Introduction
Cognitive function is regarded as the ability to
objectively understand ideas. Cognitive function is composed of
numerous cognitive domains, including memory, calculation,
orientation in time and space, structural ability, ability to
perform tasks, and language comprehension and application (1–3).
Previous studies have suggested that cognitive impairment has a
detrimental effect on the recovery of motor function and the
ability of impaired patients to perform daily activities, which is
an important factor restricting the comprehensive rehabilitation of
stroke patients (4–7). Stroke is a leading cause of mortality
and permanent disability, and two-thirds of all strokes are
considered ischemic (8). In
addition, the incidence of cognitive impairment following a stroke
may reach ≤65% (9). Furthermore,
in ~10 to 40% of patients with mild cognitive impairment, the
condition may develop into dementia within a year (10–14).
There is therefore an urgent requirement for the early detection of
cognitive impairment, so that the mental and physical functions of
patients can be fully recovered and dementia can be prevented.
Although the pathogeneses of stroke and post-stroke
disabilities are complex (15),
apoptosis has been suggested as one of the major pathways that may
lead to cell death in brain injury following an ischemic stroke
(16). In addition, oxidative
stress caused by reactive oxygen species (ROS) has long been
implicated in neurotoxicity following cerebral ischemic-reperfusion
(I/R), and may ultimately result in the initiation of pathways that
lead to apoptotic cell death (17). Since free radicals significantly
affect the pathogenesis of cerebral ischemic injuries, high levels
of free radicals may cause injury to the brain and detrimentally
influence nervous function, learning ability, and memory.
Therefore, the identification of strategies that suppress, and
increase the clearance rate, of free radicals is important in the
treatment of ischemic stroke.
The activation of the cyclic adenosine monophosphate
(cAMP) response element-binding protein (CREB) transcription factor
has been reported to protect neuronal cells in cerebral ischemia
(18,19) Furthermore, CREB and the
CRE-mediated system are associated with learning and memory, and
B-cell lymphoma 2 (Bcl-2) has a pivotal role in the control of cell
death. Bcl-2 has been shown to be upregulated by ischemic
tolerance, and its expression is regulated by CREB (20). Therefore, activation of CREB
phosphorylation can increase Bcl-2 expression, which results in
protection of the neuronal cells, and ameliorates learning and
memory following cerebral ischemia.
Acupuncture is a simple, convenient and
cost-effective treatment strategy originating from ancient China,
which has been widely used for thousands of years to treat various
diseases (21–23). Previous studies have demonstrated
the clinical efficacy of acupuncture in stroke and rehabilitation
of post-stroke cognitive impairment (24–27).
Two acupoints located on the Du meridian; Baihui (DU20) and
Shenting (DU24), are considered the most effective locations and
have been commonly used in the treatment of cognitive impairment
(28,29). In addition, electroacupuncture
(EA), which uses fixed frequency and intensity instead of the
traditional twisting and extracting techniques, has advantages
including stability, strong persistence, and reduced variability
and error between practitioners (30). However, the precise mechanism
underlying the neuroprotective effects of EA on cognitive
impairment remains unclear. Therefore, the present study aimed to
evaluate the therapeutic efficacy of EA against post-stroke
cognitive impairment. The underlying molecular mechanisms were
investigated using a focal cerebral I/R-injured rat model.
Materials and methods
Animals
Healthy adult male Sprague-Dawley (SD) rats weighing
250–280 g were purchased from the Shanghai SLAC Laboratory Animal
Co., Ltd. (Shanghai, China), and housed in pathogen-free conditions
with a 12 h light/dark cycle. All experiments were performed
strictly in accordance with the International Ethical Guidelines.
The rats had ad libitum access to food and water during the
experiment. The present study was approved by the Institutional
Animal Care and Use Committee of the Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
Establishment of the cerebral I/R-injured
rat model
Middle cerebral artery occlusion (MCAO) was used to
establish a cerebral I/R-injured rat model, as previously described
(31). Briefly, the rats were
anesthetized using 10% chloral hydrate (300 mg/kg; Shanghai
Chemical Reagent Co., Ltd., Shanghai, China) injected
intraperitoneally. The left common carotid artery, the left
external carotid artery, and the internal carotid artery (ICA) were
then carefully exposed following a midline neck incision.
