Introduction
Stroke is a major cause of morbidity and mortality
in developed and developing countries worldwide. At present, it is
recognized that rupture of the carotid atherosclerotic plaque and
subsequent formation of thrombi or emboli are the primary causes of
ischemic stroke (1–3). Treatment for carotid artery stenosis
includes carotid endarterectomy (CEA) and carotid artery stenting
(CAS), which are two approaches that have been previously
demonstrated to prevent future stroke (4–6).
Improvements in cerebral blood flow following CEA
have been observed using various imaging modalities, including
transcranial Doppler, single-photon emission computed tomography
(SPECT), perfusion computerised tomography (CT) and magnetic
resonance imaging (7–11). For the measurement of cerebral
tissue perfusion, the use of 15O-labeled water with PET
is considered the gold standard, due to the fact that it possesses
a high first-pass extraction (12). With 15O-labeled water
PET, Rijbroek et al (12)
identified that, 1 day following CEA, absolute cerebral blood flow
is increased in all arterial territories on the ipsilateral and
contralateral hemispheres. In addition, the same study demonstrated
no difference in cerebral blood flow between the ipsilateral and
contralateral hemispheres prior to and following CEA.
Following carotid artery revascularization, several
studies have observed changes in neuropsychological and cognitive
functions in humans, including memory, attention, psychomotor speed
and executive functions (8,13).
In the mammalian cerebral cortex, ≥90% of neurons are glutamatergic
or GABAergic, and neuropsychological functions depend on
neurotransmitter trafficking between neurons and astrocytes, with
ammonia involved in the glutamate/glutamine cycle (14–16).
At present, to the best of our knowledge, there have
been no reports focussing on CEA regarding the cerebral uptake of
ammonia using 13N-labeled ammonia with PET. The present
study aimed to examine the changes in neuropsychological functions
following surgery in patients with carotid artery stenosis. It was
hypothesized that cerebral ammonia metabolism is altered following
CEA, and that this alteration may be associated with GABAergic
neuron functions. Therefore, the purpose of the present PET study
was to evaluate alterations in ammonia uptake in cerebral
hemispheres prior and subsequent to CEA.
Patients and methods
Patients
The present study included 20 patients (four
females; 16 males) with a mean age of 59.5 years (range, 33–75
years), who presented with unilateral chronic stenosis between 2011
and 2012 at the Affiliated Hospital of Inner Mongolia Medical
University (Hohhot, China). The clinical characteristics of the
patients are presented in Table I.
No cortical infarctions were observed in the patients on
conventional CT prior to CEA. Of the 20 patients, six exhibited
carotid artery stenosis of 50–69%, 11 exhibited carotid artery
stenosis of 70–99% and three patients exhibited 100% stenosis,
termed thrombosis, measured by CT digital subtraction angiography
