Introduction
Hepatitis C virus (HCV) is a hepatotropic RNA virus
of the genus Hepacivirus in the Flaviviridae family. Since
it was identified in 1989, HCV has become a globally spread virus,
leading to a worldwide health problem, which requires significant
resources for its prevention and control. HCV can cause acute and
chronic hepatitis in humans and chimpanzees, and if untreated it
may progress to cirrhosis and hepatocellular carcinoma (HCC)
(1). HCV is a positive-sense,
single-stranded enveloped RNA virus ~9,600 nucleotides in length
(2). Signal transducer and
activator of transcription (STATs) factors are a family of
transcription factors that have essential roles in cell growth,
development, proliferation, immune defense and differentiation.
Seven members of the STAT family have been identified and
characterized, including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b
and STAT6 (3). Among the STAT
family, STAT3 has been identified as a critical regulator in tumor
cells (4). The activation of STAT3
may be one of the mechanisms by which human papilloma virus (HPV)
achieves oncogenic transformation. E2, an early protein of HPV16,
potentiates tumor necrosis factor (TNF)-α-mediated nuclear
factor-κB activation through activation of STAT3 in the presence of
TNF-α. In addition, this results in the reversal of E2-induced
apoptosis, leading to cell survival (5). Tacke et al found that
extracellular HCV core protein activates STAT3 in human monocytes,
macrophages and dendritic cells (DCs) through an interleukin (IL)-6
autocrine pathway (6). However,
the specific mechanism underlying how STAT3 is involved in the
regulation of HCV replication remains to be elucidated.
Non-coding RNAs (ncRNAs) are transcribed RNA
molecules with little or none protein coding capacity. Among all
types of ncRNAs, long non-coding RNAs (lncRNAs), a type of
regulatory ncRNA, are crucial in almost every aspect of cellular
processes (7). Zhang et al
found that nuclear enriched abundant transcript 1 (NEAT1) is one of
several lncRNAs whose expression is altered by human
immunodeficiency virus type 1 (HIV-1) infection and the knockdown
of NEAT1 enhanced HIV-1 production through increased
nucleus-to-cytoplasm export of Rev-dependent instability
element-containing HIV-1 mRNAs (8). According to a large number of
studies, lncRNAs are also associated with the development and
progression of HCV, which causes HCC (9–12). A
number of previous studies have implicated phosphatidylinositol
4-phosphate (PI4P) and several interacting partners in HCV
infection (13–16). It has been demonstrated that host
phosphatidylinositol 4-kinase and its product PI4P are involved in
HCV replication and exert their effect through the effector
oxysterol-binding protein (17).
However, there are no relevant studies investigating whether
lncRNAs can affect HCV replication through regulating PI4P.
Therefore, identifying the association between lncRNAs regulated by
STAT3 and PI4P expression may provide a novel pathogenic mechanism
and therapeutic target for HCV infection.
In order clarify the detailed molecular mechanism of
how STAT3 promotes the replication of HCV, human lncRNA polymerase
chain reaction (PCR) arrays were used to identify the lncRNAs
regulated by STAT3 in the current study. Finally, the mechanism
underlying the regulation of HCV replication by lnc-7SK and
lnc-IGF2-AS was investigated.
Materials and methods
Cell culture and virus
Human hepatoma cells (Huh7; American Type Culture
Collection, Manassas, VA, USA) are a cell line highly permissive
for HCV replication (18). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM) medium
(HyClone Laboratories, Inc., Logan, UT, USA) with 10% fetal bovine
serum (FBS; HyClone Laboratories, Inc.) and cell culture plates
were all placed in a humidified 5% CO2 incubator at
37°C.
The genotype 2a HCV strain JFH-1 was produced and
propagated as described by Wakita et al (19).
Lentiviral vector construction and
transfection
The lentiviral vector system contains GV-166,
pHelper1.0 and pHelper2.0. pHelper1.0 and pHelper2.0 contain
essential elements for virus packaging. The STAT3 gene was
amplified by PCR and then inserted at a unique BamHI site
and AgeI site in the vector (GV-166) to construct the
GV166-STAT3 plasmid using the following primers: STAT3, forward
ATGGGACACTGGGTGAGAGT and reverse CGGCAGGTCAATGGTATTGC. E.
coli was transformed with GV166-STAT3 and cultured overnight at
37°C. PCR of the connected product was performed and the construct
was confirmed by DNA sequencing. Huh7 cells were transfected with
GV166-STAT3, pHelper1.0 and pHelper2.0 using Lipofectamine™ 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). The virus
supernatant was collected after 48 h of cultivation and the viral
titers were determined.
Stably transfected cells were transfected with the
lentivirus (10 MOI) at 50–60% confluence in 6 well plates. The
lentivirus vector with 2 mg/ml polybrene was added to the Opti-MEM
medium and after 12–16 h, the culture medium containing lentivirus
was removed and the cell was incubated in Opti-MEM medium with 10%
FBS. The cells were selected with 2 µg/ml puromycin
(Sigma-Aldrich, St. Louis, MO, USA) for 7–10 days to produce the
GV166-STAT3 stable transfected clones. An empty GV166-control
vector was used as a negative control. The expression of STAT3 was
then identified by quantitative (q)PCR and western blot
analysis.