Approximately 18 to 22 mm of nylon surgical thread (Beijing Sunbio
Biotech Co., Ltd., Beijing, China) was inserted into the ICA until
the blunted distal end met resistance, in order to block the left
middle cerebral artery (MCA). The thread was removed following 2 h
of occlusion to restore the blood supply to the MCA area, and
reperfusion was achieved. Following awakening, the neurological
deficit scores of the rats were assessed, prior to their random
division into two groups (n=24/group): An ischemia (MCAO) control
group, and an MCAO + EA group. The rats in the sham group (n=24)
were subjected to the procedure as described above, without the
occlusion of the MCA. Following the surgery, the rats were allowed
to recover in prewarmed cages.
EA treatment
Following I/R injury, the rats in the EA group
received EA treatment. Acupuncture needles, 0.3 mm in diameter,
were inserted at a depth of 2 to 3 mm into the heads of the rats at
the Baihui (DU20) and Shenting (DU24) acupoints. Stimulation was
then generated using EA apparatus (model G6805; Suzhou Medical
Appliance Factory, Suzhou, China), and the stimulation parameters
were set as follows: 5 and 20 Hz at 1–3 mA, dispersed for 30 min
once daily. The treatment was performed 2 h following I/R treatment
and was continued until the animals were sacrificed by 10% chloral
hydrate intraperitoneal injection and decapitation, 7 days after
the operation.
Assessment of neurological deficit
scores
The neurological deficit score was assessed in a
single-blind manner, as previously described by Chen et al
(31). The neurological deficit
scores were assessed on the first, third, fifth and seventh day
following I/R injury. The scores were determined as follows: Score
0 indicated no neurological deficit; score 1, (failure to fully
extend the right forepaw) indicated mild deficits; score 2,
(circling of the right forepaw); score 3, (falling on the right
forepaw) indicated moderate deficits; and score 4, (failure to
walk) indicated severe deficits. Rats with scores 0 or 4 were
excluded from the experiment.
Measurement of cerebral infarct
volume
Following completion of the experiment, the rats
were sacrificed and their brains rapidly collected. The brain
tissue was coronally sectioned into slices 2 mm thick, prior to
being stained with a 2% solution of tetrazolium chloride (TTC;
Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 20 min. The sections
were subsequently fixed with 4% paraformaldehyde, as previously
described (32). Normal tissue was
stained deep red, whereas the infarct area was stained a pale gray
color. The stained sections were scanned using a Canon SX20
high-resolution digital camera (Canon, Inc., Tokyo, Japan), and the
infarct volume was quantified using the Motic Med 6.0 System (Motic
Incorporation, Ltd., Causeway Bay, Hong Kong). The infarct volume
was expressed as a percentage of the contralateral hemisphere
volume.
Assessment of cognitive function
From the third day following surgery, the spatial
learning and memory abilities (33–35)
of the rats were investigated by subjecting them to a Morris water
maze (Chinese Academy of Sciences, Beijing, China), a circular tank
with a diameter of 120 cm and a height of 50 cm. The tank was
filled with 22±1°C water to a depth of 30 cm. A circular escape
platform, measuring 6 cm in diameter and 28 cm in height, was
submerged 2 cm below the surface of the water. The tank was divided
into four quadrants: Northeast, southeast, southwest, and
northwest. These points served as the starting positions at which
each rat was lowered gently into the water, its head facing the
wall of the water maze. Morris water maze tasks include
orientation, navigation and space exploration trials. In the first
set of trials, each rat was placed in the water at four equidistant
locations to the platform. If the rat arrived at the platform
within the 90 sec time restriction and remained on it for 3 sec, it
was considered to have found the platform and was scored on the
time taken to complete the task, as well as the length of the
chosen route. However, if the rat was unable to find the platform
within 90 sec, it was placed on the platform for 10 sec and given a
time score of 90 sec. The computer recorded the time taken and the
length of the route by which each rat found the safe platform, and
each day the average result of the time taken and the length of the
route taken for the four quadrants were assessed for each rat. The
duration of the first set of trials was 5 days, with the experiment
performed once daily.