prior to CEA. No patients exhibited occlusion or stenosis ≥50% in
the contralateral hemisphere. All study procedures were approved by
the Ethics Committee in the Affiliated Hospital of Inner Mongolia
Medical University, and written informed consent was obtained from
all subjects prior to their enrolment in the study.
 | Table ICharacteristics of patients. |
Table I
Characteristics of patients.
| Patient | Age (years) | Gender | Side of surgery | PET (daysa) | Stenosis (%) |
|---|
| 1 | 56 | Male | Left | 23 | 70–99 |
| 2 | 75 | Female | Left | 10 | 50–69 |
| 3 | 73 | Male | Left | 30 | 50–69 |
| 4 | 66 | Female | Left | 14 | 50–69 |
| 5 | 72 | Male | Right | 13 | 50–69 |
| 6 | 36 | Male | Right | 13 | 100 |
| 7 | 46 | Male | Left | 10 | 70–99 |
| 8 | 72 | Male | Left | 10 | 70–99 |
| 9 | 57 | Male | Right | 14 | 50–69 |
| 10 | 54 | Male | Left | 11 | 50–69 |
| 11 | 58 | Male | Right | 9 | 70–99 |
| 12 | 52 | Male | Right | 10 | 70–99 |
| 13 | 67 | Male | Left | 12 | 100 |
| 14 | 62 | Female | Right | 10 | 70–99 |
| 15 | 71 | Female | Right | 10 | 70–99 |
| 16 | 57 | Male | Left | 7 | 70–99 |
| 17 | 51 | Male | Left | 9 | 70–99 |
| 18 | 58 | Male | Left | 8 | 70–99 |
| 19 | 33 | Male | Left | 12 | 70–99 |
| 20 | 75 | Male | Right | 7 | 100 |
Surgical protocol
All patients received antiplatelet therapy (aspirin,
100–200 mg/day) until the morning of the day on which CEA was
performed. All patients underwent surgery under general anaesthesia
(propofol; initial bolus 1.5–2.5 mg/kg, infusion 100–140
µg/kg/min). Blood pressure was maintained at a stable level,
in the range of their preoperative level ±20%, throughout the
procedure by adjusting the depth of anaesthesia or, if required, by
intravenous administration of a vasodilator (nitroglycerin, ~100
mg/min) or a vasoconstrictor (adrenaline, ~2 mg/min). No
intraluminal shunt was used in these procedures. The mean duration
of intracranial artery clamping was 30 min, ranging between 20 and
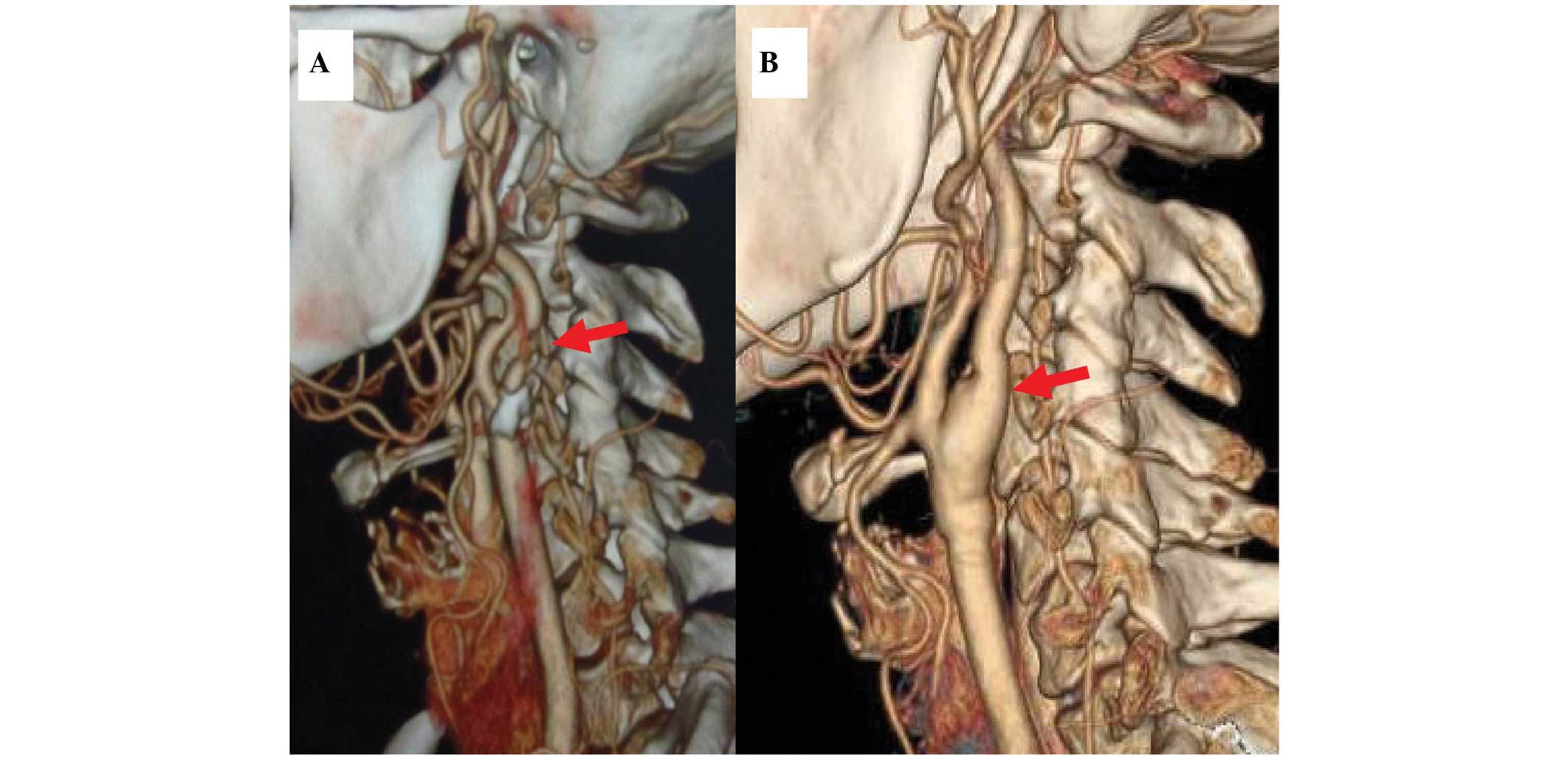
40 min. An angiograph is presented in Fig. 1 as an example prior to and
following surgery.
Ulstrasound
High resolution B-mode and Doppler ultrasonography
(Vivid 7; GE Healthcare, Milwaukee, WI, USA) of the carotid
arteries were performed for each patient prior to surgery to
evaluate the severity of vessel stenosis.
PET and CT protocol
13N-ammonia was prepared by the
16O(p,α) 13N nuclear reaction in a water
target with a cyclotron (MINItrace; GE Healthcare Life Sciences,
Pittsburgh, PA, USA). The target, containing
16O-H2O, was exposed to a 25 µA
current for 10–15 min, following which the in-line-produced
13N-NH3 was passed through a Sep-Pak CM
column (Plus Accell CM; Waters, Brehamwood, UK), and was separated
chromatically by positive ion exchange and use of a filter (EMD
Millipore, Billerica, MA, USA), prior to its delivery to patients
intravenously.
The PET imaging was performed using a PET/CT scanner
(Discovery ST8; GE Healthcare). The CT imaging was performed 3 min
prior to the injection of 137 MBq 13N-labeled ammonia.