Total RNA extraction
Total RNA from the cells was extracted using TRIzol.
TRIzol (1 ml) reagent was added to the 6-well plates and then the
homogenized samples were incubated for 10 min at room temperature.
Chloroform (0.2 ml) was added to the samples and agitated
vigorously for 15 sec. The samples were incubated for 5 min at room
temperature and then centrifuged at 12,000 × g for 10 min at 4°C.
The upper aqueous phase was transferred to new tubes and 0.5 ml
isopropyl alcohol was added. The samples were incubated for 10 min
at room temperature and were then centrifuged at 12,000 × g for 10
min at 4°C. The supernatant liquid was carefully removed without
disturbing the pellet containing the RNA. The RNA pellet was washed
with 1 ml of 75% ethanol three times and then centrifuged at 12,000
× g for 10 min at 4°C. The supernatant was carefully removed and
the RNA pellet was dried for 10–15 min. Subsequently, the RNA
pellet was redissolved in nuclease-free water according to the
pellet size. The concentration of RNA was then measured using a
NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington,
DE, USA) at an absorbance of 260 nm and 280 nm.
LncRNAs PCR array
The Human LncRNA PCR Array kit, termed the Human
LncRNA Profiler, which includes 94 lncRNAs associated with numerous
human diseases, was designed and produced by Qiagen China Co., Ltd.
(Shanghai, China). The sample preparation and Human LncRNA PCR
Array kit was prepared according to the manufacturer's
instructions. Purified RNA was reverse transcribed into cDNA with
human LncRNA Profiler cDNA synthesis buffer. qPCR and PCR array
amplification was conducted using an IQ5 machine (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the conditions were set
as follows: 15 sec at 95°C, followed by 40 cycles of 15 sec at
95°C, 30 sec at 65°C and 30 sec at 72°C. Subsequently, a melt curve
analysis was performed to determine the specificity of the PCR
reaction. Relative gene expression was calculated using the
ΔΔCt method (20).
Primers used for qPCR are summarized in Table I.
 | Table IPrimer sequences used for quantitative
polymerase chain reaction. |
Table I
Primer sequences used for quantitative
polymerase chain reaction.
| LncRNAs | Primer sequence |
|---|
| Lnc-7SK | F:
5′-AAACAAGCTCTCAAGGTC-3′ |
| R:
5′-CCTCATTTGGATGTGTCT-3′ |
| Lnc-SRA1 | F:
5′-TTACAGAGATTAGAACCACATT-3′ |
| R:
5′-GGCAAGTCAGAGTTACAAT-3′ |
| Lnc-IGF2-AS | F:
5′-CGCCACTGTGTTACCATT-3′ |
| R:
5′-TTGCCCATCCCAGATAGAA-3′ |
| LncRNA-SChLAP1 | F:
5′-ACACAAGGTCCACCTTCCAG-3′ |
| R:
5′-GGTGGTATCTGCATCCCCAG-3′ |
Reverse transcription-qPCR
Reverse transcription of RNA was performed according
to the manufacturer's instructions of the Reverse Transcription
System (Promega Corporation, Madison, WI, USA). The primers were
designed using the NCBI primer blast tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
The PCR system contained 2 µl of diluted cDNA, 12.5
µl of Fast SYBR Green Master mix (Invitrogen Life
Technologies) and 10 µM of each gene specific primer in a
total volume of 25 µl. qPCR reactions were performed in
triplicate in 96-well plates under the following conditions: 15 sec
at 95°C, followed by 40 cycles of 15 sec at 95°C, 30 sec at 65°C
and 30 sec at 72°C.
siRNA transfection
Monolayers of Huh7 cells with 70% confluence in
6-well plates were transfected with siRNA lncRNA-7SK, siRNA
lncRNA-SRA1, siRNA lncRNA-IGF2-AS and siRNA lncRNA-SChLAP1 or with
the siRNA negative control. Transfection was performed using
Lipofectamine™ 2000 (Invitrogen Life Technologies) according to the
manufacturer's instructions. In brief, Huh7 cells (in serum free
DMEM) were transfected using different quantities of Lipofectamine™
2000 according to the quantity of siRNA. At 8 h post infection, the
cells were harvested to measure the viral gene mRNA level by qPCR
or PI4P-kinase level by western blot analysis. Four pairs of siRNA
sequences were designed and synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The negative control sequence was a
missense sequence. The sequences of the four siRNAs are provided in
Table II.
 | Table IIsiRNA sequences of the four
lncRNAs. |
Table II
siRNA sequences of the four
lncRNAs.