The second part of the experiment was performed on
the seventh day following surgery. Briefly, the ability of each rat
to remember the position of the platform was evaluated by measuring
the time in which each rat found the platform within the 90 sec
time restriction. Following the trials, the rats were thoroughly
dried with a hair dryer and returned to their cages.
Determination of superoxide dismutase
(SOD) and glutathione peroxidase (GSHPx) activities, and
malondialdehyde (MDA) levels
The ischemic brain hippocampus of each rat was
collected on the seventh day following MCAO. The brains were
rinsed, weighed, and homogenized in 9 volumes of 9 g/l ice-cold
saline for 10 min using a Dounce Tissue Grinder (Kimble Chase Life
Science and Research Products LLC, Vineland, NJ, USA). The
supernatant homogenate was collected following centrifugation at
12,000 × g for 10 min at 4°C. The total protein concentrations were
then determined using a Bradford protein assay (Novagen, Inc.,
Madison, WI, USA). The SOD and GSHPx activities, and the MDA levels
were measured using assay kits according to the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The brains of the rats were removed immediately
following decapitation, and the ischemic brain tissues were
dissected and maintained at −80°C until use. Total RNA was isolated
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), and the Oligo(dT)-primed RNA (1 µg) was reverse
transcribed into cDNA, according to the manufacturer's instructions
(Fermentas, Thermo Fisher Scientific, Pittsburgh, PA, USA). The
cDNA was subsequently used to determine the expression levels of
Bcl-2 and Bax mRNA by PCR using Taq DNA polymerase (Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA), and β-actin was used as an
internal control. The primer sequence were as follows: Bcl-2,
forward, 5′-GGTGGTGGAGGAACTCTTCA-3′; and reverse,
5′-GAGCAGCGTCTTCAGAGACA-3′; Bax forward,
5′-GAGCAGCGTCTTCAGAGACA-3′; and reverse,
5′-TCACGGAGGAAGTCCAGTGT-3′; and β-actin forward,
5′-ACTGGCATTGTGATGGACTC-3′; and reverse, 5′-CAGCACTGTGTTGGCATAGA-3′
(Shanghai Institute of Bioengineering, Shanghai, China). The PCR
products were analyzed on a 1.5% agarose gel and examined using a
gel documentation system (Model Gel Doc 2000; Bio-Rad Laboratories
Inc., Hercules, CA, USA).
Western blot analysis
The left cerebral hippocampal tissues were collected
and triturated in a radioimmunoprecipitation assay buffer (Fansbio,
Guangzhou, China), and the proteins were quantified using a
bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL,
USA). The protein lysates were separated by electrophoresis by 12%
SDS-PAGE, prior to being transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA), which were blocked
for 2 h with 5% non fat dry milk at room temperature. The blots
were then incubated with primary antibodies targeting Bcl-2
(1:1,000; cat. no. 15071; Cell Signaling Technology, Inc., Danvers,
MA, USA), Bax (1:1,000; cat. no. 5023; Cell Signaling Technology,
Inc.), phosphorylated (p)-CREB (1:1,000; cat. no. 9198; Cell
Signaling Technology, Inc.), and β-actin (1:4,000; cat. no. AA-128;
Beyotime Institute of Biotechnology, Haimen, China) overnight at
4°C, prior to incubation with an appropriate horseradish peroxidase
(HRP)-conjugated secondary antibody (1:3,000; goat anti-rabbit IgG;
cat. no. 611-1322-0500; Rockland Immunochemicals Inc., Pottstown,
PA, USA) for 1 h at room temperature. The bands were visualized
with enhanced chemiluminescence (Amersham, GE Healthcare,
Piscataway, NJ, USA), and the images were captured using a
Bio-Image Analysis system (Bio-Rad Laboratories Inc.).
