Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous
group of clonal and potentially malignant bone marrow disorders
characterized by ineffective, inadequate haematopoiesis in one or
more of the lineages of the bone marrow (1). It has a variable propensity of
transformation to acute myeloid leukaemia (AML) (2) and is described as a 'pro-leukaemia
state' (3). MDS can arise de
novo or as a consequence of previous chemo- or radiotherapy in
cancer patients (4). It occurs
most frequently in aged populations with a median age of 65–70
years at diagnosis (2). However,
there is a paediatric population of MDS patients in which inherited
bone marrow-failure syndromes are associated with high-risk
factors. The median survival time of MDS patients following
diagnosis is 0.5–6 years (5,6).
Numerous types of therapy for MDS have been developed based on the
molecular mechanisms of the diseases; for example, inhibitors of
DNA methylation have been proven effective in the treatment of
patients with MDS (7). Although
available treatments have alleviated MDS-associated symptoms of
certain patients, few treatments are able to transform the natural
course of the disease (4). In
addition, numerous chemotherapeutic treatment options induce
undesirable side effects. The lack of safe and effective
therapeutic options emphasizes the urgent requirement for the
development of novel therapies. The ultimate goal is to identify an
effective treatment that can extend the overall survival of
patients with MDS.
Natural products have attracted considerable
attention as anti-cancer agents over the past few years. Several of
these compounds, including vincristine, paclitaxel and etoposide,
have been tested and used in clinical treatment (8). Fucoidan, a complex sulphated
polysaccharide natural product with a molecular weight of 5–627
kDa, was isolated from the cell wall matrix of brown seaweeds,
which have been used in Traditional Chinese Medicine for nearly
2,000 years for the treatment of a wide variety of diseases,
including thyroid disease, skin diseases, arteriosclerosis,
hypertension and cancer (9–11).
The anti-cancer effects of fucoidan are particularly promising
(12). Previous studies reported
that fucoidan effectively suppressed the proliferation and colony
formation of cancer cells in vitro (13); furthermore, fucoidan inhibited
metastasis and angiogenesis of Lewis lung adenocarcinoma and B16
melanoma xenografts in vivo (14). Natural products to attenuate or
prevent the progression of carcinogenesis via three major
mechanisms: Selective promotion of apoptosis in cancer cells,
interference with the cell cycle and inhibition of angiogenesis and
metastasis (14). However, whether
fucoidan affects the apoptosis of MDS/AML cells has remained
elusive.
The present study therefore examined the anti-cancer
effects of fucoidan as well as its underlying molecular mechanisms
of action in the human MDS/AML cell line SKM-1. For this purpose,
the effects of fucoidan on the proliferation, cell cycle,
apoptosis, generation of reactive oxygen species (ROS) and
expression of apoptosis-associated genes in SKM-1 cells were
assessed. The present study suggested that fucoidan may be a
candidate drug for the treatment of MDS.
Materials and methods
Drugs and cell culture
Fucoidan was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The human MDS/AML cell line SKM-1 was provided by
Professor Jianfeng Zhou (Department of Hematology, Tongji Medical
College of Huazhong University of Science and Technology, Wuhan,
China). Cells were maintained in RPMI-1640 (HyClone, Logan, UT,
USA) supplemented with 10% heat-inactivated foetal bovine serum
(FBS; Gibco-BRL, Invitrogen Life Technologies, Inc., Carlsbad, CA,
USA) (15).
Cell counting kit (CCK-8) assay
The CCK-8 assay (cat. no. C0038; Beyotime Institute
of Biotechnology, Shanghai, China) was performed to estimate the
effects of fucoidan on the proliferation of SKM-1 cells. Cells were
seeded (3×104 cells/ml) in a 96-well plate in 100
µl RPMI-1640 containing 10% FBS at 37°C in a 5%
CO2 incubator. After 24 h, the medium was replaced with
fresh medium containing various concentrations (50, 100, 200, 300,
400 and 500 µg/ml) of fucoidan, and the cells were incubated
for an additional 24, 48 or 72 h at 37°C in the 5% CO2
incubator. After incubation, the CCK-8 reagent (10 µl) was
added to each well, and the cells were incubated for 2 h at 37°C
and 5% CO2. The optical density (OD) values were
measured at 450 nm using a microtiter plate reader (SpectraMax M5;
Molecular Devices, LLC, Sunnyvale, CA, USA) (16). The results were expressed as the
percentage of growth inhibition calculated according to the
following formula: (ODControl −
ODExperimental group)/ODControl ×100%.
Assessment of apoptosis
For the analysis of cell apoptosis, 2×105
SKM-1 cells were seeded into six-well plates in 1 ml RPMI-1640
medium containing 10% FBS and cultured overnight. Next, the medium
was replaced with fresh RPMI-1640 or the same media containing 100
µg/ml fucoidan. After an additional incubation for 48 h, the
medium was discarded and the SKM-1 cells were washed twice in
phosphate-buffered saline (PBS). Apoptosis was evaluated using
Annexin V and propidium iodide (PI) staining (Beyotime Institute of
Biotechnology) followed by flow cytometric analysis (FACSCalibur™;
BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's instructions.
