). A false discovery rate <0.05 was
set as a cut-off criterion.
Prediction of DEG regulation
To determine the potential transcriptional and
post-transcriptional modulators, the ChIP Enrichment Analysis
(ChEA) database (http://amp.pharm.mssm.edu/lib/chea.jsp) (14) and the WEB-based GEne SeT AnaLysis
Toolkit (WebGestalt) system (http://bioinfo.vanderbilt.edu/webgestalt) (15) were used to predict the TFs and
microRNAs of DEGs, respectively. The ChEA database contains
interactions describing the regulation of TFs on target genes and
P<[0.05/Σ(TFs)] was set as the cut-off criterion. The WebGestalt
system includes interactions describing the binding of microRNAs to
the 3′ untranslated region of the target genes and an adjusted
P-value ≤0.05 was set as the cut-off criterion.
Protein-protein interaction (PPI) network
construction and analysis
The PPI pairs of the screened DEGs were analyzed
using the Search Tool for the Retrieval of Interacting Genes
(STRING) software 9.0 (16). The
pairs with combined scores >0.4 were used for the PPI network
construction using Cytoscape software 2.8 (17). Furthermore, the modules with close
internal communication were screened out with the Markov Cluster
(MCL) (18) algorithm in the
clusterMaker package (19) within
the Cytoscape software. In addition, the biological processes in
which the screened modules were enriched were identified by the
Biological Networks Gene Ontology (BiNGO) package 2.44 (20) within the Cytoscape software
package.
Results
Multiple DEGs are involved in various
pathways
A total of 482 DEGs, including 279 upregulated and
203 downregulated DEGs, were screened out in the samples from
patients with osteoporosis when compared with the healthy control
samples. The DEGs were subjected to KEGG pathway enrichment
analysis. As presented in Table I,
the upregulated genes were predominantly enriched in the pathway of
fatty acid metabolism and the downregulated genes were
predominantly enriched in the pathway of DNA replication. Notably,
cardiac muscle contraction was also a significant pathway in which
the upregulated genes were enriched.
 | Table IKey enriched pathways of the
differentially expressed genes. |
Table I
Key enriched pathways of the
differentially expressed genes.
| Regulation | Term | Count | P-value | Gene | FDR |
|---|
| Upregulated | hsa00071: fatty acid
metabolism | 8 |
4.29−6 | ACADSB, ACSL1, ACADM,
ADH5, HADH, ACSL3, ACAT1, HADHB | 0.000480 |
| hsa04260: cardiac
muscle contraction | 8 |
3.57−4 | UQCRC2, ATP1B1,
ACTC1, COX7A1, MYH7, MYH6, ATP1A2, CACNG1 | 0.019784 |
| hsa00280: valine,
leucine and isoleucine degradation | 6 |
8.84−4 | ALDH6A1, ACADSB,
ACADM, HADH, ACAT1, HADHB | 0.032468 |
| hsa04920:
adipocytokine signaling pathway | 7 |
9.69−4 | LEP, PRKCQ, PPARA,
ACSL1, PRKAA2, ACSL3, CHUK | 0.02678 |
| Downregulated | hsa03030: DNA
replication | 5 |
5.98−4 | MCM7, LIG1, MCM3,
RNASEH2C, MCM5 | 0.047308 |
Up and downregulated DEGs are modulated
by TFs and microRNAs
The transcriptional and post-transcriptional
modulators of the DEGs were also predicted. The ChEA analysis
included 94 TFs, therefore P<0.0005 was set as the criterion.
