Introduction
Chronic heart failure (CHF) is the final stage of
various heart diseases, and is the primary cause of mortality in
elderly individuals worldwide (1).
CHF affects approximately four million individuals in China, and an
increasing number of new cases are diagnosed annually (2). CHF is recognized as a major and
increasing health problem in China. However, there is a shortage of
studies regarding the underlying pathogenesis, diagnosis and
treatment of CHF. Numerous factors, including chronic myocardial
lesions, hypertension and atherosclerotic disease, are important in
CHF (3).
B-type-natriuretic peptide (BNP) is a cardiac
hormone secreted from the atrial and ventricular myocardium,
following ventricular overloading or volume expansion. At present,
the association between BNP and the risk of CHF is
well-established, and BNP is the most established biomarker of CHF
(4,5). The measurement of BNP expression
levels is used clinically as a diagnosis for CHF, as BNP levels are
elevated in the plasma of patients with CHF (6,7). BNP
has also been investigated as a potential therapeutic target of CHF
(8). Although extensive studies on
BNP have been conducted, the detailed molecular mechanism
underlying the involvement of BNP in CHF is only partially
understood.
Apoptosis is the process of programmed cell death
often characterized by cell shrinkage, nuclear fragmentation, and
chromosomal DNA fragmentation (9).
Cell apoptosis has been identified to be important in numerous
human diseases, including cancer (10), Alzheimer's disease (11) and tuberculosis (12). Previous studies have demonstrated
that apoptosis of human myocardial cells has an important role in
the pathogenesis of CHF (13,14).
It is evident that myocardial apoptosis results from numerous
factors, including hyperlipidemia, oxidized lipoproteins,
myocardial ischemic-reperfusion injury, hypoxia, and other heart
injury-inducing factors (15–18).
However, the detailed molecular mechanisms underlying the effect of
myocardial apoptosis on CHF remain to be elucidated. Deng et
al (19) reported that BNP
affects myocardial cell apoptosis during myocardial
ischemia-reperfusion injury. However, whether BNP is also
associated with myocardial cell apoptosis in CHF remains
unclear.
Protein-coding genes only constitute a small portion
of the human genome, and the majority of transcripts are non-coding
RNA (ncRNAs) (20). ncRNAs include
small ncRNAs and long ncRNAs (lncRNAs). Although small ncRNAs, such
as microRNAs, small interfering (si)RNAs and piwi-interacting RNAs
have been well-studied, lncRNAs are less well-characterized.
However, an increasing number of studies have reported that lncRNAs
have important roles in cancer progression and metastasis, as well
as cellular processes, such as cell proliferation and apoptosis
(21,22). Therefore, identifying the
association between lncRNAs regulated by BNPs and myocardial
apoptosis may aid in understand the function of BNP in the
pathogenesis of CHF.
The present study aimed to demonstrate how increased
BNP may induce myocardial cell apoptosis. Human lncRNA polymerase
chain reaction (PCR) arrays were used to compare the lncRNA
expression profiles between BNP-treated cells and control cells.
Finally, the mechanism underlying the regulation of myocardial cell
apoptosis in vitro by lncRNA LSINCT5 was investigated.
Materials and methods
Reagents
The following mouse monoclonal antibodies were
purchased from Abcam (Cambridge, MA, USA): Anti-caspase-1 (cat. no.
ab17815), anti-caspase-3 (cat. no. ab158030), anti-caspase-7 (cat.
no. ab1580933), anti-caspase-8 (cat. no. ab39731) and
anti-interleukin (IL)-1β (cat. no. ab2105). Rabbit anti-mouse
immunoglobulin G (IgG) polyclonal horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. no. ZB-2305) and mouse
anti-human GAPDH monoclonal antibodies (cat. no. TA-08) were
purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.
(Beijing, China). BNP was purchased from Sigma-Aldrich (St. Louis,
MO, USA). All others chemical reagents were purchased from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Cell culture
HCM human myocardial cells were purchased from
Sciencell Research Laboratories (Carlsbad, CA, USA). The HCM cells
were cultured in Dulbecco's modified Eagle's medium (Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Gibco Life Technologies), 100 U/ml penicillin and 100
µg/ml streptomycin (both HyClone, GE Healthcare Life
Sciences, Logan, UT, USA), and incubated at 37°C in an atmosphere
containing 5% CO2.
Determination of apoptosis levels
The levels of apoptosis were determined by flow
cytometry using an Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (Beyotime Institute of Biotechnology,
Haimen, China), according to the manufacturer's instructions. The
cells were added to various concentrations of BNP (50, 100, 150 and
200 pg/ml) in the treated groups, and untreated groups served as
controls. After 48 h, the cells were harvested and washed twice
with phosphate-buffered saline (PBS). The cells were then stained
with 1 ml Annexin V binding buffer containing 10 µl
propidium iodide (PI) solution and 5 µl Annexin
V-fluorescein isothiocyanate, for 10 min at room temperature, and
analyzed by flow cytometry (FACScan; BD Biosciences, San Jose, CA,
USA).