Cell cycle analysis
Cells were incubated with fucoidan (100
µg/ml) for 48 h, harvested and washed twice with PBS. The
medium was discarded, the SKM-1 cells were washed twice with PBS
and fixed with 70% alcohol for 4 h. The cell cycle was determined
by flow cytometry following PI staining of the nuclei.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using RNAiso Plus (Takara
Biotechnology, Otsu, Japan) following incubation of the cells with
fucoidan (100 µg/ml) for 48 h. Subsequently, cDNA was
generated using a two-step RT-PCR kit (cat. no. RR037A; Takara
Biotechnology). The quantitative PCR reaction was performed in a
mixture with a total volume of 20 µl and contained SYBR
Premix Ex Taq (10 µl), 1 µl of each primer (10
µmol/l), 2 µl cDNA template and double distilled
H2O (6 µl). A 7500 Real-Time PCR System (Applied
Biosystems Life Technologies, Foster City, CA, USA) was used and
the primers were designed using Primer 5 software (version 5; Jikai
Co., Shanghai, China) and synthesized by Sangon Biotech (Shanghai,
China; Table I). The
thermocy-cling conditions were as follows: 95°C for 10 min, 40
cycles of 95°C for 15 sec, 65°C for 30 sec and 72°C for 30 sec, and
a final extension at 72°C for 10 min. The 2−ΔΔCT method
was used to quantify the PCR products.
 | Table IPrimers used for polymerase chain
reaction. |
Table I
Primers used for polymerase chain
reaction.
| Gene | Primers |
|---|
| Caspase3 | F:
5′-ATGACATCTCGGTCTGGT-3′ |
| R:
5′-AGAAACATCACGCATCAA-3′ |
| Caspase8 | F:
5′-AAGGAAGCAAGAACCCAT-3′ |
| R:
5′-TGACCCTGTAGGCAGAAA-3′ |
| Fas | F:
5′-TCCCATCCTCCTGACCAC-3′ |
| R:
5′-TCGTAAACCGCTTCCCTC-3′ |
| Caspase9 | F:
5′-CCAAGCCTCTTCTTACTTCACC-3′ |
| R:
5′-CATCGTTCTGCCATCACTCA-3′ |
| Actin | F:
5′-CCACGAAACTACCTTCAACTAA-3′ |
| R:
5′-GTGATCTCCTTCTGCATCCTGT-3′ |
| AKT | F:
5′-GCAAGGTGATCCTGGTGAA-3′ |
| R:
5′-TCGTGGGTCTGGAAAGAGTA-3′ |
Western blot analysis
SKM-1 cells were treated with fucoidan (100
µg/ml) for 48 h. Cells were harvested and washed twice with
PBS, and the total protein was obtained after cell lysis using
lysis buffer [components: 50 mM Tris (pH 7.4), 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS and sodium
orthovanadate sodium fluoride, EDTA and leupeptin (Beyotime
Institute of Biotechnology)]. Total protein was quantified using a
BCA protein Assay kit (cat. no. P0012S; Beyotime Institute of
Biotechnology) and 50 µg protein was loaded per lane and
separated using 10% SDS-PAGE, then transferred onto a
polyvinylidene fluoride membrane with glycine transfer buffer
(composed of 3.05 g Tris, 14.4 g glycine, 200 ml methanol and 800
ml H2O; Beyotime Institute of Biotechnology). After
blocking with 5% nonfat milk, the membrane was incubated with the
following primary antibodies: Rabbit polyclonal Fas (cat. no.
YT1676), rabbit polyclonal caspase-8 (cat. no. YT0660), rabbit
poly-clonal caspase-9 (cat. no. YT0664), rabbit polyclonal
caspase-3 (cat. no. YT5204; Immunoway Biotechnology Co., Newark,
DE, USA) all at a dilution of 1:500, for 12 h at 4°C. Subsequently
the membrane was incubated with the following secondary antibodies:
Rabbit polyclonal anti-β-actin [dilution, 1:200 (cat. no.
Ab119716); Abcam, Cambridge, UK], rabbit polyclonal
phosphorylated-Akt [dilution, 1:100 (cat. no. Sc-135651); Santa
Cruz Biotechnology, Inc., Dallas, TX, USA], rabbit polyclonal Akt
[dilution, 1:100 (cat. no. Sc-8312); Santa Cruz Biotechnology,
Inc.], all incubated at 4°C for 12 h, as well as horseradish
peroxidase-conjugated goat polyclonal immuno-globulin G [dilution,
1:7,000 (cat. no. ZDR-5036); ZSGB-BIO, Beijing, China] at 37°C for
2 h. Protein bands were visualized using an enhanced
chemiluminescence kit (cat. no. P0018; Beyotime Institute of
Biotechnology) and analyzed using Quantity One software (version
4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Measurement of intracellular ROS
The T-AOC detection assay kit (cat. no. S0119;
Beyotime Institute of Biotechnology) was used to assess
intracellular ROS production. SKM-1 cells were collected after
incubation with fucoidan (100 µg/ml) for 48 h and labelled
with 10 µM dichloro-dihydro-fluorescein diacetate at 37°C in
the dark for 30 min. After washing the cells twice with RPMI-1640,
the cellular fluorescence intensity was measured using a flow
cytometer (excitation and emission wavelength, 488 and 525 nm,
respectively).