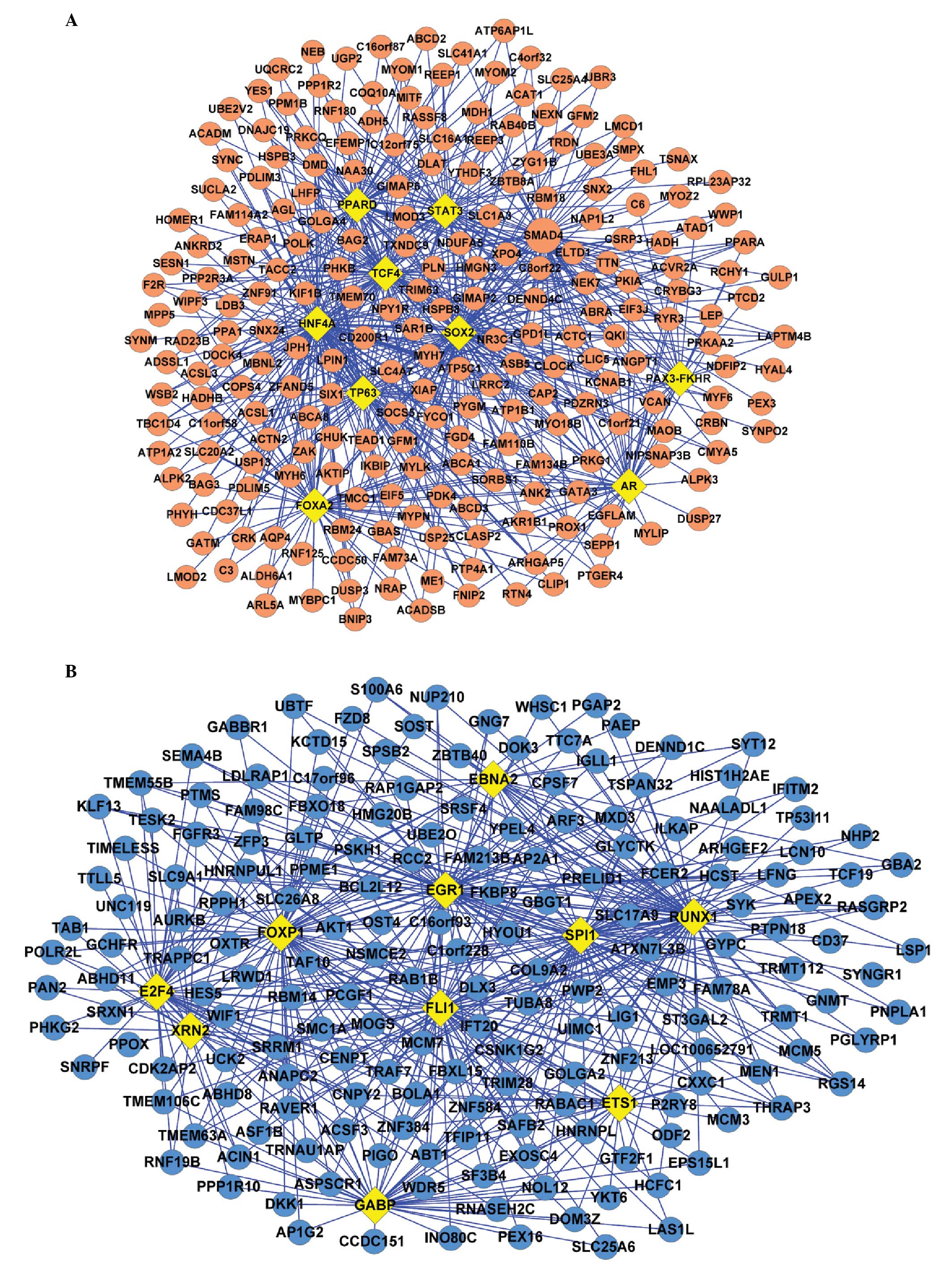
This analysis demonstrated that the TFs, including HNF4A, SMAD4 and
SOX2, were significantly associated with the upregulated DEGs.
HNFA4 had the most significant P-value, therefore, may regulate 110
genes. SOX2, which exhibited the second most significant P-value
was suggested to regulate 72 genes, followed by SMAD4, which may
regulate 59 genes (Fig. 1A).
Additionally, FOXP1 and SPI1 were significantly associated with the
downregulated DEGs and may regulate 65 and 55 genes, respectively
(Fig. 1B).