Following injection, PET images were captured immediately for 10
min. The PET images were reconstructed using an ordered subset
expectation maximization iterative algorithm, comprising eight
subsets; a 128×128 matrix; 3 mm slice thickness and no overlap
(12). The PET investigation and
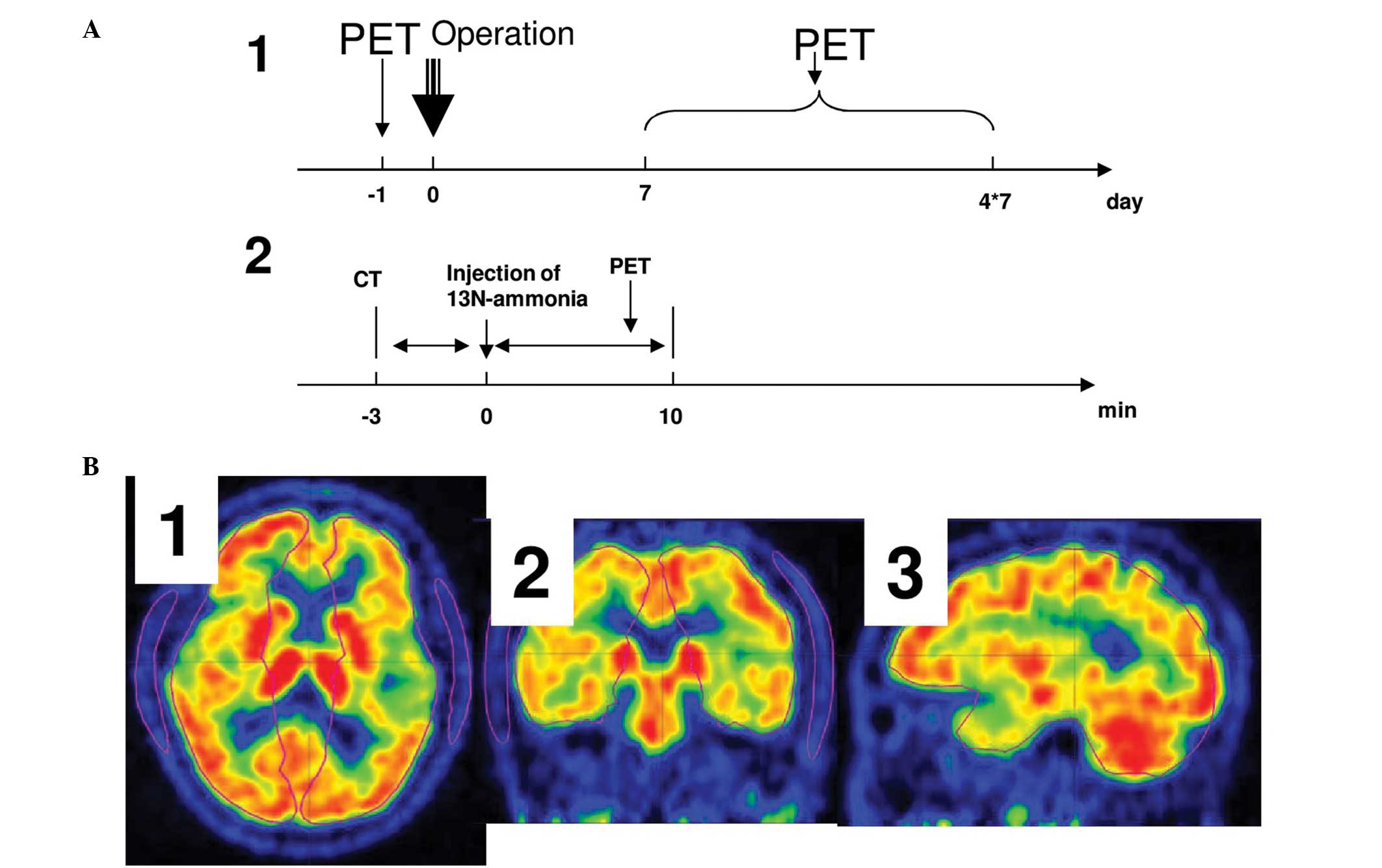
scanning protocols used in the present study are illustrated in
Fig. 2A.
PET data analysis
Human cerebral hemisphere templates were created
covering the majority of the cerebral tissue, including the white
matter, grey matter and basal ganglia, as shown in Fig. 2B, in Carimas (a PET data analysis
package developed in Turku PET Centre of Finland; http://www.turku-petcentre.fi/carimas).
For individual cases, these templates were loaded and modified
accordingly to fit the cerebral hemispheres. Additional volume of
interest (VOI), covering the lateral skin, was created for each
case. The preoperative and postoperative PET image data were loaded
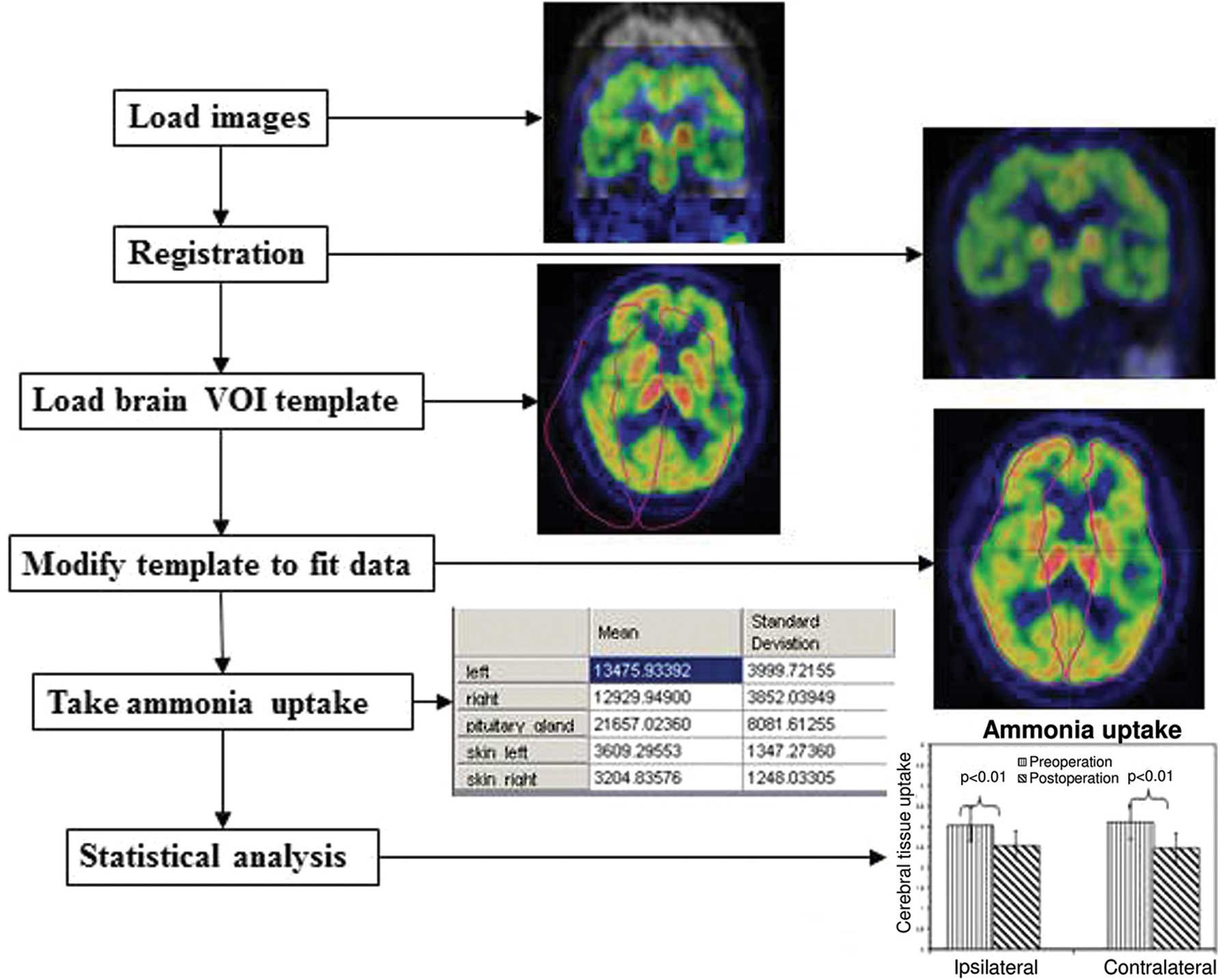
into Carimas. Automatic registration using an algorithm developed
by Maes et al (17)
implemented in Carimas was performed, and all cases exhibited
geometric alignment of the PET image with the CT image, as shown in
Fig. 2. Ammonia uptake was