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD) and statistically analyzed by one-way analysis of
variance using SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA), prior to being subjected to a post hoc least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
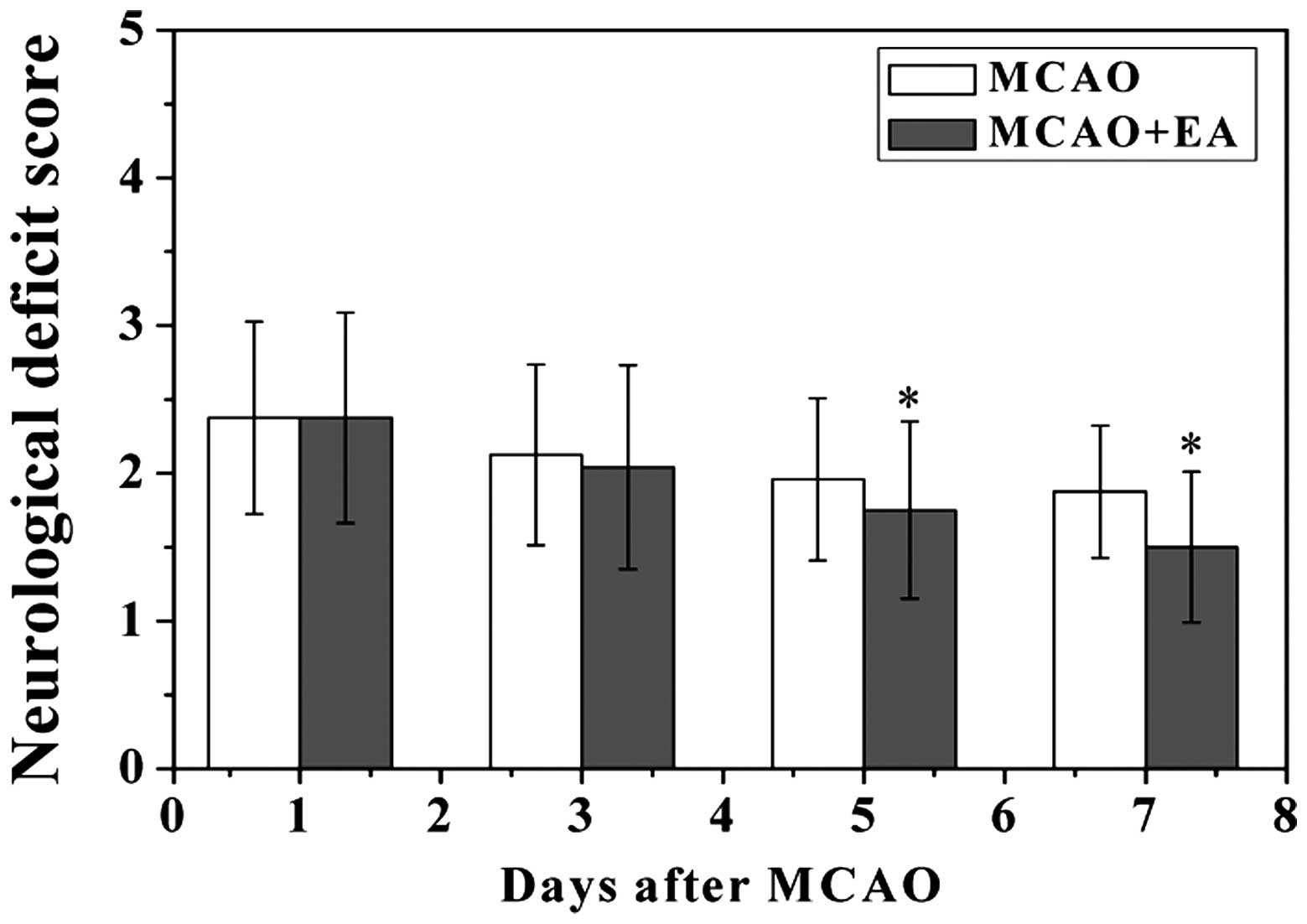
EA reduces neurological deficits and
infarct volume in rats following MCAO
To evaluate whether EA at the Baihui (DU20) and
Shenting (DU24) acupoints attenuated ischemic brain injury, the
neurological scores were determined at various time points
following a stroke. As hypothesized, the rats in the sham group did
not exhibit any manifestations of neurological deficits, whereas
all of the rats in the MCAO and MCAO + EA groups exhibited clear
symptoms of cerebral injury (Fig.