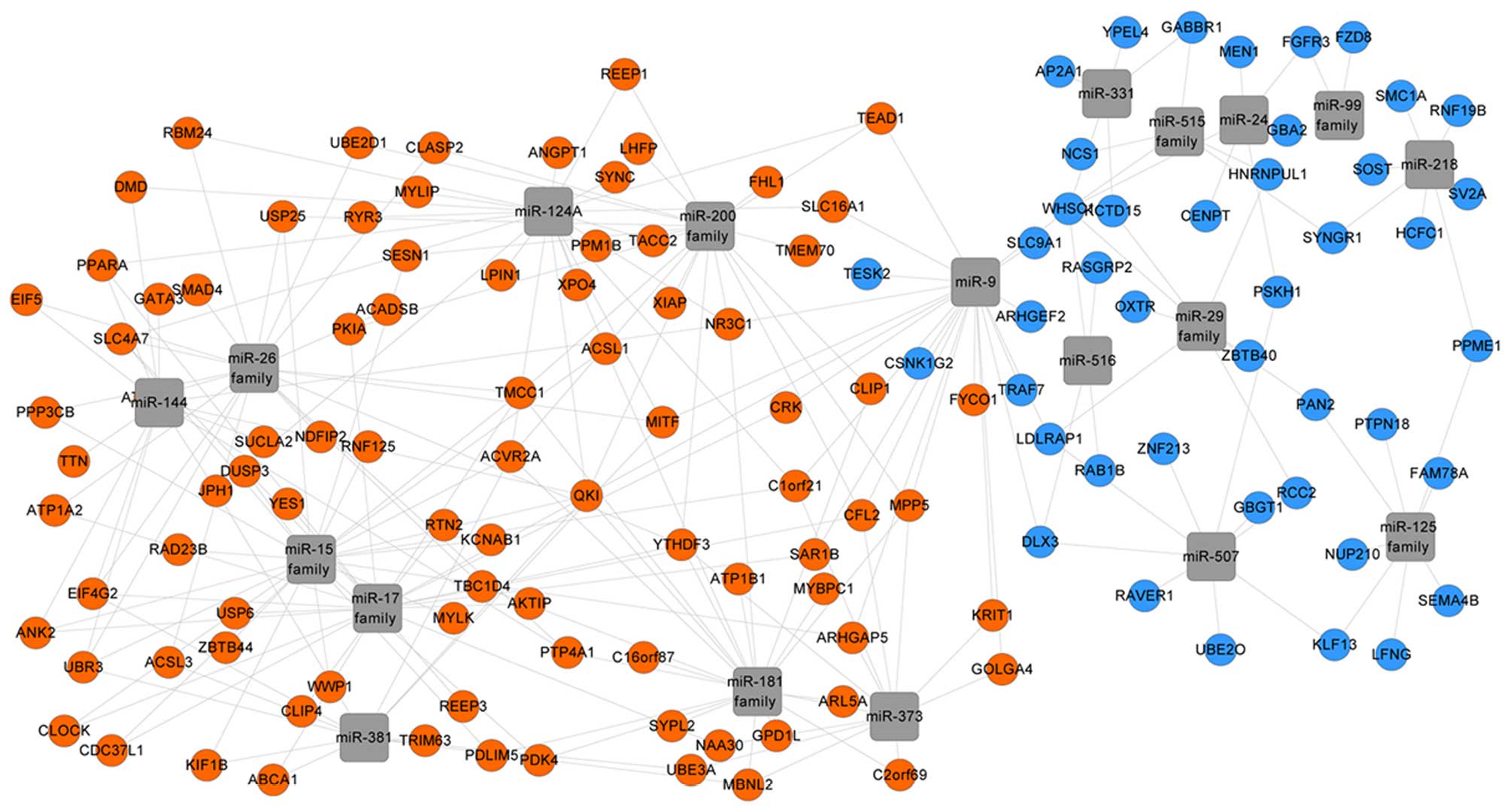
The WebGestalt analysis indicated that the
microRNAs, including the microRNA-125 (miR-125) family, miR-331 and
miR-24, potentially modulated the downregulated DEGs. This
suggested that these microRNAs may be in an active state in PO.
Additionally, the miR-26, miR-15 and miR-200 families were
identified to possibly modulate the upregulated DEGs, which
suggested their inactive state in PO (Fig. 2).
PPI network construction
Interactions between protein pairs were identified
using STRING software. The PPI network of the DEGs in PO was
constructed using Cytoscape software while the nodes with no
connections were filtered out. A total of 146 DEGs with 271
interactions were detected. TTN, MYOZ2 and LDB3 were identified to
possess the highest degrees of connectivity and were observed to be
involved in 22, 19 and 17 pairs of interactions, respectively.