| LncRNAs | siRNA sequences |
|---|
| LncRNA-7SK |
5′-GCTCTCAAGGTCCATTTGTAGGAGA-3′ |
| LncRNA-SRA1 |
5′-CCACACAAGGAAGCAGGTATGTGAT-3′ |
| LncRNA-IGF2-AS |
5′-CCTCTGGTTATTACCTCAATTCTGA-3′ |
| LncRNA-SChLAP1 |
5′-CACCATGTACCTGGAAGCAACATCT-3′ |
Western blot analysis
Cells were collected by trypsinization. Total
protein was extracted using RIPA plus phenylmethyl-sulfonyl
fluoride according to the manufacturer's instructions (Beyotime
Institute of Biotechnology, Shanghai, China). The protein samples
were diluted in 5X loading buffer, boiled for 10 min, subjected to
a 10% polyacrylamide gel (Sangon Biotech Co., Ltd., Shanghai,
China) and then underwent electrophoresis. The proteins were
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Following transfer, the membranes were blocked
with 5% (w/v) non-fat dry milk in Tris-buffered saline and Tween 20
(TBST; 10 mM Tris-HCl, pH 8.0, 150 mM NaCl containing 0.1% v/v
Tween 20) for at least 1 h and then the membranes were incubated
overnight at 4°C with the following monoclonal antibodies from
Abcam (Cambridge, MA, USA) in blocking buffer: Mouse monoclonal
STAT3 (ab119352; 1:5,000), mouse monoclonal HCV-E2 (ab20852; 1:100)
and rabbit monoclonal PI4K-β (ab109418; 1:1,000). Following being
washed three times with TBST, the membranes were incubated with the
following secondary antibodies (CST) from Abcam: Goat
anti-mouse(ab131368; 1:5,000), goat anti-rabbit (ab7085; 1:1,000)
for 30 min and were revealed using an ECL PLUS detection kit
(Biomiga, Inc., San Diego, CA, USA). β-actin was obtained from
Sigma-Aldrich.
Results
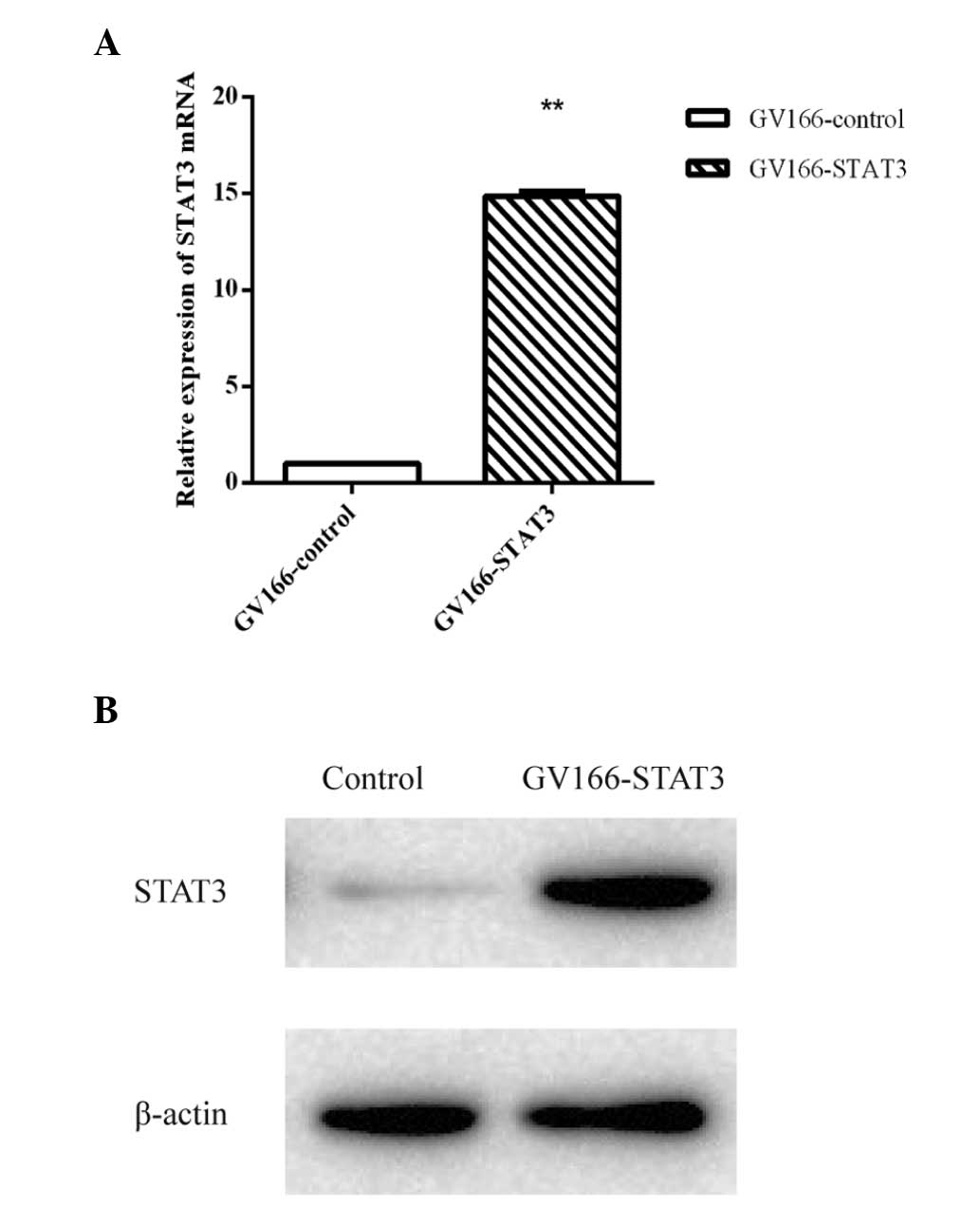
Overexpression of STAT3
In order to identify the lncRNAs regulated by STAT3,
a stable transfection cell line of STAT3 was established. qPCR
demonstrated that the quantity of STAT3 mRNA in the cells
transfected with GV166-STAT3 was significantly higher than that in
the GV166-control transfected cells. The western blot assay of
STAT3 demonstrated that the concentration of STAT3 in the
GV166-STAT3 transfected cells was higher than that of the control
cells (Fig. 1).