Western blot analysis
The cells were harvested and homogenized using cell
lysis buffer (Beyotime Institute of Biotechnology). The homogenates
were then centrifuged for 30 min at 4°C, 10,500 x g, and the
supernatants were collected as protein samples. Protein levels were
measured using a Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology). Equal quantities of protein samples
were separated by 10% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred onto polyvinylidene difluoride
membranes (Beyotime Institute of Biotechnology). The membranes were
subsequently incubated in 5% skimmed milk in Tris-buffered saline
with Tween-20 (Sangon Biotech Co., Ltd., Shanghai, China) blocking
solution at room temperature for 1 h. The membranes were then
washed three times with TBST (20 mM Tris-HCl, pH 7.6; 137 mM NaCl;
0.1% Tween-20). The membranes were incubated with agitation at 4°C
overnight with the following specific mouse anti-human monoclonal
primary antibodies: Anti-caspase-1 (1:1,000), anti-caspase-3
(1:1,000), anti-caspase-7 (1:2,000), anti-caspase-8 (1:1,500),
anti-IL-1β (1:1,000) and anti-GAPDH (1:1,000). The membranes were
washed again, and then incubated with rabbit anti-mouse IgG
polyclonal HRP-conjugated secondary antibodies (1:1,000), at room
temperature for 50 min. Finally, the protein bands were visualized
using an Enhanced Chemiluminescence Western Blotting Detection
system (GE Healthcare Life Sciences, Chalfont, UK).
Human lncRNA PCR array and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and
purified with an RNeasy MinElute Cleanup kit (Qiagen GmbH, Hilden,
Germany). RT-qPCR was subsequently performed using a One Step
SYBR® PrimeScript™ RT-PCR kit II (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer's
instructions.
The Human lncRNA Profiler, which included 94 lncRNAs
associated with numerous human diseases, was designed and produced
by the Shanghai Funeng Biological Technology Co., Ltd. (Shanghai,
China). The sample preparation and lncRNA PCR array were performed
according to the manufacturer's instructions. Purified RNA was
reversed transcribed into cDNA using a Human lncRNA Profiler cDNA
synthesis buffer (Shanghai Funeng Biological Technology Co.,
Ltd).
RT-qPCR and PCR array amplification were conducted
using an IQ5 machine (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), with the following thermocycling conditions: 42°C for 5 min,
95°C for 10 sec, 40 cycles at 95°C for 15 sec, 60°C for 15 sec, and
72°C for 20 sec. PCR was followed by melt curve analysis to
determine the reaction specificity. Relative gene expression levels
were calculated using the ΔΔCt method (23). Primers used in RT-qPCR are shown in
Table I.
 | Table ISequences of the RT-qPCR primers. |
Table I
Sequences of the RT-qPCR primers.
| Primer name | Primer sequence |
|---|
| Caspase-1 | F:
5′-AGGAGGGAATATGTGGG-3′ |
| R:
5′-AACCTTGGGCTTGTCTT-3′ |
| Caspase-3 | F:
5′-ATCCACGAGCAGAGTCAAAG-3′ |
| R:
5′-CCTGGTCAACTGTCACAAAAC-3′ |
| Caspase-7 | F:
5′-CAACGGGACAGACAAAGAT-3′ |
| R:
5′-TCAGGATGGAGCAGAGGG-3′ |
| Caspase-8 | F:
5′-TACTACCGAAACTTGGACC-3′ |
| R:
5′-GTGAAAGTAGGTTGTGGC-3′ |
| IL-1β | F:
5′-CCTTGTCGAGAATGGGCAGT-3′ |
| R:
5′-TTCTGTCGACAATGCTGCCT-3′ |
| GAPDH | F:
5′-GGACCAATACGACCAAATCCG-3′ |
| R:
5′-AGCCACATCGCTCAGACAC-3′ |
| LSINCT5 | F:
5′-TTCGGCAAGCTCCTTTTCTA-3′ |
| R:
5′-GCCCAAGTCCCAAAAAGTTCT-3′ |
Small interfering (si)RNA
In order to investigate the mechanism underlying the
regulation of myocardial cell apoptosis by lncRNA LSINCT5, the HCM
cells were transfected with siRNA targeting lncRNA LSINCT5
(si-LSINCT5) or negative control (si-Scramble) using
Lipofectamine® 2000 transfection reagent (Invitrogen
Life Technologies). Briefly, HCM cells at 50% confluence were
transfected with 100 nM of either siRNAs targeting LSINCT5 or
scrambled negative controls using Lipofectamine® 2000,
according to the manufacturer's instructions. siRNAs were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences
of si-LSNCT5 were as follows: si-LSNCT5-1,
5′-TACAGCACTTTAGTGGCAGAGATTT-3′; si-LSNCT5-2, 5′-
GACAACTTACTTAACCTCATGATCA-3′; and si-LANCT5-3,
5′-CAATACAGTCAGCACTGAATCTTCT-3′.
Immunofluorescence
The cells were washed three times with cold PBS, and
fixed for 10 min with 4% paraformaldehyde dissolved in PBS at room
temperature. The fixed cells were blocked for 1 h in 5% skimmed
milk dissolved in PBS. The cells were then incubated with
anti-GAPDH antibody overnight at 4°C in blocking solution, and
washed three times with PBS with Tween-20. Following washing, the
cells were incubated with goat anti-mouse IgG monoclonal antibody
conjugated to FITC dye (cat no. KC-MM-095; 1:50; Kangchen,
Shanghai, China)for 1 h at room temperature. Finally, the cells
were stained with 10 µg/ml DAPI (Beyotime Institute of
Biotechnology) for 10 min. Images were observed and captured using
a Nikon fluorescence microscope (TE2000-U; Nikon Corporation,
Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was determined using Student's t-test
(two-tailed) and one-way analysis of variance using SPSS version
20.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicated a statistically significant difference.