Statistical analysis
Values are expressed as the mean ± standard
deviation and one-way analysis of variance, Dunnett's test and
independent samples t-test were performed to evaluate statistical
significance. Statistical analyses were performed using SPSS
version 20.0 (IBM SPSS, Armonk, NY, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
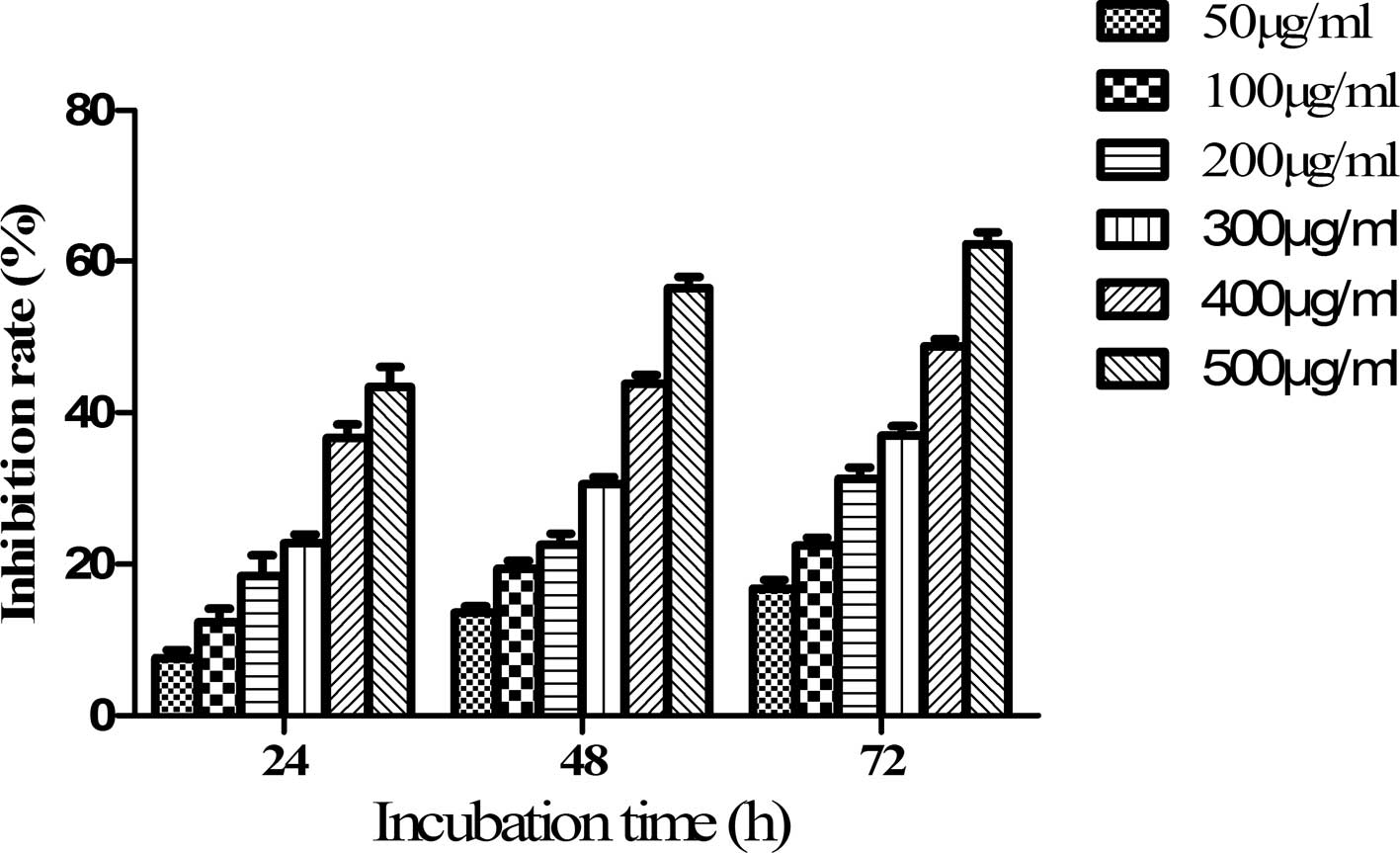
Fucoidan inhibits the proliferation of
SKM-1 cells in a dose-and time-dependent manner
The present study first examined the effects of
fucoidan on the proliferation of SKM-1 cells using a CCK-8 assay.
SKM-1 cells were treated with various concentrations of fucoidan
(50, 100, 200, 300, 400 and 500 µg/ml) for 24, 48 or 72 h.
The OD values were determined and the inhibition rate was
calculated. The inhibition rate of SKM-1 cells treated with 50
µg/ml fucoidan for 24 h was 7.5±1.11%, which increased to
43.4±2.72% at a concentration of 500 µg/ml fucoidan.