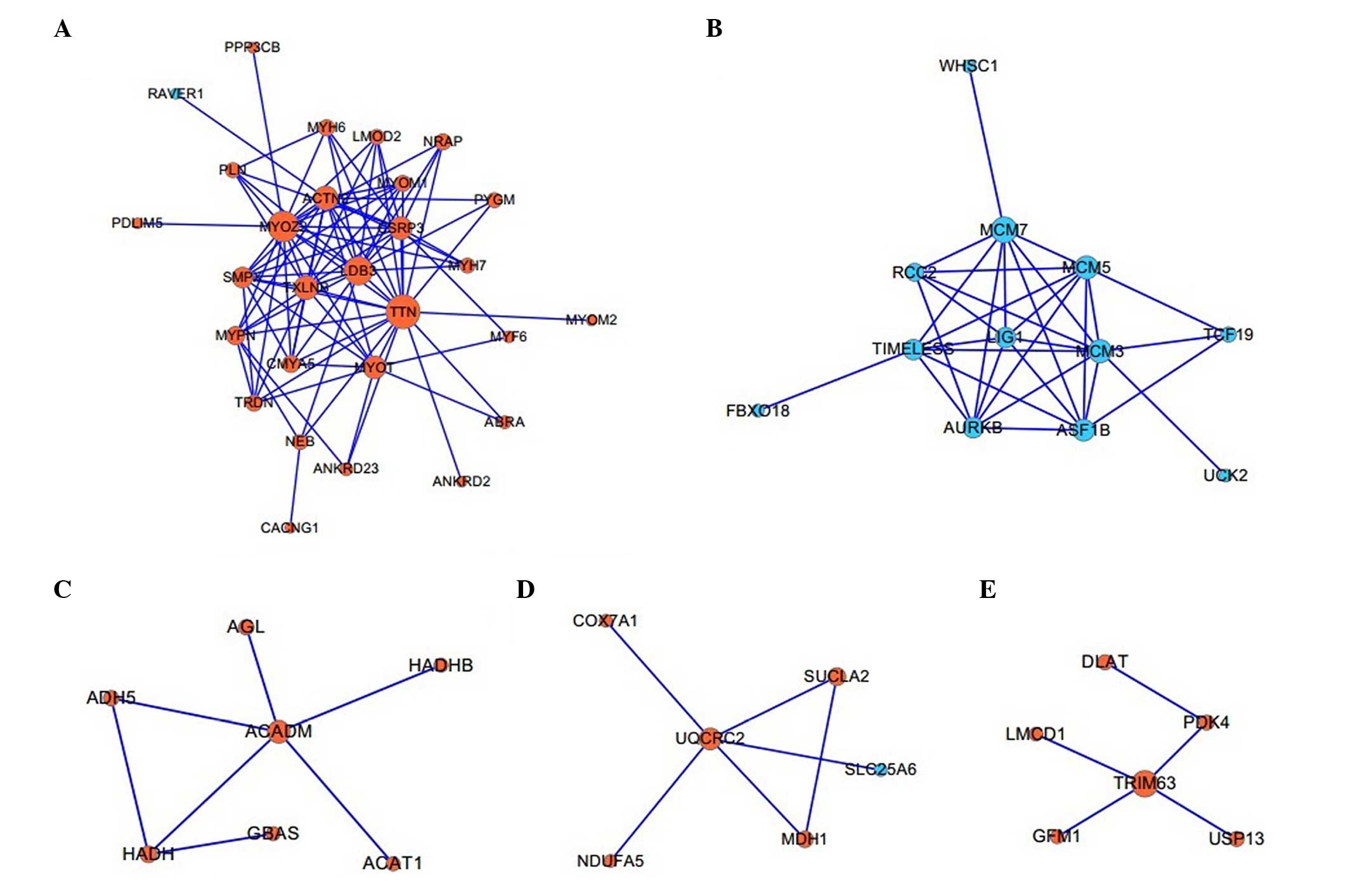
Twelve functional modules associated with
biological processes
A total of 12 modules, which contained at least
three DEGs in the PPI network, were constructed, of which the five
largest modules were identified with TTN (Fig. 3A), L1G1 (Fig. 3B), ACADM (Fig. 3C), UQCRC2 (Fig. 3D) and TRIM63 (Fig. 3E) as the hub proteins,
respectively. BiNGO analysis demonstrated that these five modules
were associated with the biological processes of muscle
contraction, DNA-dependent DNA replication initiation, lipid
modification, generation of precursor metabolites and energy, and
regulation of the acetyl-CoA biosynthetic process from pyruvate,
respectively (Table II).