Results
Increased BNP induces human myocardial
cell apoptosis
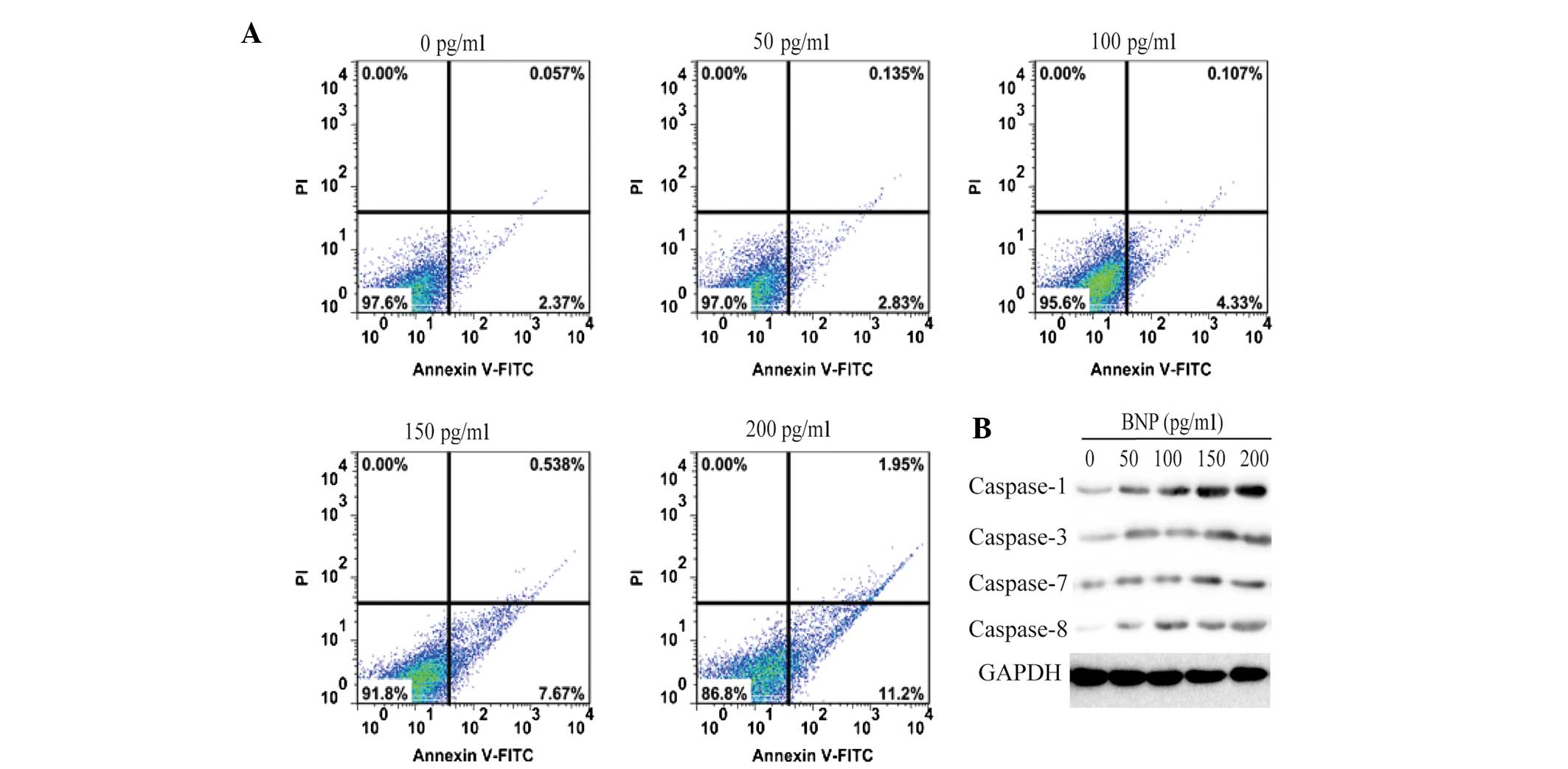
HCM cell apoptosis was measured by an Annexin-V
FITC/PI double staining assay. As shown in Fig. 1A, the BNP-induced increase in HCM
cell apoptosis levels occurred in a dose-dependent manner. In the
BNP-untreated group, the percentage of apoptotic cells was ~2.4%.
However, following cell treatment with various concentrations of
BNP for 24 h, the percentage of apoptotic cells increased to 3.0,
4.4, 8.1 and 13.1%, respectively. These results suggest that BNP
significantly promotes the apoptosis of HCM cells.
Caspase-1 is involved in BNP-induced
apoptosis of HCM cells
To determine the mechanism underlying BNP-induced
apoptosis, the expression levels of caspase-1, -3, -7 and -8 were
analyzed in 200 pg/ml BNP-treated HCM cells. As shown in Fig. 1B, only the expression levels of
caspase-1 were upregulated by BNP in a dose-dependent manner. No
marked changes were identified in the expression levels of the
other caspases in BNP-stimulated HCM cells. These data provide
evidence that BNP-induced apoptosis is associated with the
caspase-1 signaling pathway.