Furthermore, the inhibition rate increased by 9.2% when the cells
were exposed to 50 µg/ml fucoidan for 72 h (Fig. 1). The growth inhibition of SKM-1
cells by fucoidan was therefore concentration- and
time-dependent.
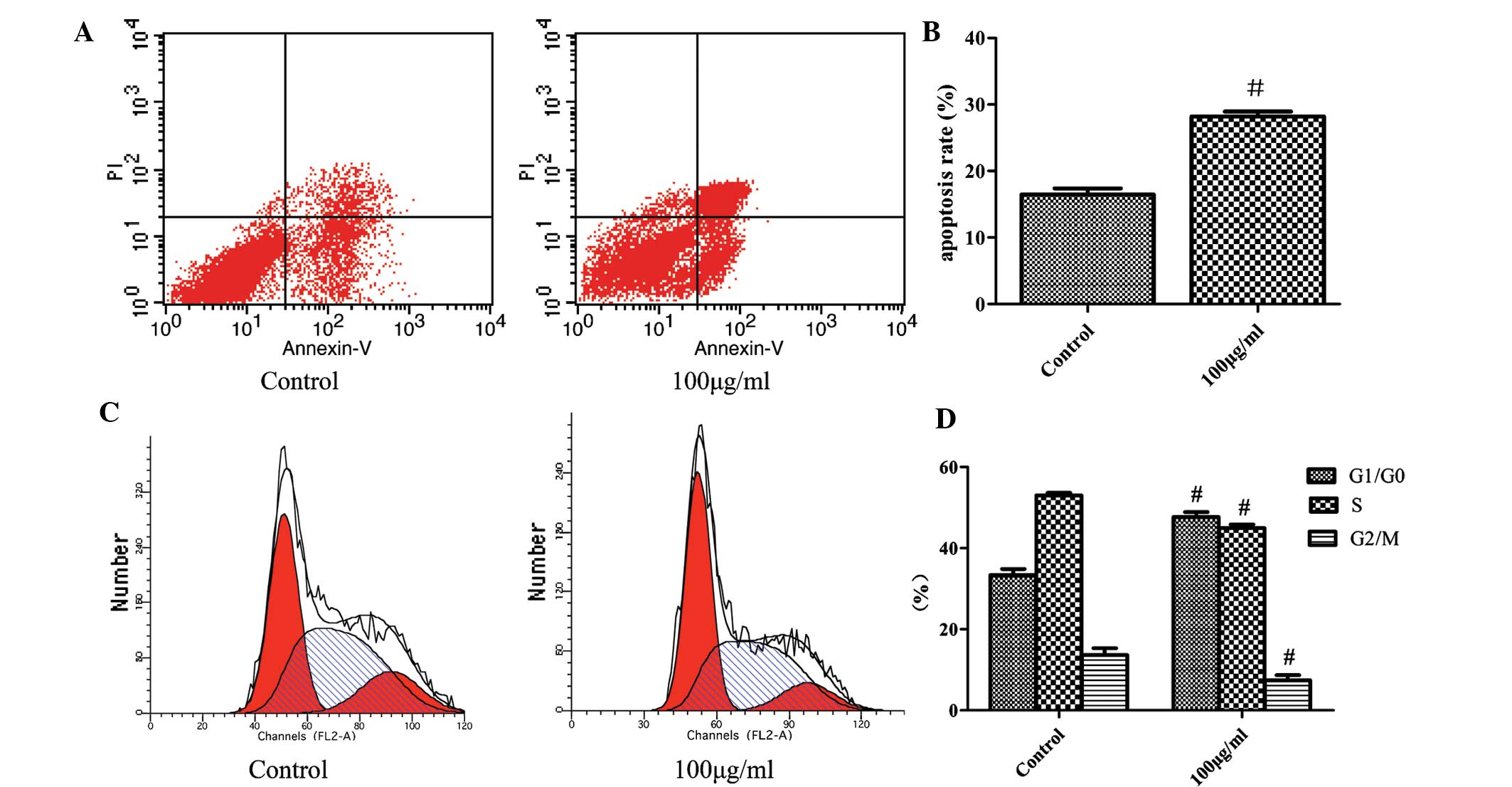
Fucoidan induces apoptosis of SKM-1 cells
and blocks the cell cycle in G1 phase
To determine whether the inhibitory effects of
fucoidan on cell proliferation resulted from its induction of
apoptotic cell death, SKM-1 cells treated with fucoidan (100
µg/ml) for 48 h were stained with Annexin V and PI, and the
apoptotic rate was detected using flow cytom-etry. The apoptotic
rate of SKM-1 cells following treatment with fucoidan (100
µg/ml) was 28.2±0.94% compared with 16.4±0.75% in the
control (Fig. 2A). The underlying
mechanism of the anti-proliferative effects of fucoidan was further
investigated by determining its effect on the cell cycle of SKM-1
cells. Following incubation with fucoidan (100 µg/ml) for 48
h, cells were stained with PI and cell cycle analysis was performed
using flow cytometry. The G1/G0-phase population in the
fucoidan-treated group was 47.71±1.23% compared with 33.38±1.52% in
the control group. However, the S-phase population decreased from
53.01±0.67 to 44.94±0.99% and the G2/M-phase population decreased
from 13.61±1.69 to 7.36±1.35% following treatment with fucoidan
compared with the control-group populations (Fig. 2B). These findings demonstrated that
treatment with fucoidan increased the apoptotic rate and induced
cell cycle arrest in G1/G0 phase.