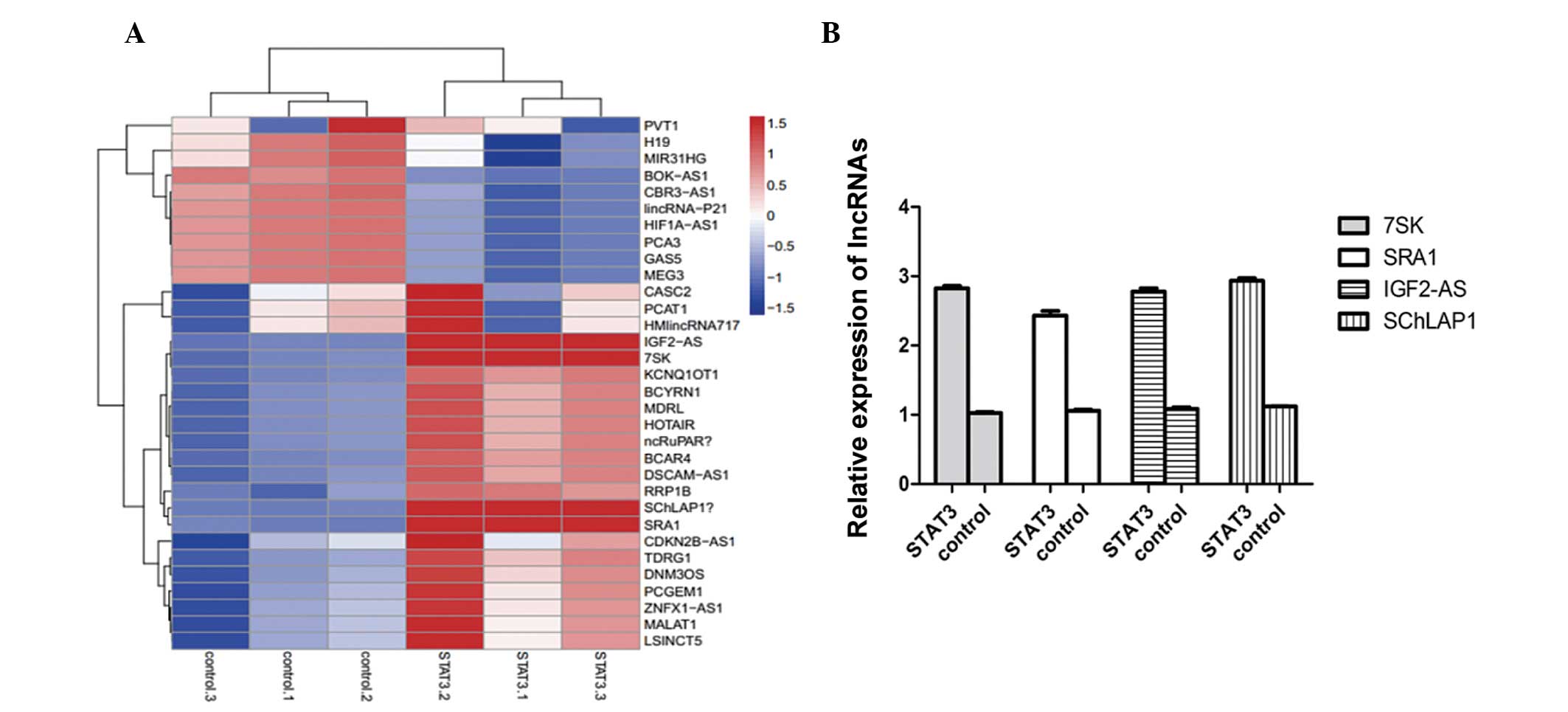
STAT3 upregulates four lncRNAs
Following identification of the stable transfection
of STAT3, lncRNA PCR array method was used to detect the different
expression levels of lncRNAs in STAT3 overexpressing and control
cells. As shown in Fig. 2A,
lnc-IGF2-AS, lnc-7SK, lnc-SChLAP1 and lnc-SRA1 were upregulated in
STAT3 overexpressing cells compared with the control cells. To
further certify the preliminary result, qPCR was performed. The
histogram in Fig. 2B confirmed
that STAT3 is able to increase these four lncRNAs.
LncRNA-7SK and lncRNA-IGF2-AS siRNA
inhibits HCV replication
As these four lncRNAs can be upregulated by STAT3,
the present study aimed to determine whether these four lncRNAs
have a direct association with HCV replication. To verify the
involvement of these four lncRNAs in HCV replication, the Huh7
cells were transfected with siRNA lncRNA-IGF2-AS, siRNA lncRNA-7SK,
siRNA lncRNA-SChLAP1 and siRNA lncRNA-SRA1, and the expression of
all these four lncRNAs were analyzed at the mRNA level. The qPCR
data shown include at least three independent experiments. As shown
in Fig. 3A, the relative
expression of the four lncRNAs in Huh7 cells transfected with siRNA
lncRNA-IGF2-AS, siRNA lncRNA-7SK, siRNA lncRNA-SChLAP1 and siRNA
lncRNA-SRA1, respectively, were significantly lower than cells
transfected with the control siRNA (P<0.005).