 | Table IIBiological processes involved in the
five largest functional modules. |
Table II
Biological processes involved in the
five largest functional modules.
| Module hub | GO-term (biological
process) | P-value | Adjusted P-value | Gene |
|---|
| TNN | Muscle
contraction |
5.04−17 |
2.11−14 | TRDN, MYOM2,
ANKRD2, SMPX, MYH7, ACTN2, MYH6, MYOM1, CACNG1, TTN, MYOT |
| L1G1 | DNA-dependent DNA
replication initiation |
2.51−7 |
4.14−5 | MCM7, MCM3,
MCM5 |
| ACADM | Lipid
modification |
5.78−9 |
1.04−6 | ACADM, HADH, ACAT1,
HADHB |
| UQCRC2 | Generation of
precursor metabolites and energy |
2.82−8 |
1.33−6 | UQCRC2, NDUFA5,
COX7A1, SUCLA2, MDH1 |
| TRIM63 | Regulation of
acetyl-CoA biosynthetic process from pyruvate |
9.66−6 |
5.77−4 | PDK4, DLAT |
Discussion
In order to elucidate the molecular mechanisms of
PO, the gene expression profiles were systematically analyzed using
bioinformatic approaches. A total of 482 DEGs, including 279
upregulated and 203 downregulated DEGs, were screened out in
patients with PO. The biological functions of these DEGs were
further assessed based on pathway enrichment data. Further
modulator prediction identified the potential TFs and microRNAs,
which may regulate the DEGs in PO. In addition, the functional
modules in the PPI network of the DEGs were identified, of which
certain modules were clearly involved in PO.
TFs prediction in the present study identified that
59 of the upregulated DEGs were targets of SMAD4, which is the only
member of common-mediator SMAD (co-SMAD) class. A previous study
demonstrated that defects in bone morphogenetic protein (BMP)-SMAD
signaling led to bone-associated disorders, including osteoporosis
(21). Association of BMPs to BMP
receptors on the cell surface leads to the activation of the
formation of Smad4 and Smad1/5/8 complexes (22). The complexes subsequently
translocate into the nucleus and bind to the consensus DNA sequence
to modulate the transcription of BMP target genes (23). In addition, SMAD4 was suggested to
function as a transcriptional co-repressor for estrogen receptor α
(ERα) by forming a complex when ERα binds to the
estrogen-responsive element within the promoters of estrogen target
genes (24). Estrogen-associated
therapies are widely used for the treatment of PO (25–27)
and antiestrogens are able to enhance the endogenous interactions
between SMAD4 and ER (24).
According to the data from the present study, the expression of
SMAD4 itself was upregulated in PO, suggesting that it may be
activated by a mechanism of cross-talk between BMP-SMAD signaling
and ERα-estrogen interaction to subsequently regulate downstream
target genes involved in this disease.
Several microRNAs have been identified to serve
important roles in PO, including miR-148a, which promotes
osteoclastogenesis (28), miR-133a
(29) and miR-422a (30), which are upregulated with low BMD
in human circulating monocytes (osteoclast precursors). However,
the roles of microRNAs in PO remain to be elucidated. The microRNA
prediction in the present study suggested that the miR-24 and
miR-125 families may be activated in PO to suppress the expression
levels of a series of genes, which is consistent with a previous
observation that miR-24 and miR-125b were significantly upregulated
in the serum and bone tissue of patients with PO (31). Another microRNA suggested to be
activated was miR-331, which may be associated with miR-24 by its
interactions with Wolf-Hirschhorn Syndrome Candidate 1 (32). This suggested that miR-331 may be a
novel potential biomarker for PO.
In addition, the three hub proteins identified in
the PPI network of DEGs were TTN, MYO2 and LDB3, which were
demonstrated to be associated in one functional module of muscle
contraction. Notably, KEGG pathway enrichment analysis demonstrated
that the cardiac muscle contraction pathway was significantly
associated with the upregulated DEGs. Voltage-dependent calcium
channel γ1 (CACNG1) is a DEG, which was observed to be associated
with the muscle contraction module and the cardiac muscle
contraction pathway. It has been previously reported that CACNG1 in
different cell types may be important in the mechanism of the
regulation of Ca2+ channel function (33). A previous study indicated that
patients with PO often exhibit hypercalciuria with normal blood
Ca2+ levels (34).