calculated by taking the average radioactivity in all pixels inside
the hemisphere or skin VOIs from the preoperative images and
postoperative images. Furthermore, ammonia uptake in the cerebral
hemisphere was normalized by the corresponding contralateral skin
VOI. The reason for replacing standardized uptake value (SUV) with
contralateral skin VOI normalization was due to the fact that the
remains of radioactivity in the syringe following the injection
were not measured for all cases. Without the measurement of
remaining radioactivity, the introduction of bias requires
inclusion in the calculation of SUV. All the ammonia uptake
numerical data generated in Carimas were saved in ASCII format
files for further statistical analysis. An overview of the PET data
analysis protocol is presented in Fig.
3.
Statistical analysis
All statistical analyses were performed using Origin
7.0 (OriginLab Corporation, Northampton, MA, USA). Student's t-test
was used for the analysis of differences in paired data. P<0.01
was considered to indicate a statistically significant
difference.
Results
A total of 20 patients were involved in the present
study. The patients' characteristics, including age, gender, side
of surgery, postoperative PET scanning time (in days) and stenosis,
estimated by ultrasound, are listed in Table I. In total, >50% of the patients
(13 patients) suffered from stenosis >80%.
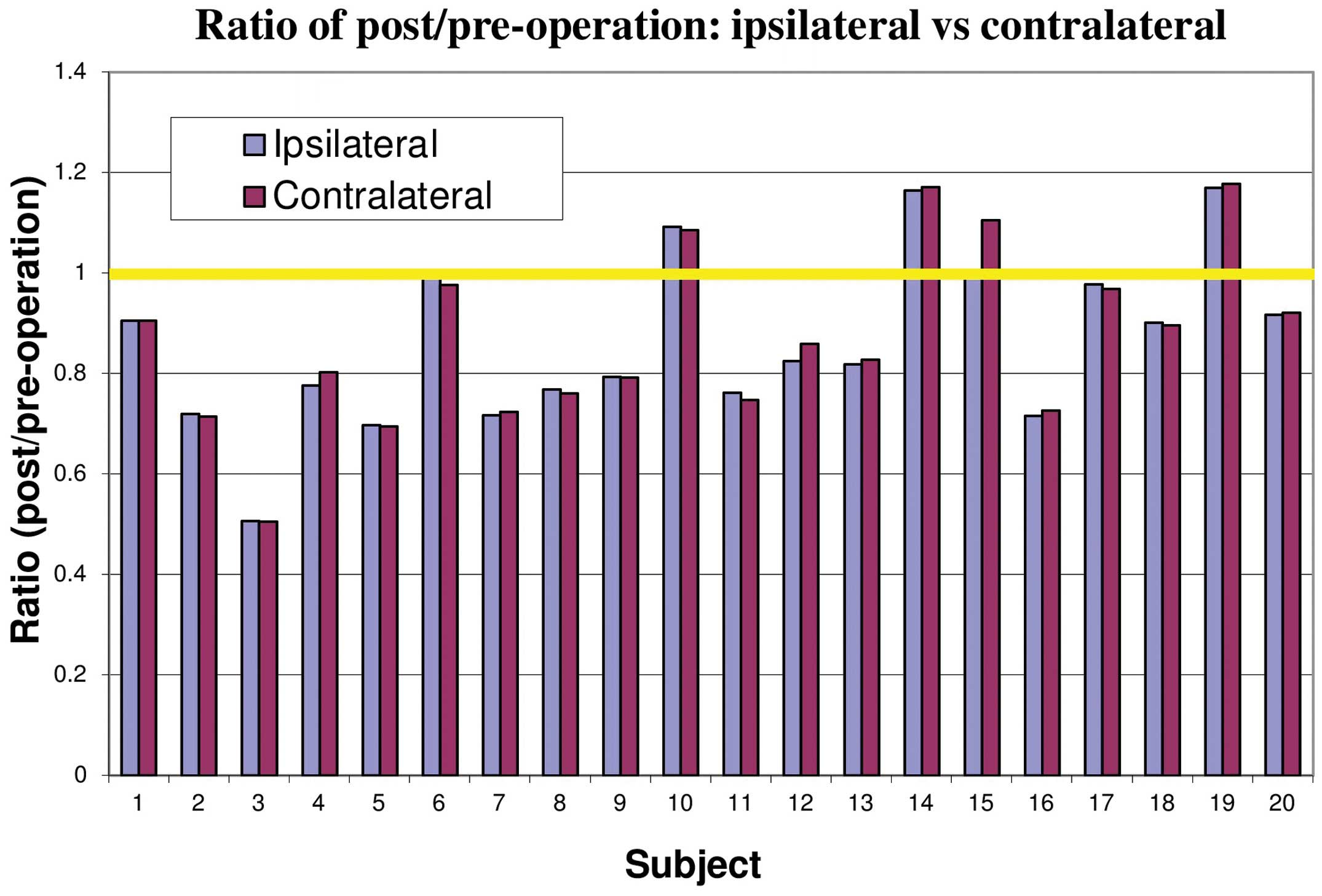
The ratio of ammonia uptake prior to and following
CEA for the ipsilateral and contralateral hemispheres is shown in
Fig. 4 for each individual
patient. The changes in the uptake of ammonia were not
significantly different between the ipsilateral and contralateral
hemispheres. Ammonia uptake was observed to be higher following CEA
in four patients, whereas the uptake in two patients remained
almost the same and the remaining patients exhibited significantly
reduced uptake.
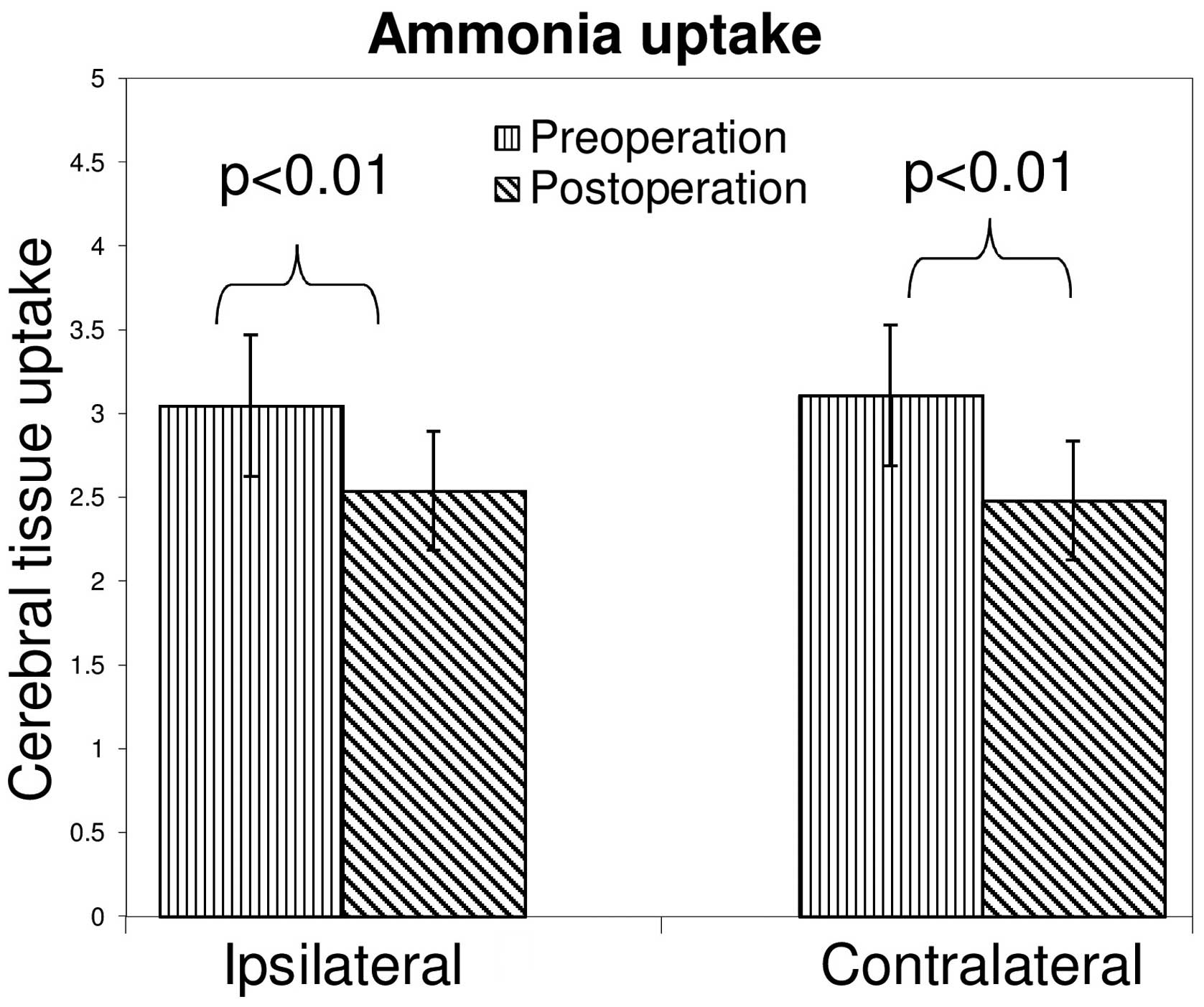
The mean values of ammonia uptake prior and
subsequent to CEA for the ipsilateral and contralateral hemispheres
were examined (Fig. 5). Following
surgery, the uptake of ammonia was observed to decrease between
3.04±0.42 and 2.54±0.35 (23.2%) in the ipsilateral hemisphere, and
between 3.10±0.47 and 2.48±0.36 (23.5%) in the contralateral
hemisphere. Changes in ammonia uptake were statistically
significant for the right and left hemispheres between the
preoperative and postoperative states. However, no significant
difference was identified between the two hemispheres
preoperatively and postoperatively.