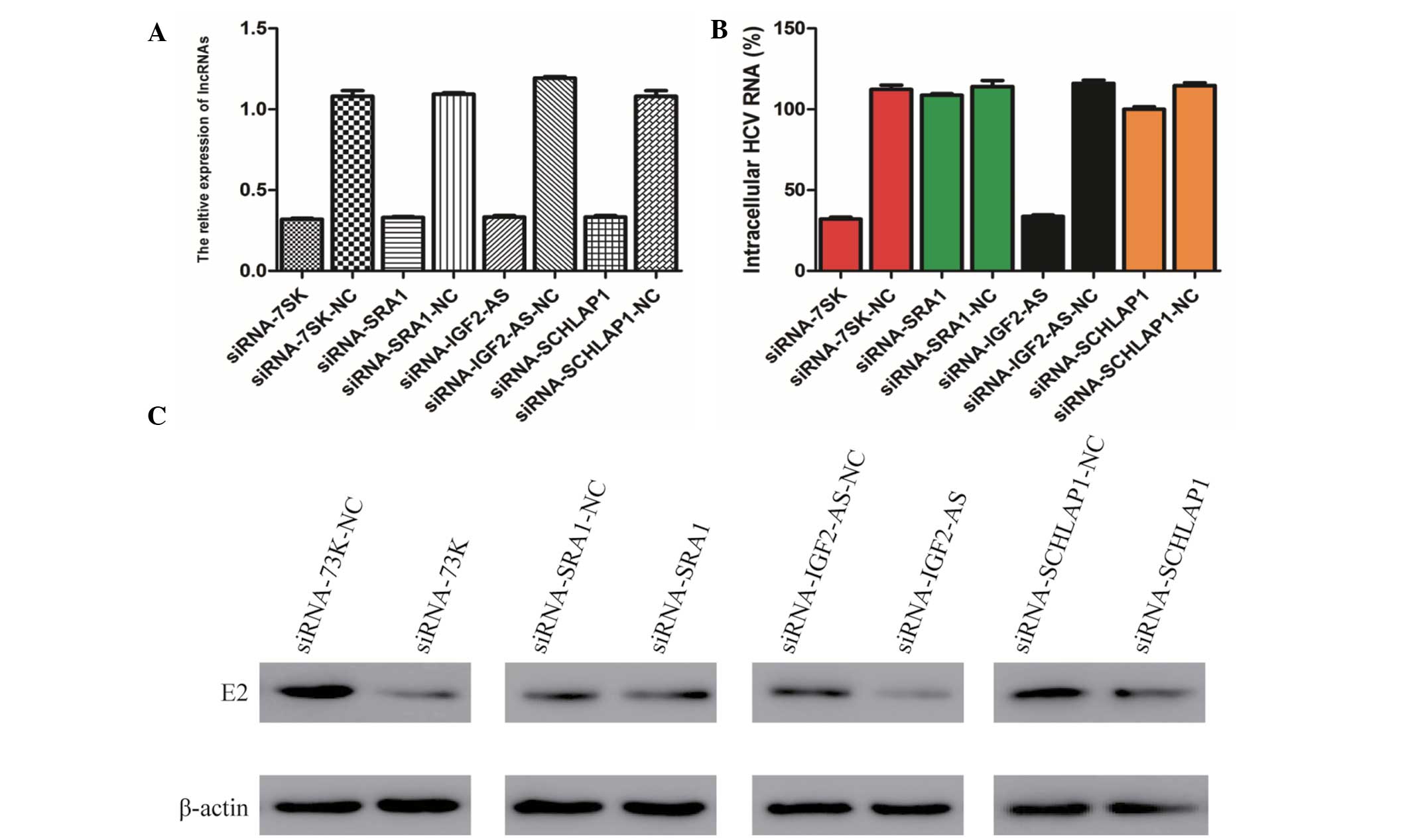 | Figure 3Knockdown of lnc-7SK and lnc-IGF2-AS
by siRNA inhibit HCV replication. (A) Silencing efficiency of
lnc-7SK, lnc-SRA1, lnc-IGF2-AS and lnc-SChLAP1 were verified
through quantitative polymerase chain reaction. (B) Effects of
lnc-7SK, lnc-SRA1, lnc-IGF2-AS and lnc-SChLAP1 knockdown on the
mRNA level of the envelope glycoprotein E2. The results are
presented as the mean ± standard deviation from three independent
experiments performed in triplicate. Relative expression is
presented in arbitrary units. (C) Effects of lnc-7SK, lnc-SRA1,
lnc-IGF2-AS and lnc-SChLAP1 knockdown on the expression level of E2
were verified through western blot analysis. β-actin was used as an
internal control. IGF, insulin-like growth factor; SRA1, steroid
receptor RNA activator 1; siRNA, small interfering RNA; HCV,
hepatitis C virus; lncRNAs, long non-coding RNAs; NC, negative
control. |
Subsequently, the siRNA-transfected Huh7 cells were
infected with the HCV JFH-1 strain and the cell lysates were
analyzed by qPCR and western blot analysis to measure the
expression level of the envelope glycoprotein E2. Notably, it was
observed that only following transfection of siRNA lncRNA-7SK and
siRNA lncRNA-IGF2-AS, the mRNA level of E2 was reduced by 71.5 and
70.9%, respectively, compared with the negative control. While the
transfection of siRNA lncRNA-SAR1 and siRNA lncRNA-SChLAP1 had no
pronounced effect on the E2 mRNA level (Fig. 3B). Subsequently, the western blot
assay also demonstrated that knockdown of lncRNA-7SK and
lncRNA-IGF2-AS affects the expression of envelope glycoprotein E2
(Fig. 3C). These results suggest
that lncRNA-7SK and lncRNA-IGF2-AS rather than lncRNA-SChLAP1 and
lncRNA-SRA1 are associated with the replication of HCV.