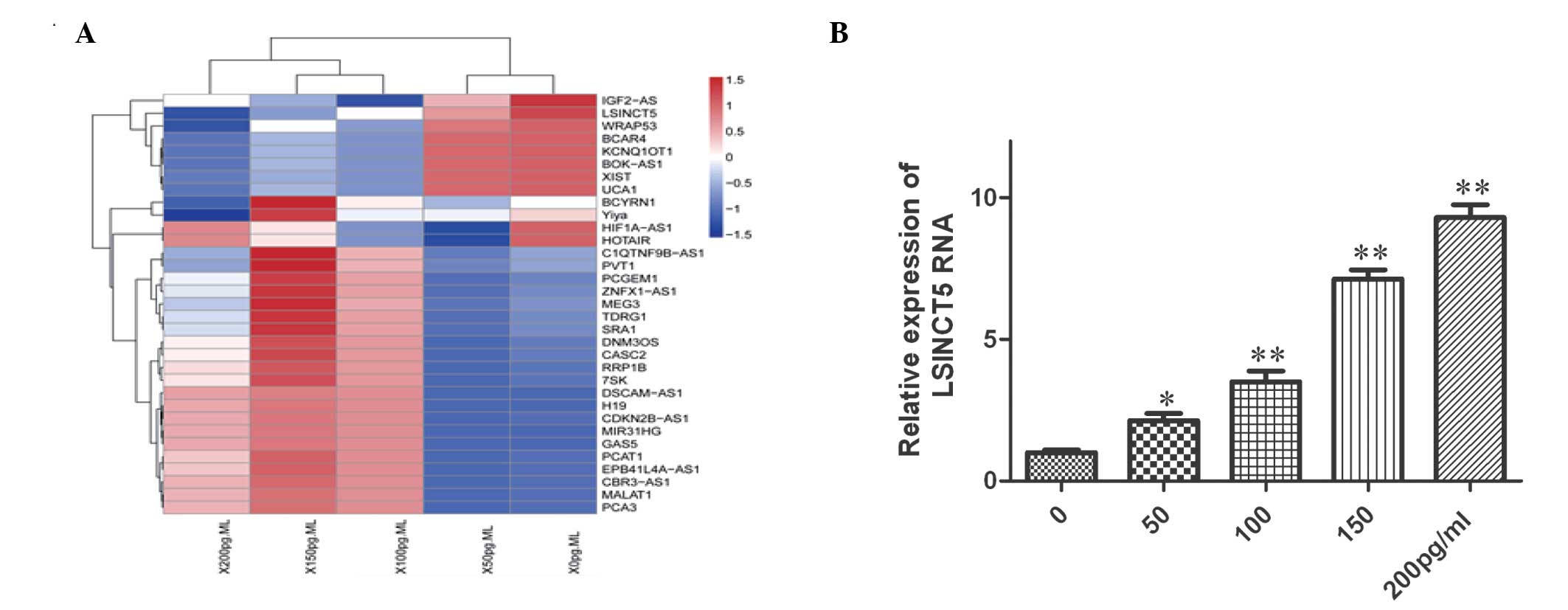
lncRNA expression profiles of BNP-treated
HCM cells
To further investigate the molecular mechanism
underlying BNP-induced apoptosis, a human lncRNA PCR array was used
to compare the lncRNA expression profiles between BNP-treated and
untreated cells. A heat map of differential lncRNA expression was
generated using a method of hierarchical clustering by GeneSpring
GX software 7.3 (Agilent Technologies, Inc., Santa Clara, CA, USA).
As shown in Fig. 2A, a total of 33
lncRNAs were found to be differentially expressed between the two
groups. Among them, eight and 25 lncRNA probes were upregulated and
downregulated, respectively, in BNP-treated cells. lncRNA LSINCT5
exhibited the greatest change in expression in BNP-treated
cells.
RT-qPCR was used to further investigate the
upregulation of LSINCT5 in BNP-treated cells. As shown in Fig. 2B, compared with untreated cells,
the expression levels of LSINCT5 were significantly increased
(~10-fold) in a dose-dependent manner in BNP-treated cells.
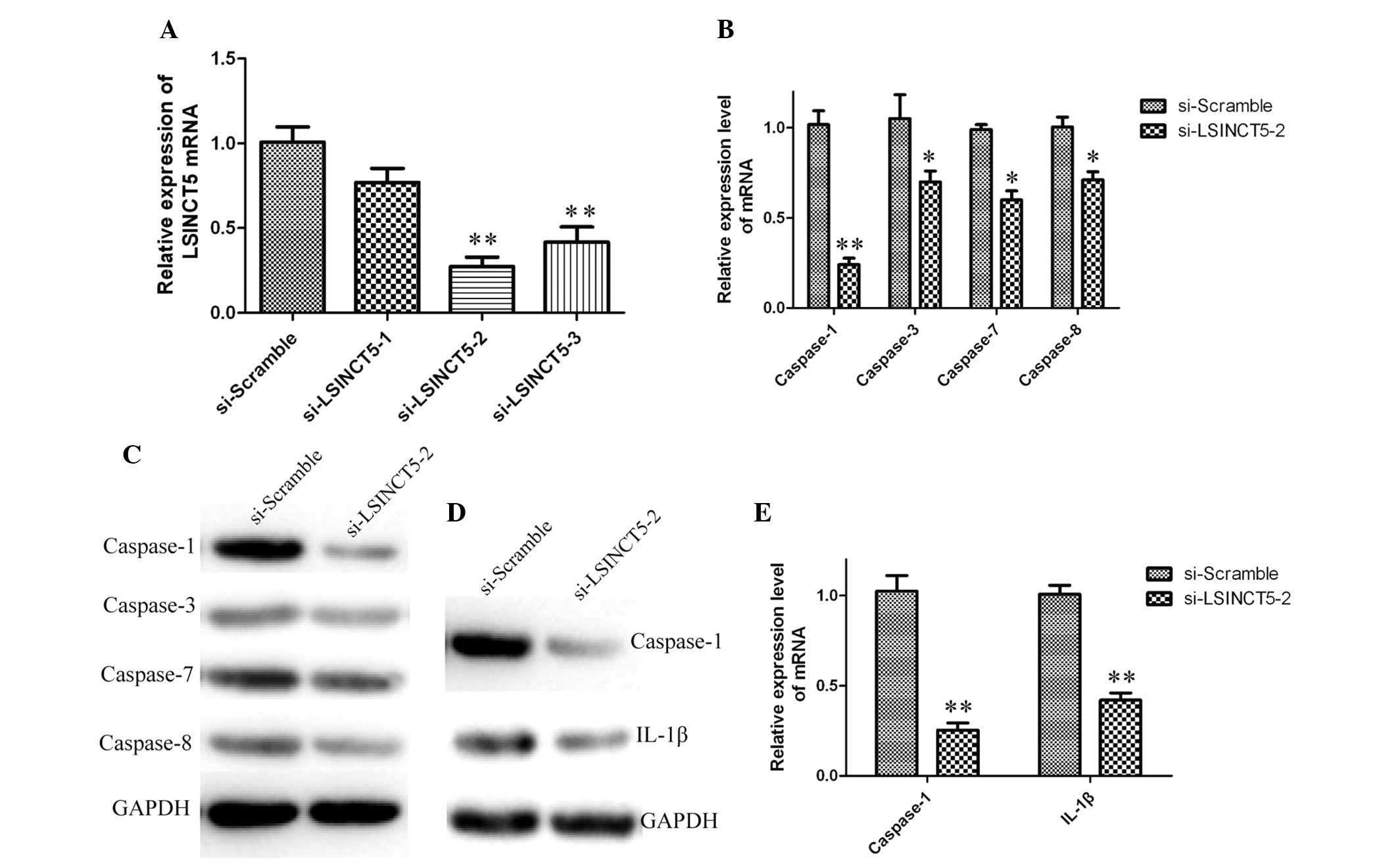
LSINCT5 silencing inhibits
caspase-1/IL-1β signaling in BNP-treated HCM cells
To investigate the role of lncRNA LSINCT5 in the
apoptosis of HCM cells, LSINCT5 was silenced in BNP-treated cells
using siRNA. Cells transfected with si-scramble were used as
negative controls. As shown in Fig.