Discussion
In the present study, 13N-labeled ammonia
PET was used to examine cerebral ammonia metabolism in patients
with severe carotid artery stenosis, by comparing the cerebral
ammonia uptake prior to and following CEA. The two key observations
were as follows: i) Uptake of ammonia in the ipsilateral and
contralateral cerebral hemispheres reduced significantly following
treatment; ii) no significant difference in the uptake of ammonia
was identified between the ipsilateral and contralateral
hemispheres prior to and following CEA.
As an effective treatment approach for symptomatic
and asymptomatic carotid artery stenosis, CEA has been in use since
the 1950s (18), and CAS has been
used more widely and frequently since the 1990s, as an alternative
treatment option for this disease (18). A large clinical randomized trial,
termed the Carotid Revascularization Endarterectomy vs. Stenting
Trial (CREST), sponsored by the National Institutes of Health,
demonstrated that carotid stenting and endarterectomy were
associated with similar rates of mortality and disabling stroke
(19). However, at least among
symptomatic patients in the CREST trial, CEA exhibited notably low
stroke and mortality rates (20).
Several studies have demonstrated the increase of
cerebral blood flow in ipsilateral cerebral territories or
hemispheres following CEA or CAS in the short- and long-term,
including a study by Yang et al (10) using perfusion CT and a study by
Araki et al (21) using
transcanial Doppler, SPECT and PET. Of note, using
15O-labeled water, which is known to be the gold
standard for measurement of cerebral blood/perfusion, Rijbroek
et al (12) observed that
blood flow and oxygen consumption are increased in the ipsilateral
and contralateral hemispheres following CEA, with no significant
difference of these parameters between the ipsilateral and
contralateral hemispheres, either preoperatively or
postoperatively. The present study demonstrated that the effects of
carotid artery stenosis and the improvements of CEA on cerebral
blood flow and metabolism were equal to the two hemispheres. This
observation can be explained by the effective collateral
circulation, or circle of Willis, in the human brain (12). Furthermore, since blood supply is
the basis of all metabolism, the observation that ammonia uptake
did not change in the present study, was reasonable, based on
uniform hemodynamic changes pre- and postoperatively. Combining the
findings of the present study with those of Rijbroek et al
(12) suggests that unilateral
surgery for carotid artery stenosis improves cerebral blood supply
and metabolism, not only for the ipsilateral hemisphere, but also
for the contralateral hemisphere.
Several studies have investigated the neurological
and neuropsychological effects of carotid artery revascularisation,
including carotid artery stenting and endarterectomy, in patients
with severe carotid artery stenosis or occlusion (22,23).
For example, in patients with chronic internal carotid artery
occlusion, Lin et al (22)
identified that successful carotid artery stenting significantly
improves global cognitive function, and attention and psychomotor
processing speed. Another study (23) demonstrated that unilateral carotid
artery stenting is beneficial to patients with severe asymptomatic
stenosis by improving executive function. In humans,
neurotransmitters are important in the modulation of neurological
and neuropsychological functions, therefore, neurotransmitters are
likely to be involved in the neurological and neuropsychological
effects following CEA.
13N-ammonia has been validated as a
perfusion tracer for use in cardiac PET (24). In brain tissue, the uptake of
ammonia is not as closely associated with blood flow as in the
myocardium. For example, Phelps et al (24) demonstrated that doubling or halving
basal cerebral blood flow resulted in alterations of ammonia uptake
of 30–50% in the human brain, and further increases of blood flow
were associated with progressively smaller alterations in ammonia
uptake. Other studies have demonstrated that the uptake of ammonia
is more dependent on the process of glutamine synthesis in the
brain (14,16).
In the human cerebral cortex, >90% of neurons are
glutamergic or GABAergic, and the high in vivo flux of the
glutamate/glutamine cycle in brain cortex puts a significant demand
on the return of ammonia, mediated by phosphate-activated
glutaminase, between the neurons and astrocytes in order to
maintain the nitrogen balance (16). Therefore, changes in the uptake of
13N-labeled ammonia in the human brain are likely to be
more sensitive than glutamine metabolism.