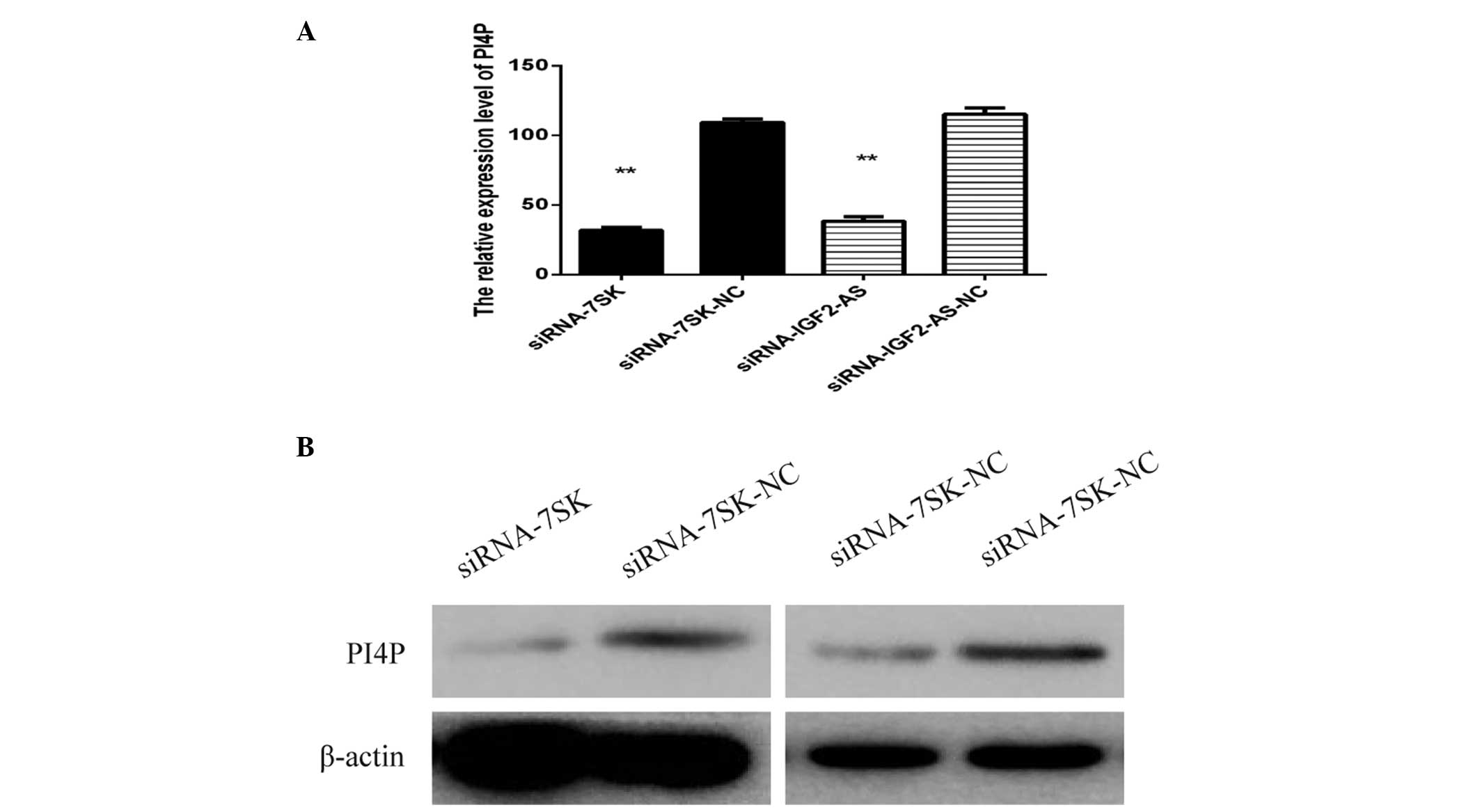
LncRNA-7SK and lncRNA-IGF2-AS siRNA
inhibit HCV replication by reducing the expression of PI4P
In order to investigate the potential mechanism by
which siRNA lncRNA-7SK and siRNA lncRNA-IGF2-AS inhibited HCV
replication, the expression level of PI4P-kinase, which has been
verified to be involved in HCV replication, was measured by western
blot analysis. Huh7 cell lysates were obtained following
transfection with siRNA lncRNA-IGF2-AS, siRNA lncRNA-7SK as well as
infected JFH-1. As expected, the expression of PI4P-kinase was
reduced in the cells transfected with siRNA lncRNA-7SK and siRNA
lncRNA-IGF2-AS as compared with the negative control. The results
were verified through qPCR and western blot analysis (Fig. 4).