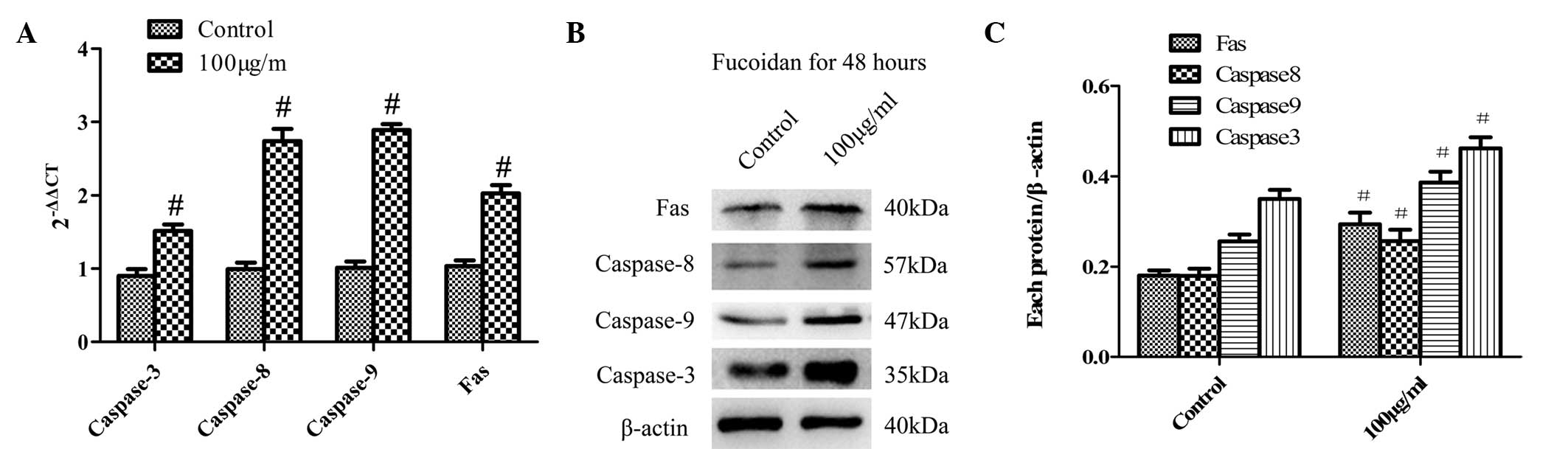
Fucoidan induces apoptosis in SKM-1 cells
via the extrinsic and intrinsic pathways
To gain further insight into the mechanism of
fucoidan-induced apoptosis of SKM-1 cells, the expression of the
apoptosis-associated molecules Fas, caspase-8, caspase-9 and
caspase-3 was detected at the mRNA and protein level using RT-qPCR
(Fig. 3A) and western blot
analysis (Fig. 3B), respectively.
The expression levels of the extrinsic pathway-associated molecules
Fas and caspase-8 as well as the intrinsic pathway-associated
molecule caspase-9 were gradually increased in response to fucoidan
treatment (100 µg/ml for 48 h). In addition, the downstream
effector caspase-3 was also activated.
Fucoidan blocks phosphoinositide-3 kinase
(PI3K)/Akt signaling in SKM-1 cells
To investigate whether the PI3K/Akt signaling
pathway is involved in fucoidan-induced apoptosis in SKM-1 cells,
the mRNA levels of AKT were examined using RT-qPCR (Fig. 4A). The mRNA expression of AKT
decreased by 0.2–0.5 times compared with that in the normal control
group. Furthermore, the PI3K/Akt signaling pathway protein
phospho-Akt and Akt were quantified using western blot analysis
(Fig. 4B). The protein expression
of phospho-Akt was decreased in SKM-1 cells treated with fucoidan
(100 µg/ml for 48 h). These results indicated that fucoidan
inactivated the PI3K/Akt signaling pathway.