3A, the expression levels of LSINCT5 in siRNA-transfected cells
decreased by 0.8, 0.3 and 0.4-fold, respectively, as compared with
the scramble group. These results indicate that LSINCT5 was
efficiently silenced in HCM cells by siRNA-LSINCT5-2. Therefore,
siRNA-LSINCT5-2 was used for all subsequent LSINCT5 silencing
experiments.
The expression levels of caspase-1, -3, -7 and -8 in
siRNA-LSINCT5-2-transfected cells were analyzed using RT-qPCR and
western blotting. Compared with cells treated with si-scramble, the
expression levels of caspase-1 were significantly downregulated in
siRNA-LSINCT5-2-treated groups; however, the expression levels of
the other caspases were not significantly altered (Fig. 3B and C).
In order to investigate the role of LSINCT5 in the
caspase-1/IL-1β signaling pathway, the expression levels of
caspase-1 and IL-1β were quantified in HCM cells containing
silenced LSINCT5. As shown in Fig. 3D
and E, caspase-1 and IL-1β were down-regulated in LSINCT5
knockdown cells. The data suggest that LSINCT5 silencing inhibits
caspase-1/IL-1β signaling in BNP-treated HCM cells.
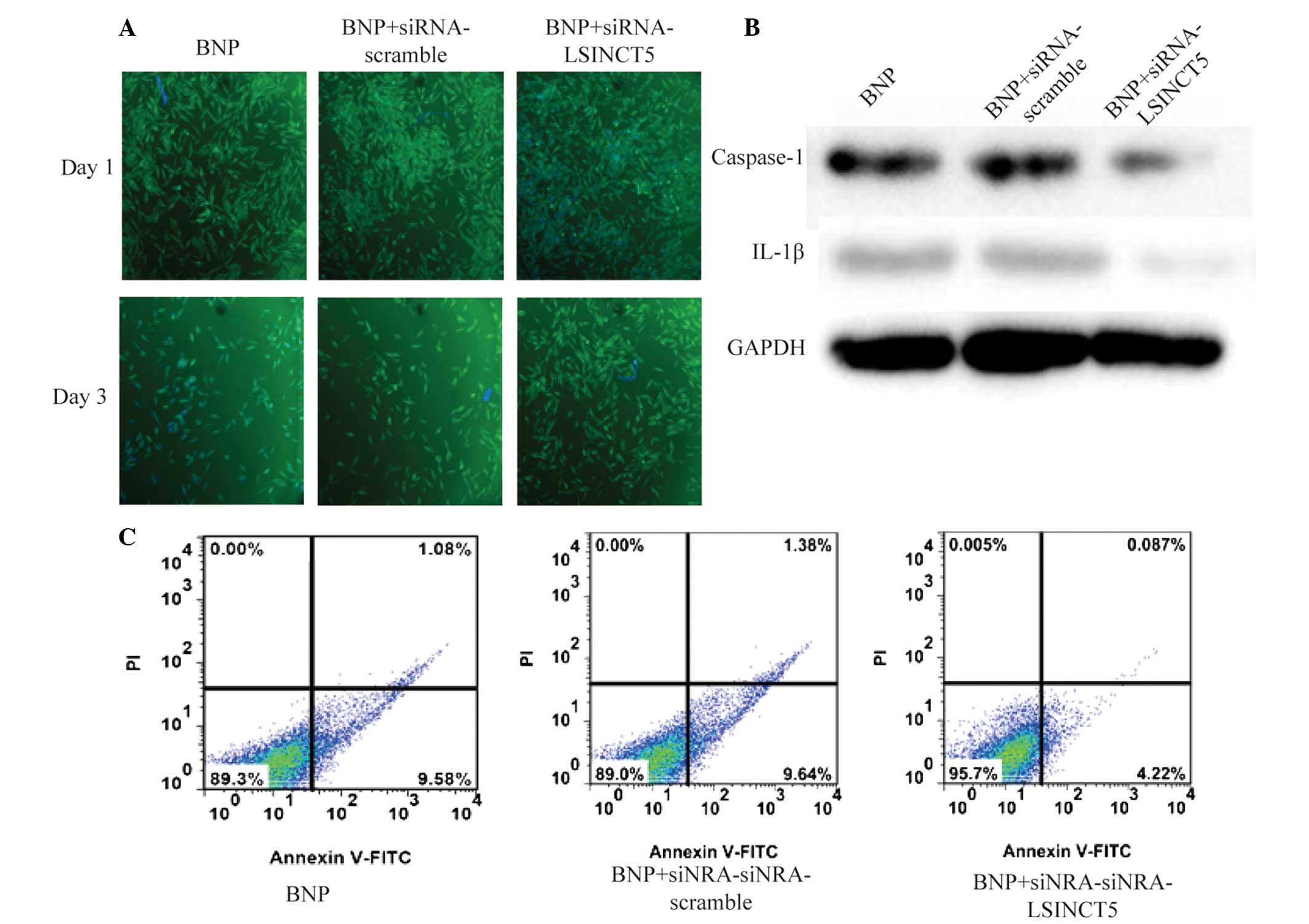
Knockdown of LSINCT5 prevents apoptosis
in BNP-treated HCM cells
The expression levels of LSINCT5 were increased in
BNP-treated HCM cells. Therefore, LSINCT5 may be important in HCM
apoptosis. To investigate this hypothesis, siRNA knockdown was
performed in HCM cells.