Discussion
In the present study, two novel findings were
reported. Overexpression of STAT3 upregulated lnc-IGF2-AS, lnc-7SK,
lnc-SChLAP1 and lnc-SRA1 in Huh7 cells and among these four types
of lncRNAs, only lnc-IGF2-AS and lnc-7SK regulated HCV replication
through affecting the expression of PI4P. A previous study
demonstrated that genetic or pharmacological activation of STAT3 in
glioma cells enhanced oncolytic herpes simplex virus replication
and cytotoxicity (21). In
addition, McCartney et al also verified that HCV replication
increases STAT3 activation and in turn activation of STAT3
increases HCV replication by modulating microtubule dynamics
(22). Activation or
overexpression of STAT3 is relevant for various types of human
cancer, including Epstein-Barr virus (EBV)-associated cancer. The
expression of STAT3 rapidly increases upon EBV infection, which
mediates relaxation of the intra-S phase cell-cycle checkpoint;
this facilitates viral oncogene-driven cell proliferation (23). HCV core is capable of upregulating
the expression of NANOG, which is a member of the homeobox family
of DNA binding transcription factors and was identified as a
pluripotency promoting gene. In addition, this core-induced NANOG
expression enhanced cell growth and cell cycle progression through
increased expression of phosphorylated STAT3 protein (24).
LncRNAs are RNAs >200 bp in length. Previous
findings have revealed that lncRNAs are involved in several steps
of cancer development (25,26).
These lncRNAs interact with DNA, RNA and proteins, acting as an
essential regulator of cell differentiation and organogenesis.
Their misexpression may affect epigenetic information and may
result in the progressive and uncontrolled growth of a tumor
(11,27). Lnc-DC, which was exclusively
expressed in human conventional DCs was involved in DC
differentiation and stimulated T-cell activation through
interacting with STAT3 (28). The
lncRNA-HULC, highly upregulated in liver cancer, is markedly
upregulated in HCC, which functions through suppressing the tumor
suppressor gene p18 (29).
Prensner et al initially identified lnc-SChLAP1 and found
that it contributes to the development of lethal prostate cancer at
least in part by antagonizing the tumor-suppressive functions of
the SWI/SNF chromatin-modifying complex (30). Castelo-Branco et al
indicated that the snRNA 7SK, a single non-coding RNA, is a
gatekeeper of transcriptional termination and bidirectional
transcription in embryonic stem cells and mediates transcriptional
poising through a mechanism independent of chromatin bivalency
(31).
In the present study, a stable expression cell line
of STAT3 was constructed. Subsequently, a human lncRNA PCR array
kit was used. Human LncRNA Profiler was used to compare the LncRNA
expression profile differences between Huh7 cells and control
cells. It was found that four lncRNAs, including lnc-7SK, lnc-SRAI,
lnc-IGF2-AS and lnc-SChLAP1, can be upregulated by STAT3. Among the
four lncRNAs, only lnc-7SK and lnc-IGF2-AS are associated with the
replication of HCV as the transfection of siRNA lnc-7SK and siRNA
lnc-IGF2-AS reduced the expression level of mRNA coding the
envelope glycoprotein E2. The expression level of PI4P was then
measured in cells following transfection with siRNA lnc-7SK and
siRNA lnc-IGF2-AS. Compared with cells transfected with the
siRNA-control, the expression level of PI4P in cells transfected
with siRNA lnc-7SK and siRNA lnc-IGF2-AS were highly reduced.
In conclusion, these findings increase our
understanding of the molecular mechanisms of HCV replication and
the effect of STAT3 on HCV replication. Furthermore, a preliminary
study was performed regarding the mechanism underlying the function
of lnc-7SK and lnc-IGF2-AS. Future studies are required to
determine whether lnc-7SK and lnc-IGF2-AS are potential diagnostic
or even therapeutic targets for HCV infection.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant nos. 30872230 and 81171643).
References
|
1
|
Lavanchy D: Evolving epidemiology of
hepatitis C virus. Clin Microbiol Infect. 17:107–115. 2011.