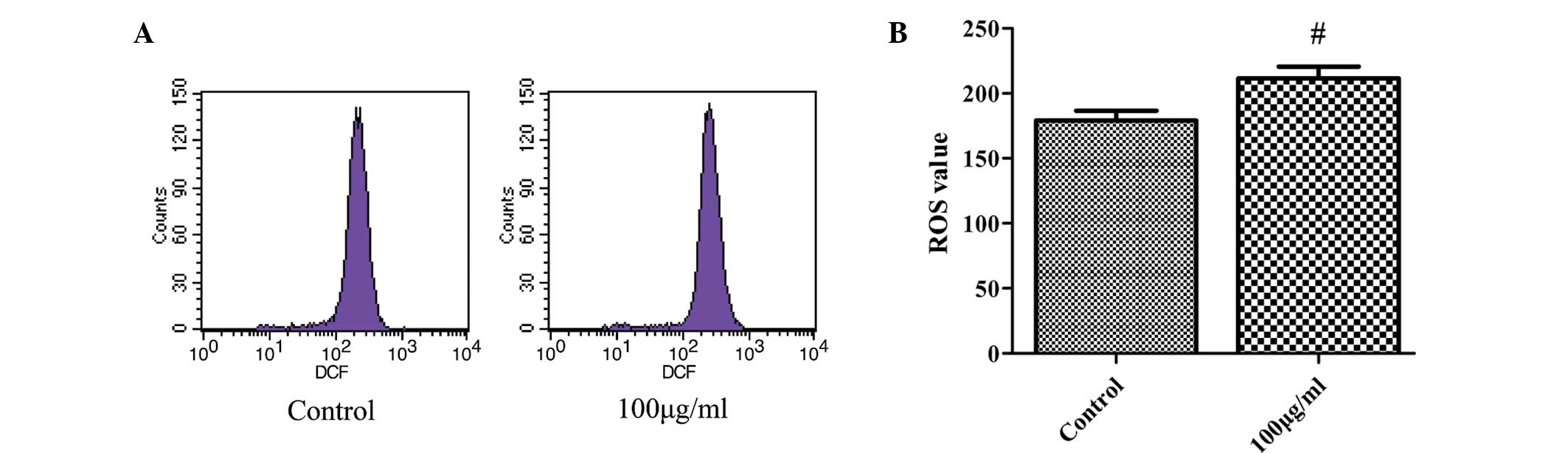
Fucoidan increases intracellular ROS
production
The present study investigated the generation of
intracellular ROS to determine whether changes in ROS levels have a
role in fucoidan-induced apoptosis in SKM-1 cells. Cells were
incubated with 100 µg/ml fucoidan for 48 h and ROS were
measured using flow cytometry. The mean value of ROS production was
264.3×103/mg protein in the fucoidan-treated group
compared with 179.1×103/mg in the control group
(Fig. 5). As ROS production was
markedly enhanced in SKM-1 cells following fucoidan treatment, the
generation of ROS may, at least in part, be the underlying
molecular mechanism of the induction of cancer-cell apoptosis by
fucoidan.
Discussion
Fucoidan, a complex sulphated polysaccharide
extracted from brown seaweeds, has been shown to exhibit
anti-cancer activity in a wide variety of tumour cell types and is
therefore considered to be a promising novel candidate for cancer
therapy with low toxicity to normal cells (17-20).
In the present study, treatment with fucoidan inhibited the
proliferation and induced apoptosis in the MDS/AML cell line
SKM-1.
Previous studies have also indicated that fucoidan
directly inhibited the proliferation of various cancer cell lines,
including that of PC-3 cells at 10-200 µg/ml (21), MCF-7 cells at 82-820 µg/ml
(22) and U937 cells at 20-100
µg/ml (23) where the
incubation times were between 12 and 96 h. In the present study,
fucoidan inhibited the proliferation of SKM-1 cells at all studied
concentrations (50, 100, 200, 300, 400, 500 µg/ml) according
to a CCK-8 assay. The inhibition rate of cells was 7.5±1.11% when
the concentration of fucoidan was 50 µg/ml, which increased
to 43.4±2.72% at a concentration of 500 µg/ml. Additionally,
the inhibition rate increased by 9.2% when cells were exposed to 50
µg/ml fucoidan for 72 h. Thus, fucoidan treatment inhibited
the proliferation of SKM-1 cells in a dose- and time-dependent
manner. Furthermore, flow cytometric analysis was performed to
determine whether the inhibitory effects of fucoidan on cell
proliferation resulted from apoptotic cell death and cell cycle
arrest. The apoptotic rate of SKM-1 cells treated with fucoidan
(100 µg/ml) for 48 h was 28.2% compared to 16.4% in the
control group. In addition, following treatment with fucoidan, the
G1/G0 phase population of SKM-1 cells was markedly increased
compared with that in the normal control group, while the S-phase
and G2/M-phase populations were significantly decreased. Therefore,
flow cytometric analysis confirmed that the inhibition of
proliferation by fucoidan was based on the induction of cell cycle
arrest and apoptosis.