To facilitate the observation of cell proliferation,
immunocytochemistry was used for visual observation. As shown in
Fig. 4A, cell proliferation was
significantly suppressed in the BNP and BNP +
siRNA-scramble-treated groups following three days treatment.
However, in BNP-treated cells transfected with siRNA-LSINCT5, a
marked increase in cell proliferation was observed, as compared
with the BNP-treated group. HCM cell apoptosis was measured by an
Annexin-V FITC/PI double staining assay. The expression levels of
caspase-1 and IL-1β were quantified in all groups. The expression
of caspase-1 and IL-1β was overexpressed in BNP-treated and BNP +
siRNA-scramble-treated cells. However, these values were markedly
downregulated in cells transfected with siRNA-LSINCT5 (Fig. 4B). As shown in Fig. 4C, the percentage of apoptotic cells
in the BNP and BNP + siRNA-scramble-treated groups was ~10.6 and
11.0%, respectively. However, this value in the BNP +
siRNA-LSINCT5-treated group decreased to 4.3%. These results
suggest that knockdown of LSINCT5 prevents apoptosis in BNP-treated
HCM cells via the inhibition of caspase-1/IL-1β signaling
pathway.
Discussion
To the best of our knowledge, the present study is
the first to report that BNP promotes apoptosis of myocardial cells
through lncRNA LSINCT5 in vitro.
Previous studies have suggested that BNP, a peptide
neurohormone secreted predominantly in the heart, has an important
role in numerous cardiovascular diseases (24,25).
Patients with type 2 diabetes mellitus exhibit elevated levels of
BNP in plasma (26). In acute
coronary syndromes, elevated levels of BNP are a prognostic factor
for adverse outcome (27). It is
well-established that BNP is useful as a biomarker for the
diagnosis of CHF, and even as a therapeutic target in CHF (6,8).
This implies that BNP may have an important role in the development
and progression of CHF.
An increasing number of studies have demonstrated
that myocardial cell apoptosis is involved in the pathogenesis of
CHF (28,29). Hirota et al (30) reported the presence of considerable
myocardial cell apoptosis in rats, which was induced by the absence
of membrane protein gp130, resulting in the rapid development of
CHF. Song et al (31)
showed that inhibition of myocardial cell apoptosis may prevent CHF
progression in the rat model. Although numerous studies on
myocardial cell apoptosis have been conducted, the molecular
mechanisms underlying myocardial cell apoptosis remain to be
elucidated.
Caspases, a family of cysteine proteases, have a
regulatory function in cell apoptosis by cleaving their specific
substrates. Previous studies have indicated that caspases are
implicated in the development and progression of heart failure
(32,33). Narula et al (34) proposed that caspase-3 activated by
the release of mitochondrial cytochrome c is a predictive
factor of adverse outcomes in patients with CHF. Liu et al
(35) reported that manipulation
of the caspase-12 and c-Jun N-terminal kinase signaling pathways
may alter the outcome of heart failure. Recent studies have
reported concordant results, demonstrating that caspase-1 has an
important role in cardiovascular disease (36,37).
Merkle et al (38)
demonstrated that overexpression of cardiomyocyte-specific
caspase-1 in mice may lead to heart failure. All these findings
support a critical role for caspase-1-mediated myocardial apoptosis
in the progression of CHF. However, little is known regarding the
mechanism underlying the regulation of caspase-1 in myocardial
apoptosis in patients with CHF.
Accumulating evidence suggests that lncRNAs have
important roles in the regulation of cell apoptosis in numerous
diseases. DeOcesano-Pereira et al (39) revealed that lncRNA INXS,
transcribed from the opposite genomic strand of B-cell
lymphoma-extra large, induces apoptosis in kidney tumor cell lines.
Xu et al (40) reported
that high expression levels of lncRNA URHC may inhibit apoptosis by
suppressing ZAK expression, a regulator of the extracellular
signal-regulated kinase/mitogen-activated protein kinase signaling
pathway, in hepatocellular carcinoma. Wang et al (22) demonstrated that lncRNA CARL
inhibits anoxia-induced apoptosis in cardiomyocytes by impairing
microRNA-539-dependent downregulation of prohibitin 2, which is
able to inhibit mitochondrial fission and apoptosis. Although
lncRNAs have been recognized as regulators of gene expression in
apoptosis, the possible regulation of caspase-1 by lncRNAs has yet
to be determined.