Therefore, CACNG1 may be important in the underlying mechanisms of
PO through Ca2+ regulation in the muscle contraction
process.
Another functional module of the PPI network
identified in the present study with TRIM63 as the hub, was
demonstrated to be associated with the regulation of the acetyl-CoA
biosynthetic process from pyruvate, which is an important pathway
in the human metabolic process. TRIM63, also termed muscle-specific
ring finger protein 1, has been reported as an E3 ubiquitin ligase
expressed predominantly in muscular tissue. Azuma et al
(35) proposed that the
overexpression of TRIM63 increased the expression of an
osteoblastic differentiation marker gene, alkaline phosphatase,
resulting in reduced proliferation. In addition, TRIM63 was
identified to be involved in the two major bone remodeling
activities, osteoblastic bone formation and osteoclastic bone
resorption (36). According to the
present study, the four genes, LMCD1, PDK4, USP13 and GFM1,
encoding the proteins which interacted with TRIM63, were all
upregulated in PO, indicating a potential synergistic effect of
these proteins with TRIM63 in the bone remodeling activities in
PO.
In conclusion, the DEGs in PO were screened
comparing them with the normal controls and further intensive
bioinformatic analysis, including pathway enrichment, modulator
prediction of TFs and microRNAs, PPI network analysis and
functional module identification was performed on the DEGs. It was
suggested that SMAD4, CACNG1 and TRIM63 may have important roles in
the molecular mechanism of PO and that miR-331 may be novel
potential biomarker for PO. The present study may provide
bioinformatic support for further investigations into the
mechanisms of PO. However, associated experimental data are
necessary to confirm the conclusions of the present study.
References
|
1
|
Bouillon R, Burckhardt P, Christiansen C,
et al: Consensus development conference: Prophylaxis and treatment
of osteoporosis. Am J Med. 90:107–110. 1991. View Article : Google Scholar
|
|
2
|
Marcus R: Post-menopausal osteoporosis.
Best Pract Res Clin Obstet Gynaecol. 16:309–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michaëlsson K, Melhus H, Ferm H, Ahlbom A
and Pedersen NL: Genetic liability to fractures in the elderly.
Arch Intern Med. 165:1825–1830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mullin BH, Prince RL, Dick IM, Hart DJ,
Spector TD, Dudbridge F and Wilson SG: Identification of a role for
the ARHGEF3 gene in postmenopausal osteoporosis. Am J Hum Genet.
82:1262–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capulli M, Olstad OK, Önnerfjord P,
Tillgren V, Muraca M, Gautvik KM, Heinegård D, Rucci N and Teti A:
The C-terminal domain of chondroadherin: A new regulator of
osteoclast motility counteracting bone loss. J Bone Miner Res.
29:1833–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reppe S, Refvem H, Gautvik VT, Olstad OK,
Høvring PI, Reinholt FP, Holden M, Frigessi A, Jemtland R and
Gautvik KM: Eight genes are highly associated with BMD variation in
postmenopausal Caucasian women. Bone. 46:604–612. 2010. View Article : Google Scholar
|
|
7
|
Jemtland R, Holden M, Reppe S, Olstad OK,
Reinholt FP, Gautvik VT, Refvem H, Frigessi A, Houston B and
Gautvik KM: Molecular disease map of bone characterizing the
postmenopausal osteoporosis phenotype. J Bone Miner Res.
26:1793–1801. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brazma A, Parkinson H, Sarkans U,
Shojatalab M, Vilo J, Abeygunawardena N, Holloway E, Kapushesky M,
Kemmeren P, Lara GG, et al: ArrayExpress-a public repository for
microarray gene expression data at the EBI. Nucleic Acids Res.
31:68–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and computational biology solutions
using R and Bioconductor. Springer; pp. 397–420. 2005, View Article : Google Scholar
|
|
11
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Stat Soc Series B Stat Methodol. 289–300.
1995.
|
|
12
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
14
|
Lachmann A, Xu H, Krishnan J, Berger SI,
Mazloom AR and Ma'ayan A: ChEA: Transcription factor regulation
inferred from integrating genome-wide ChIP-X experiments.
Bioinformatics. 26:2438–2444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Duncan D, Shi Z and Zhang B:
WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013.