It is well known that apoptosis, the process of
programmed cell death, has an important role in the normal
development and differentiation of multicellular organisms; it is
characterized by distinct morphological features and
energy-dependent biochemical mechanisms (24). Apoptosis also serves as a critical
protective mechanism against carcinogenesis caused by genetic
mutations of normal cells, which may occur spontaneously or which
may be induced by various stimuli or carcinogens. Fucoidan has been
previously demonstrated to induce apoptosis in leukemia cells via
B-cell lymphoma 2 and mitogen-activated protein kinase signaling
(23). In the present study, the
observed fucoidan-induced cell cycle arrest in G1/G0-phase, which
may have been a response to cellular damage by ROS, may have caused
the activation of apoptotic pathways, which was further confirmed
by the increased apoptotic rate. Furthermore, the extrinsic pathway
(death receptor-mediated) and the intrinsic pathway (mitochondrial
mediated) of apoptosis represent two major pathways implicated in
the induction of apoptotic cell death (25). Activation of caspases is a pivotal
step in the extrinsic as well as the intrinsic apoptotic pathways
and is triggered by signals from death factors, mitochondrial
alterations or DNA damage due to external and/or internal insults
(26). Fas, one of these death
receptor factors, results in the clustering and formation of a
death-inducing signaling complex. The results of the present study
showed that treatment of SKM-1 cells with fucoidan increased the
expression of Fas, which actives the extrinsic pathway. Further
downstream in the apoptotic signaling cascade, initiator caspases,
including caspase-8, significantly amplify the complex
death-inducing signaling (24).
The results of the present study showed that incubation of SKM-1
cells with fucoidan decreased the mRNA expression of AKT as well as
the levels of phosphorylated AKT protein, therefore inhibiting the
PI3K/Akt signaling pathway. The PI3K/Akt signaling pathway is
another regulator of cell survival, cell growth and apoptosis, and
inactivation of the PI3K/Akt signaling pathway inhibits the
proliferation and induces the apoptosis of cancer cells by
activating apoptotic signals through caspase-9 (19,27).
The present study further demonstrated that treatment of SKM-1
cells with fucoidan increased caspase-9 levels, which was likely to
be mediated via decreases of PI3K/AKT signaling via the intrinsic
pathway of apoptosis. In addition, activation of caspase-3 was
observed, which is a downstream effector of caspase-8 and
caspase-9. Upon its activation, the executioner caspase-3
disassembles the cytoskeleton, leading to cell-morphological
changes associated with apoptosis. These results indicated that
fucoidan induced apoptosis of SKM-1 cells via activation of
extrinsic as well as intrinsic apoptotic pathways.
Furthermore, ROS have a key role in oxidative stress
and are generated as by-products of cellular metabolism, primarily
in the mitochondria (28). The
maintenance of an appropriate level of intracellular ROS is
important for the maintenance of a redox balance and signaling
associated with cellular proliferation (29). However, upon its overproduction,
ROS can degrade cellular proteins, DNA and lipids, resulting in a
state of oxidative stress (30).
Previous studies have demonstrated that cancer cells can be
effectively killed using natural products with the ability to
increase intracellular ROS levels (29,31).
In the present study, fucoidan treatment of SKM-1 cells for 48 h
caused a rapid accumulation of intracellular ROS. High levels of
ROS increase the vulnerability of tumor cells to apoptosis;
furthermore, ROS may have initiated the cellular damage signaling
cascade, leading to cell cycle arrest and apoptosis. The
observations of the present study suggested that the generation of
ROS is involved in fucoidan-induced apoptosis in SKM-1 cells.
In conclusion, fucoidan, a natural product from the
cell wall of brown seaweeds, caused cell cycle arrest and induced
apoptosis via the activation of apoptotic pathways. Furthermore,
the induction of apoptosis by fucoidan was likely to be associated
with enhanced production of ROS. The results of the present study
suggested that fucoidan is a promising candidate drug for the
treatment of MDS.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30971277 and
81250034), the Chongqing Natural Science Foundation (grant no.
CSTC2009BB5070), the Chongqing Health Bureau Foundation (grant no.
2013-2-023) and the Chongqing Education Commission Foundation
(grant no. 2013).
References
|
1
|
Foran JM and Shammo JM: Clinical
presentation, diagnosis and prognosis of myelodysplastic syndromes.
Am J Med. 125(Suppl 7): S6–S13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou T, Hasty P, Walter CA, Bishop AJ,
Scott LM and Rebel VI: Myelodysplastic syndrome: An inability to
appropriately respond to damaged DNA? Exp Hematol. 41:665–674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pilo F, Di Tucci AA, Dessalvi P, Caddori A
and Angelucci E: The evolving clinical scenario of myelodysplastic
syndrome: The need for a complete and up to date upfront diagnostic
assessment. Eur J Intern Med. 21:490–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JN: Myelodysplastic syndrome
hematopoietic stem cell. Int J Cancer. 133:525–533. 2013.
View Article : Google Scholar
|
|
5
|
Garcia-Manero G: Myelodysplastic
syndromes: 2012 update on diagnosis, risk-stratification and
management. Am J Hematol. 87:692–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma X: Epidemiology of myelodysplastic
syndromes. Am J Med. 125(Suppl 7): S2–S5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voso MT, Santini V, Finelli C, Musto P,
Pogliani E, Angelucci E, Fioritoni G, Alimena G, Maurillo L,
Cortelezzi A, et al: Valproic acid at therapeutic plasma levels may
increase 5-azacytidine efficacy in higher risk myelodysplastic
syndromes. Clin Cancer Res. 15:5002–5007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Teruya K, Eto H and Shirahata S:
Induction of apoptosis by low-molecular-weight fucoidan through
calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231
breast cancer cells. Biosci Biotechnol Biochem. 77:235–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Heinrich M, Myers S and Dworjanyn
SA: Towards a better understanding of medicinal uses of the brown
seaweed sargassum in traditional Chinese medicine: A phytochemical
and pharmacological review. J Ethnopharmacol. 142:591–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu C, Cao R, Zhang SX, Man YN and Wu XZ:
Fucoidan inhibits the growth of hepatocellular carcinoma
independent of angiogenesis. Evid Based Complement Alternat Med.