In the present study, a human lncRNA PCR array kit,
the Human lncRNA Profiler, was used to compare the lncRNA
expression profile between BNP-treated and control cells. The
results demonstrated that the expression levels of lncRNA LSINCT5
were upregulated by BNP in cardiomyocytes. In addition, suppression
of LSINCT5 by siRNA was able to reduce cell apoptosis. Therefore,
LSINCT5 may have an important role in cardiomyocyte apoptosis. In
order to further investigate the molecular mechanisms underlying
myocardial cell apoptosis, caspase expression levels were
quantified in myocardial cells with LSINCT5 knockdown. The results
indicated that the expression of caspase-1 was regulated by LSINCT5
during myocardial apoptosis. To further investigate these results,
the present study also quantified the expression levels of IL-1β,
cleaved by caspase-1 from pro-IL-1β. The results demonstrated that
the expression levels of IL-1β were concordant with those of
caspase-1.
In conclusion, the results of the present study
demonstrated that lncRNA LSINCT5 was upregulated in apoptotic
myocardial cells induced by BNP. In addition, LSINCT5 has an
important role in the regulation of cardiomyocyte apoptosis through
the regulation of caspase-1/IL-1β expression. These findings may
aid in improving the understanding of the molecular mechanism
underlying CHF. Future research may focus on whether lncRNA LSINCT5
can be used as a potential diagnostic tool, or even a therapeutic
target for CHF.
References
|
1
|
Granger CB, McMurray JJ, Yusuf S, Held P,
Michelson EL, Olofsson B, Ostergren J, Pfeffer MA and Swedberg K;
CHARM Investigators Committees: Effects of candesartan in patients
with chronic heart failure and reduced left-ventricular systolic
function intolerant to angiotensin-converting-enzyme inhibitors:
The CHARM-Alternative trial. Lancet. 362:772–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu D, Huang G and He J: Investigation of
prevalence and distributing feature of chronic heart failure in
Chinese adult population. Zhonghua Xin Xue Guan Bing Za Zhi.
31:3–6. 2002.In Chinese.
|
|
3
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al American College of Cardiology Foundation; American Heart
Association: Task Force on Practice Guidelines: 2013 ACCF/AHA
guideline for the management of heart failure: A report of the
American college of cardiology foundation/american heart
association task force on practice guidelines. J Am Coll Cardiol.
62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowie MR: BNP-guided therapy for chronic
heart failure: Anything more than just an attractive concept? Eur
Heart J. 35:1507–1509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishino M, Takeishi Y, Niizeki T, Watanabe
T, Nitobe J, Miyamoto T, Miyashita T, Kitahara T, Suzuki S, Sasaki
T, et al: Risk stratification of chronic heart failure patients by
multiple biomarkers: Implications of BNP, H-FABP and PTX3. Circ J.
72:1800–1805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickstein K, Cohen-Solal A, Filippatos G,
McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van
Veldhuisen DJ, Atar D, Hoes AW, et al ESC Committee for Practice
Guidelines (CPG): ESC Guidelines for the diagnosis and treatment of
acute and chronic heart failure 2008. The Task Force for the
diagnosis and treatment of acute and chronic heart failure 2008 of
the European Society of Cardiology. Developed in collaboration with
the Heart Failure Association of the ESC (HFA) and endorsed by the
European Society of Intensive Care Medicine (ESICM). Eur J Heart
Fail. 10:933–989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Shea P, Daly R, Kasim S and Tormey WP:
B-Type natriuretic peptide in the cardiology department. Ir Med J.
105:341–343. 2012.
|
|
8
|
Porapakkham P, Zimmet H, Billah B and Krum
H: B-type natriuretic peptide-guided heart failure therapy: A
meta-analysis. Arch Intern Med. 170:507–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szondy Z: Methylprednisolone and
2-chloroadenosine induce DNA fragmentation at different stages of
human T-lymphocyte development. Immunol Lett. 58:59–65. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klein G: Cancer, apoptosis, and nonimmune
surveillance. Cell Death Differ. 11:13–17. 2004. View Article : Google Scholar
|
|
11
|
Shimohama S: Apoptosis in Alzheimer's
disease - an update. Apoptosis. 5:9–16. 2000. View Article : Google Scholar
|
|
12
|
Li M, Wu Z, Niu W, Wan Y, Zhang L, Shi G
and Xi X: The protective effect of curcumin against the 19-kDa
Mycobacterium tuberculosis protein-induced inflammation and
apoptosis in human macrophages. Mol Med Rep. 10:3261–3267.
2014.PubMed/NCBI
|
|
13
|
Olivetti G, Abbi R, Quaini F, Kajstura J,
Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski
S, et al: Apoptosis in the failing human heart. N Engl J Med.
336:1131–1141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narula J, Haider N, Virmani R, DiSalvo TG,
Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW and Khaw BA:
Apoptosis in myocytes in end-stage heart failure. N Engl J Med.