Nucleic Acids Res. 41:4392013.
|
|
16
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar :
|
|
17
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: new features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar :
|
|
18
|
Enright AJ, Van Dongen S and Ouzounis CA:
An efficient algorithm for large-scale detection of protein
families. Nucleic Acids Res. 30:1575–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morris JH, Apeltsin L, Newman AM, Baumbach
J, Wittkop T, Su G, Bader GD and Ferrin TE: ClusterMaker: A
multi-algorithm clustering plugin for Cytoscape. BMC
Bioinformatics. 12:4362011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li B: Bone morphogenetic protein-Smad
pathway as drug targets for osteoporosis and cancer therapy. Endocr
Metab Immune Disord Drug Targets. 8:208–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
David L, Mallet C, Mazerbourg S, Feige JJ
and Bailly S: Identification of BMP9 and BMP10 as functional
activators of the orphan activin receptor-like kinase 1 (ALK1) in
endothelial cells. Blood. 109:1953–1961. 2007. View Article : Google Scholar
|
|
23
|
Li B: Bone morphogenetic protein-Smad
pathway as drug targets for osteoporosis and cancer therapy. Endocr
Metab Immune Disord Drug Targets. 8:208–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L, Wu Y, Gathings B, Wan M, Li X,
Grizzle W, Liu Z, Lu C, Mao Z and Cao X: Smad4 as a transcription
corepressor for estrogen receptor alpha. J Biol Chem.
278:15192–15200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Genant HK, Lucas J, Weiss S, Akin M, Emkey
R, McNaney-Flint H, Downs R, Mortola J, Watts N, Yang HM, et al:
Low-dose esterified estrogen therapy: Effects on bone, plasma
estradiol concentrations, endometrium and lipid levels.
Estratab/Osteoporosis Study Group. Arch Intern Med. 157:2609–2615.
1997. View Article : Google Scholar
|
|
26
|
Cummings SR, Ensrud K, Delmas PD, LaCroix
AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J,
et al: Lasofoxifene in postmenopausal women with osteoporosis. N
Engl J Med. 362:686–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G
and Naujoks C: The impact of compliance with osteoporosis therapy
on fracture rates in actual practice. Osteoporos Int. 15:1003–1008.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie
H, Zhu W, Dai RC, Wu XP, Liao EY, et al: miR-148a regulates
osteoclastogenesis by targeting V-maf musculoaponeurotic
fibrosarcoma oncogene homolog B. J Bone Miner Res. 28:1180–1190.
2013. View Article : Google Scholar
|
|
29
|
Wang Y, Li L, Moore BT, Peng XH, Fang X,
Lappe JM, Recker RR and Xiao P: MiR-133a in human circulating
monocytes: a potential biomarker associated with postmenopausal
osteoporosis. PLoS One. 7:e346412012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Z, Moore BT, Wang Y, Peng XH, Lappe
JM, Recker RR and Xiao P: MiR-422a as a potential cellular microRNA
biomarker for postmenopausal osteoporosis. Plos One. 9:e970982014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Vizio D, Freeman MR and Morello M:
Large oncosomes in human tumors and in circulation in patients with
cancer. U.S. Patent Application 13/975,059. 2013 8 23
|
|
33
|
Burgess DL, Gefrides LA, Foreman PJ and
Noebels JL: A cluster of three novel Ca2+ channel gamma subunit
genes on chromosome 19q13. 4: evolution and expression profile of
the gamma subunit gene family. Genomics. 71:339–350. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giannini S, Nobile M, Dalle Carbonare L,
Lodetti MG, Sella S, Vittadello G, Minicuci N and Crepaldi G:
Hypercalciuria is a common and important finding in postmenopausal
women with osteoporosis. Eur J Endocrinol. 149:209–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azuma K, Urano T, Ouchi Y and Inoue S:
Glucocorticoid-induced gene tripartite motif-containing 63 (TRIM63)
promotes differentiation of osteoblastic cells. Endocr J.
57:455–462. 2009. View Article : Google Scholar
|
|
36
|
Kondo H, Ezura Y, Nakamoto T, Hayata T,
Notomi T, Sorimachi H, Takeda S and Noda M: MURF1 deficiency
suppresses unloading-induced effects on osteoblasts and osteoclasts
to lead to bone loss. J Cell Biochem. 112:3525–3530. 2011.
View Article : Google Scholar : PubMed/NCBI
|