2013:6925492013.PubMed/NCBI
|
|
12
|
Yang L, Wang P, Wang H, Li Q, Teng H, Liu
Z, Yang W, Hou L and Zou X: Fucoidan derived from Undaria
pinnatifida induces apoptosis in human hepatocellular carcinoma
SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar
Drugs. 11:1961–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ermakova S, Sokolova R, Kim SM, Um BH,
Isakov V and Zvyagintseva T: Fucoidans from brown seaweeds
Sargassum hornery, Eclonia cava, Costaria costata: Structural
characteristics and anticancer activity. Appl Biochem Biotechnol.
164:841–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fulda S: Modulation of apoptosis by
natural products for cancer therapy. Planta Med. 76:1075–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Luo J, Nian Q, Xiao Q, Yang Z and
Liu L: Ribosomal protein S14 silencing inhibits growth of acute
myeloid leukemia transformed from myelodysplastic syndromes via
activating p53. Hematology. 19:225–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thinh PD, Menshova RV, Ermakova SP,
Anastyuk SD, Ly BM and Zvyagintseva TN: Structural characteristics
and anticancer activity of fucoidan from the brown alga sargassum
mcclurei. Mar Drugs. 11:1456–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue M, Ge Y, Zhang J, Wang Q, Hou L, Liu
Y, Sun L and Li Q: Anticancer properties and mechanisms of fucoidan
on mouse breast cancer in vitro and in vivo. Plos One.
7:e434832012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teruya T, Konishi T, Uechi S, Tamaki H and
Tako M: Anti-proliferative activity of oversulfated fucoidan from
commercially cultured Cladosiphon okamuranus Tokida in U937 cells.
Int J Biol Macromol. 41:221–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boo HJ, Hong JY, Kim SC, Kang JI, Kim MK,
Kim EJ, Hyun JW, Koh YS, Yoo ES, Kwon JM and Kang HK: The
anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar
Drugs. 11:2982–2999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue M, Ge Y, Zhang J, Liu Y, Wang Q, Hou L
and Zheng Z: Fucoidan inhibited 4T1 mouse breast cancer cell growth
in vivo and in vitro via downregulation of Wnt/beta-catenin
signaling. Nutr Cancer. 65:460–468. 2013. View Article : Google Scholar
|
|
21
|
Boo HJ, Hong JY, Kim SC, Kang JI, Kim MK,
Kim EJ, Hyun JW, Koh YS, Yoo ES, Kwon JM and Kang HK: The
anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar
Drugs. 11:2982–2999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Teruya K, Eto H and Shirahata S:
Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism
involving the ROS-dependent JNK activation and
mitochondria-mediated pathways. PLoS One. 6:e274412011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HS, Hwang HJ, Kim GY, Cha HJ, Kim WJ,
Kim ND, Yoo YH and Choi YH: Induction of apoptosis by fucoidan in
human leukemia U937 cells through activation of p38 MAPK and
modulation of Bcl-2 family. Mar Drugs. 11:2347–2364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Senthilkumar K, Manivasagan P, Venkatesan
J and Kim SK: Brown seaweed fucoidan: Biological activity and
apoptosis, growth signaling mechanism in cancer. Int J Biol
Macromol. 60:366–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin S, Pang RP, Shen JN, Huang G, Wang J
and Zhou JG: Grifolin induces apoptosis via inhibition of PI3K/AKT
signalling pathway in human osteosarcoma cells. Apoptosis.
12:1317–1326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quan Z, Gu J, Dong P, Lu J, Wu X, Wu W,
Fei X, Li S, Wang Y, Wang J and Liu Y: Reactive oxygen
species-mediated endoplasmic reticulum stress and mitochondrial
dysfunction contribute to cirsimaritin-induced apoptosis in human
gallbladder carcinoma GBC-SD cells. Cancer Lett. 295:252–259. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pelicano H, Feng L, Zhou Y, Carew JS,
Hileman EO, Plunkett W, Keating MJ and Huang P: Inhibition of
mitochondrial respiration: A novel strategy to enhance drug-induced
apoptosis in human leukemia cells by a reactive oxygen
species-mediated mechanism. J Biol Chem. 278:37832–37839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Wang P, Wang H, Li Q, Teng H, Liu
Z, Yang W, Hou L and Zou X: Fucoidan derived from Undaria
pinnatifida induces apoptosis in human hepatocellular carcinoma
SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar
Drugs. 11:1961–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang P, Feng L, Oldham EA, Keating MJ and
Plunkett W: Superoxide dismutase as a target for the selective
killing of cancer cells. Nature. 407:390–395. 2000. View Article : Google Scholar : PubMed/NCBI
|