View Article : Google Scholar
|
|
2
|
Kim CW and Chang KM: Hepatitis C virus:
Virology and life cycle. Clin Mol Hepatol. 19:17–25. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi QR and Yang ZM: Regulation and function
of signal transducer and activator of transcription 3. World J Biol
Chem. 5:231–239. 2014.PubMed/NCBI
|
|
4
|
Nguyen AV, Wu YY and Lin EY: STAT3 and
sphin-gosine-1-phosphate in inflammation-associated colorectal
cancer. World J Gastroenterol. 20:10279–10287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prabhavathy D, Prabhakar BN and
Karunagaran D: HPV16 E2-mediated potentiation of NF-κB activation
induced by TNF-α involves parallel activation of STAT3 with a
reduction in E2-induced apoptosis. Mol Cell Biochem. 394:77–90.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tacke RS, Tosello-Trampont A, Nguyen V,
Mullins DW and Hahn YS: Extracellular hepatitis C virus core
protein activates STAT3 in human monocytes/macrophages/dendritic
cells via an IL-6 autocrine pathway. J Biol Chem. 286:10847–10855.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kambara H, Niazi F, Kostadinova L, Moonka
DK, Siegel CT, Post AB, Carnero E, Barriocanal M, Fortes P, Anthony
DD and Valadkhan S: Negative regulation of the interferon response
by an interferon-induced long non-coding RNA. Nucleic Acids Res.
42:10668–10680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Chen CY, Yedavalli VS and Jeang
KT: NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1
post-transcriptional expression. MBio. 4:e00596–e00512. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou W and Bonkovsky HL: Non-coding RNAs in
hepatitis C-induced hepatocellular carcinoma: Dysregulation and
implications for early detection, diagnosis and therapy. World J
Gastroenterol. 19:7836–7845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Pu R, Du Y, Han Y, Su T, Wang H
and Cao G: Non-coding RNAs in hepatitis B or C-associated
hepatocellular carcinoma: Potential diagnostic and prognostic
markers and therapeutic targets. Cancer Lett. 321:1–12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu ZQ, Li RJ, Mei JZ and Li XH:
Downregulation of long non-coding RNA GAS5 is associated with the
prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol.
7:4303–4309. 2014.
|
|
13
|
Bishè B, Syed G and Siddiqui A:
Phosphoinositides in the hepatitis C virus life cycle. Viruses.
4:2340–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bishé B, Syed GH, Field SJ and Siddiqui A:
Role of phosphatidylinositol 4-phosphate (PI4P) and its binding
protein GOLPH3 in hepatitis C virus secretion. J Biol Chem.
287:27637–27647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berger KL, Cooper JD, Heaton NS, Yoon R,
Oakland TE, Jordan TX, Mateu G, Grakoui A and Randall G: Roles for
endocytic trafficking and phosphatidylinositol 4-kinase III alpha
in hepatitis C virus replication. Proc Natl Acad Sci USA.
106:7577–7582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berger KL, Kelly SM, Jordan TX, Tartell MA
and Randall G: Hepatitis C virus stimulates the
phosphatidylinositol 4-kinase III alpha-dependent
phosphatidylinositol 4-phosphate production that is essential for
its replication. J Virol. 85:8870–8883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Perry JW, Lauring AS, Neddermann
P, De Francesco R and Tai AW: Oxysterol-binding protein is a
phosphatidylinositol 4-kinase effector required for HCV replication
membrane integrity and cholesterol trafficking. Gastroenterology.
146:1373–1385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blight KJ, McKeating JA and Rice CM:
Highly permissive cell lines for subgenomic and genomic hepatitis C
virus RNA replication. J Virol. 76:13001–13014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakita T, Pietschmann T, Kato T, Date T,
Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami
M, et al: Production of infectious hepatitis C virus in tissue
culture from a cloned viral genome. Nat Med. 11:791–796. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Zhang S, Chen J, Jiang K, Zhang Q,
Guo K and Liu Y: The transcriptional profiling of glycogenes
associated with hepatocellular carcinoma metastasis. PLoS One.
9:e1079412014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okemoto K, Wagner B, Meisen H, Haseley A,
Kaur B and Chiocca EA: STAT3 activation promotes oncolytic HSV1
replication in glioma cells. PLoS One. 8:e719322013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCartney EM, Helbig KJ, Narayana SK, Eyre
NS, Aloia AL and Beard MR: Signal transducer and activator of
transcription 3 is a proviral host factor for hepatitis C virus.
Hepatology. 58:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koganti S, Hui-Yuen J, McAllister S,
Gardner B, Grasser F, Palendira U, Tangye SG, Freeman AF and
Bhaduri-McIntosh S: STAT3 interrupts ATR-Ch k1 signaling to allow
oncovirus-mediated cell proliferation. Proc Natl Acad Sci USA.
111:4946–4951. 2014. View Article : Google Scholar
|
|
24
|
Zhou JJ, Chen RF, Deng XG, Zhou Y, Ye X,
Yu M, Tang J, He XY, Cheng D, Zeng B, et al: Hepatitis C virus core
protein regulates NANOG expression via the stat3 pathway. FEBS
Lett. 588:566–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar
|
|
28
|
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S,
Jiang Z, Xu J, Liu Q and Cao X: The STAT3-binding long noncoding
RNA lnc-DC controls human dendritic cell differentiation. Science.
344:310–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du Y, Kong G, You X, Zhang S, Zhang T, Gao
Y, Ye L and Zhang X: Elevation of highly upregulated in liver
cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell
proliferation via downregulating p18. J Biol Chem. 287:26302–26311.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castelo-Branco G, Amaral PP, Engström PG,
Robson SC, Marques SC, Bertone P and Kouzarides T: The non-coding
snRNA 7SK controls transcriptional termination, poising and
bidirectionality in embryonic stem cells. Genome Biol. 14:R982013.
View Article : Google Scholar
|