The reduced uptake of ammonia in the human brain
following carotid CEA observed in the present study suggested two
potential hypotheses: Washout by increased blood flow or a negative
association between blood flow and uptake of ammonia in human brain
tissue.
Washout effect
In human cortex tissue, neurons are surrounded by
astrocytes. This anatomical structure in cerebral histology
suggests that the exchange of contents between the blood and
neurons is modulated by astrocytes. In the glutamate/glutamine and
GABA/glutamine cycles, glutamate and ammonia are released in
neurons and taken up by astrocytes, where glutamine is synthesized
by glutamine synthetase using glutamate and ammonia (14). Previous studies have demonstrated
that there is a high flux of ammonia trafficking between neurons
and astrocytes. Therefore, the present study hypothesized that
increased levels of cerebral blood flow following CEA wash out more
ammonia from astrocytes, resulting in a reduction in
13N-labeled ammonia uptake in the patients.
Negative association of cerebral blood
flow with ammonia uptake
Hepatic encephalopathy is a worsening of brain
function resulting from liver failure, due to the fact that the
liver is no longer able to remove ammonia from the blood (25). In patients with hepatic
encephalopathy and healthy individuals, blood-borne ammonia readily
enters brain tissue and the metabolic trapping in it is correlated
with the blood concentration of ammonia (26). However, how ammonia leaves the
brain had been debated for a long time. Using
13N-labeled ammonia PET. Sørensen et al revealed
that backflux of ammonia from the brain to the blood does indeed
occur in healthy individuals and patients with and without hepatic
encephalopathy (27). Therefore,
combining this finding with the present results, demonstrating a
reduction of ammonia uptake in cerebral tissue, it is reasonable to
assume that, after CEA, the improved cerebral blood flow
accelerates the backflux of ammonia from the brain to the blood, or
a negative association between cerebral blood flow and the ammonia
uptake in brain tissue.
Certain limitations were identified in the present
study. Firstly, no 15O-labeled water was used to measure
the cerebral blood flow simultaneously with 13N-labeled
ammonia. In addition, dynamic PET scanning was not performed, which
has been reported to provide more accurate results for ammonia
uptake in brain tissue. These two limitations are due to the lack
of a 15O-labeled water device and blood radioactivity
measurement devices at the Department of Nuclear Medicine of Inner
Mongolia University Hospital of China. Finally, the patients were
only scanned 1 month following CEA and no long-term follow up, for
example PET scanning 6 months or 1 year-post CEA, was performed.
Therefore, whether the reduction of cerebral ammonia uptake is
temporary or permanent following CEA remains to be elucidated.
These limitations require attention in further investigations.
In conclusion, using 13N-labeled ammonia
PET to evaluate cerebral ammonia metabolism following CEA in
patients with severe carotid artery stenosis, the present study
demonstrated that the uptake of ammonia in the ipsilateral and
contralateral hemispheres is significantly reduced. However, no
significant differences in ammonia uptake were identified between
the two hemispheres, or between ipsilateral and contralateral
hemispheres, pre- or postoperatively.
Acknowledgments
The present study was funded by grants to Dr Tao
Wang from the Key project from Affiliated Hospital of Inner
Mongolia Medical University (grant. no. NYFYBZ2010005), the Natural
Science Foundation of Inner Mongolia (grant. no. 20080404MS1125)
and the Medical Research Plan of Inner Mongolia Public Health in
2010 (grant. no. 2010024); to Dr Xia Bai by the Scientific and
Technological Project of Inner Mongolia Education and to Dr Chunlei
Han by the Centre of Excellence in Cardiovascular and Metabolic
Imaging of Academy of Finland. The authors would like to thank Mr.
Vesa Oikone of Turku PET Centre of Finland for his assistance with
the preparation of this manuscript. The abstract of the present
study was previously published in the Annual Congress of the
Association of Nuclear Medicine, Lyon, France, pS339, 2013.
References
|
1
|
Silver FL, Mackey A, Clark WM, Brooks W,
Timaran CH, Chiu D, Goldstein LB, Meschia JF, Ferguson RD, Moore
WS, et al CREST Investigators: Safety of stenting and
endarterectomy by symptomatic status in the carotid
revascularization endarterectomy versus stenting trial (CREST).
Stroke. 42:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennekamp CW, Immink RV, den Ruijter HM,
Kappelle LJ, Ferrier CM, Bots ML, Buhre WF, Moll FL and de Borst
GJ: Near-infrared spectroscopy can predict the onset of cerebral
hyperperfusion syndrome after carotid endarterectomy. Cerebrovasc
Dis. 34:314–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russjan A, Goebell E, Havemeister S,
Thomalla G, Cheng B, Beck C, Krützelmann A, Fiehler J, Gerloff C
and Rosenkranz M: Predictors of periprocedural brain lesions
associated with carotid stenting. Cerebrovasc Dis. 33:30–36. 2012.
View Article : Google Scholar
|
|
4
|
Buczek J, Karliński M, Kobayashi A, Białek
P and Członkowska: Hyperperfusion syndrome after carotid
endarterectomy and carotid stenting. Cerebrovasc Dis. 35:531–537.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantese VA, Timaran CH, Chiu D, Begg RJ
and Brott TG; CREST investigators: The Carotid Revascularization
Endarterectomy versus Stenting Trial (CREST): Stenting versus
carotid endarterectomy for carotid disease. Stroke. 41(Suppl):
S31–S34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bond R, Rerkasem K, Cuffe R and Rothwell
PM: A systematic review of the associations between age and sex and
the operative risks of carotid endarterectomy. Cerebrovasc Dis.