335:1182–1189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishino S, Kuge Y, Takai N, Tamaki N,
Strauss HW, Blackenberg FG, Shiomi M and Saji H: 99mTc-Annexin A5
for noninvasive characterization of atherosclerotic lesions:
Imaging and histological studies in myocardial infarction-prone
Watanabe heritable hyperlipidemic rabbits. Eur J Nucl Med Mol
Imaging. 34:889–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Williams V, Liu L, Chen H, Sawamura
T, Romeo F and Mehta JL: Expression of lectin-like oxidized
low-density lipoprotein receptors during ischemia-reperfusion and
its role in determination of apoptosis and left ventricular
dysfunction. J Am Coll Cardiol. 41:1048–1055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung KP, Qu YH, Qiao DF, Xie WB, Li DR,
Xu JT, Wang HJ and Yue X: Critical role of insulin-like growth
factor binding protein-5 in methamphetamine-induced apoptosis in
cardiomyocytes. Mol Med Rep. 10:2306–2312. 2014.PubMed/NCBI
|
|
19
|
Deng YJ, Tan N, Zeng HK, Fu YH and Dong
XL: Effects of BNP preconditioning on myocardial cell apoptosis and
expressions of bcl-2 and Bax during myocardial ischemia-reperfusion
injury in rats. Zhonghua Yi Xue Za Zhi. 90:3431–3434. 2010.In
Chinese.
|
|
20
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun.
5:35962014.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Henriksen JH, Gøtze J, Fuglsang S,
Christensen E, Bendtsen F and Møller S: Increased circulating
pro-brain natriuretic peptide (proBNP) and brain natriuretic
peptide (BNP) in patients with cirrhosis: Relation to
cardiovascular dysfunction and severity of disease. Gut.
52:1511–1517. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smit B, Spoelstra-de Man AM, Girbes AR and
de Waard MC: NT-proBNP in cardiopulmonary resuscitated patients
treated with mild therapeutic hypothermia is not independently
associated with mortality: a retrospective observational study. BMC
anesthesiology. 15:482015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beer S, Golay S, Bardy D, Feihl F,
Gaillard RC, Bachmann C, Waeber B and Ruiz J: Increased plasma
levels of N-terminal brain natriuretic peptide (NT-proBNP) in type
2 diabetic patients with vascular complications. Diabetes Metab.
31:567–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber M, Bazzino O, Navarro Estrada JL,
Fuselli JJ, Botto F, Perez de Arenaza D, Möllmann H, Nef HN,
Elsässer A and Hamm CW: N-terminal B-type natriuretic peptide
assessment provides incremental prognostic information in patients
with acute coronary syndromes and normal troponin T values upon
admission. J Am Coll Cardiol. 51:1188–1195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Song P, Zhu Q, Yin QY, Ji JW, Li W
and Bian HM: Liguzinediol improved the heart function and inhibited
myocardial cell apoptosis in rats with heart failure. Acta
Pharmacol Sin. 35:1257–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suo C, Sun L and Yang S: Alpinetin
activates the δ receptor instead of the κ and µreceptor pathways to
protect against rat myocardial cell apoptosis. Exp Ther Med.
7:109–116. 2014.
|
|
30
|
Hirota H, Chen J, Betz UA, Rajewsky K, Gu
Y, Ross J Jr, Müller W and Chien KR: Loss of a gp130 cardiac muscle
cell survival pathway is a critical event in the onset of heart
failure during biomechanical stress. Cell. 97:189–198. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song XJ, Yang CY, Liu B, Wei Q, Korkor MT,
Liu JY and Yang P: Atorvastatin inhibits myocardial cell apoptosis
in a rat model with post-myocardial infarction heart failure by
downregulating ER stress response. Int J Med Sci. 8:564–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang PM and Izumo S: Apoptosis in heart:
basic mechanisms and implications in cardiovascular diseases.
Trends Mol Med. 9:177–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mital S, Barbone A, Addonizio LJ,
Quaegebeur JM, Mosca RJ, Oz MC and Hintze TH: Endogenous
endothelium-derived nitric oxide inhibits myocardial caspase
activity: Implications for treatment of end-stage heart failure. J
Heart Lung Transplant. 21:576–585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narula J, Pandey P, Arbustini E, Haider N,
Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A,
Dec GW, et al: Apoptosis in heart failure: Release of cytochrome c
from mitochondria and activation of caspase-3 in human
cardiomyopathy. Proc Natl Acad Sci USA. 96:8144–8149. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Wang J, Qi SY, Ru LS, Ding C, Wang
HJ, Zhao JS, Li JJ, Li AY and Wang DM: Reduced endoplasmic
reticulum stress might alter the course of heart failure via
caspase-12 and JNK pathways. Can J Cardiol. 30:368–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prescimone T, D'Amico A, Caselli C,
Cabiati M, Viglione F, Caruso R, Verde A, Del Ry S, Trivella MG and
Giannessi D: Caspase-1 transcripts in failing human heart after
mechanical unloading. Cardiovasc Pathol. 24:11–18. 2015. View Article : Google Scholar
|
|
37
|
Syed FM, Hahn HS, Odley A, Guo Y, Vallejo
JG, Lynch RA, Mann DL, Bolli R and Dorn GW II: Proapoptotic effects
of caspase-1/interleukin-converting enzyme dominate in myocardial
ischemia. Circ Res. 96:1103–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merkle S, Frantz S, Schön MP, Bauersachs
J, Buitrago M, Frost RJ, Schmitteckert EM, Lohse MJ and Engelhardt
S: A role for caspase-1 in heart failure. Circ Res. 100:645–653.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DeOcesano-Pereira C, Amaral MS, Par reira
KS, Ayupe AC, Jacysyn JF, Amarante-Mendes GP, Reis EM and
Verjovski-Almeida S: Long non-coding RNA INXS is a critical
mediator of BCL-XS induced apoptosis. Nucleic Acids Res.
42:8343–8355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu WH, Zhang JB, Dang Z, Li X, Zhou T, Liu
J, Wang DS, Song WJ and Dou KF: Long non-coding RNA URHC regulates
cell proliferation and apoptosis via ZAK through the ERK/MAPK
signaling pathway in hepatocellular carcinoma. Int J Biol Sci.
10:664–676. 2014. View Article : Google Scholar : PubMed/NCBI
|