20:69–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito H, Kuroda S, Hirata K, Magota K,
Shiga T, Tamaki N, Yoshida D, Terae S, Nakayama N and Houkin K:
Validity of dual MRI and F-FDG PET imaging in predicting vulnerable
and inflamed carotid plaque. Cerebrovasc Dis. 35:370–377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nanba T, Ogasawara K, Nishimoto H,
Fujiwara S, Kuroda H, Sasaki M, Kudo K, Suzuki T, Kobayashi M,
Yoshida K and Ogawa A: Postoperative cerebral white matter damage
associated with cerebral hyperperfusion and cognitive impairment
after carotid endarterectomy: A diffusion tensor magnetic resonance
imaging study. Cerebrovasc Dis. 34:358–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vanninen E, Kuikka JT, Aikiä M, Könönen M
and Vanninen R: Heterogeneity of cerebral blood flow in symptomatic
patients undergoing carotid endarterectomy. Nucl Med Commun.
24:893–900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B, Chen W, Yang Y, Lin Y, Duan Y, Li
J, Wang H, Fu F, Zhuge Q and Chen X: Short- and long-term
hemodynamic and clinical effects of carotid artery stenting. AJNR
Am J Neuroradiol. 33:1170–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trojanowska A, Drop A, Jargiello T,
Wojczal J and Szczerbo-Trojanowska M: Changes in cerebral
hemodynamics after carotid stenting: Evaluation with CT perfusion
studies. J Neuroradiol. 33:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rijbroek A, Boellaard R, Vermeulen EG,
Lammertsma AA and Rauwerda JA: Hemodynamic changes in ipsi- and
contralateral cerebral arterial territories after carotid
endarterectomy using positron emission tomography. Surg Neurol.
71:668–676. 2009. View Article : Google Scholar
|
|
13
|
Gaudet JG, Meyers PM, McKinsey JF, Lavine
SD, Gray W, Mitchell E, Connolly ES Jr and Heyer EJ: Incidence of
moderate to severe cognitive dysfunction in patients treated with
carotid artery stenting. Neurosurgery. 65:325–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rothman DL, De Feyter HM, Maciejewski PK
and Behar KL: Is there in vivo evidence for amino acid shuttles
carrying ammonia from neurons to astrocytes? Neurochem Res.
37:2597–2612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adeva MM, Souto G, Blanco N and Donapetry
C: Ammonium metabolism in humans. Metabolism. 61:1459–1511. 2012.
View Article : Google Scholar
|
|
16
|
Cooper AJ: 13N as a tracer for studying
glutamate metabolism. Neurochem Int. 59:456–464. 2011. View Article : Google Scholar :
|
|
17
|
Maes F, Collignon A, Vandermeulen D,
Marchal G and Suetens P: Multimodality image registration by
maximization of mutual information. IEEE Trans Med Imaging.
16:187–198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gröschel K, Schnaudigel S, Pilgram SM,
Wasser K and Kastrup A: A systematic review on outcome after
stenting for intracranial atherosclerosis. Stroke. 40:e340–e347.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gahremanpour A, Perin EC and Silva G:
Carotid artery stenting versus endarterectomy: A systematic review.
Tex Heart Inst J. 39:474–487. 2012.PubMed/NCBI
|
|
20
|
Timaran CH, Mantese VA, Malas M, Brown OW,
Lal BK, Moore WS, Voeks JH and Brott TG; CREST Investigators:
Differential outcomes of carotid stenting and endarterectomy
performed exclusively by vascular surgeons in the Carotid
Revascularization Endarterectomy versus Stenting Trial (CREST). J
Vasc Surg. 57:303–308. 2013. View Article : Google Scholar :
|
|
21
|
Araki CT, Babikian VL, Cantelmo NL and
Johnson WC: Cerebrovascular hemodynamic changes associated with
carotid endarterectomy. J Vasc Surg. 13:854–859. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin MS, Chiu MJ, Wu YW, Huang CC, Chao CC,
Chen YH, Lin HJ, Li HY, Chen YF, Lin LC, et al: Neurocognitive
improvement after carotid artery stenting in patients with chronic
internal carotid artery occlusion and cerebral ischemia. Stroke.
42:2850–2854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mendiz OA, Sposato LA, Fabbro N, Lev GA,
Calle A, Valdivieso LR, Fava CM, Klein FR, Torralva T,
Gleichgerrcht E, et al: Improvement in executive function after
unilateral carotid artery stenting for severe asymptomatic
stenosis. J Neurosurg. 116:179–184. 2012. View Article : Google Scholar
|
|
24
|
Phelps ME, Huang SC, Hoffman EJ, Selin C
and Kuhl DE: Cerebral extraction of N-13 ammonia: Its dependence on
cerebral blood flow and capillary permeability - surface area
product. Stroke. 12:607–619. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butterworth RF: Pathophysiology of hepatic
encephalopathy: A new look at ammonia. Metab Brain Dis. 17:221–227.
2002. View Article : Google Scholar
|
|
26
|
Keiding S, Sørensen M, Bender D, Munk OL,
Ott P and Vilstrup H: Brain metabolism of 13N-ammonia during acute
hepatic encephalopathy in cirrhosis measured by positron emission
tomography. Hepatology. 43:42–50. 2006. View Article : Google Scholar
|
|
27
|
Sørensen M, Munk OL and Keiding S:
Backflux of ammonia from brain to blood in human subjects with and
without hepatic encephalopathy. Metab Brain Dis. 24:237–242. 2009.
View Article : Google Scholar
|