1). However, the neurological function scores were
significantly improved in the MCAO + EA group, as compared with the
MCAO group (P<0.05). To further verify these results, the
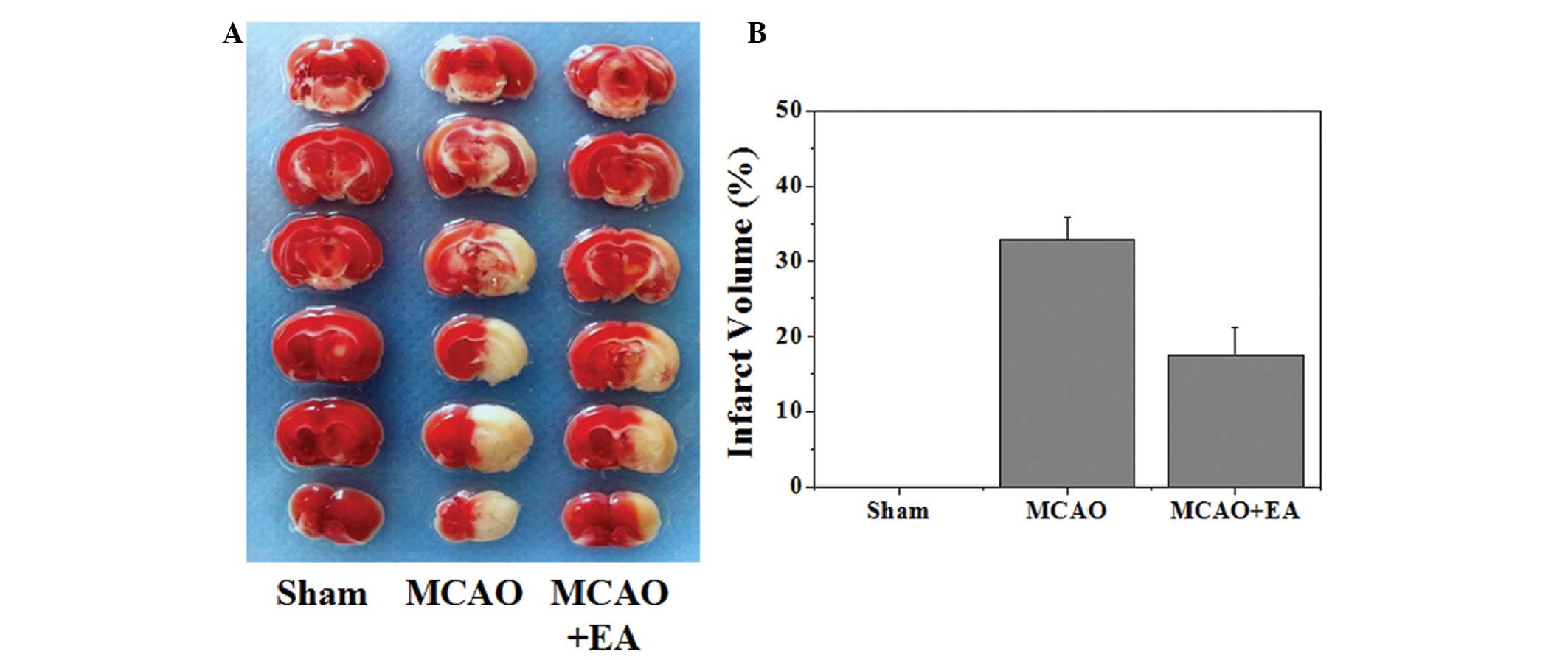
effects of EA on cerebral infarction were evaluated. As shown in
Fig. 2, the infarct volume was
measured using TTC staining. Normal tissue was stained deep red,
whereas the infarct area was a pale cream color. There was a
statistically significant decrease in infarct volume in the MCAO +
EA group, as compared with the MCAO group (P<0.05).
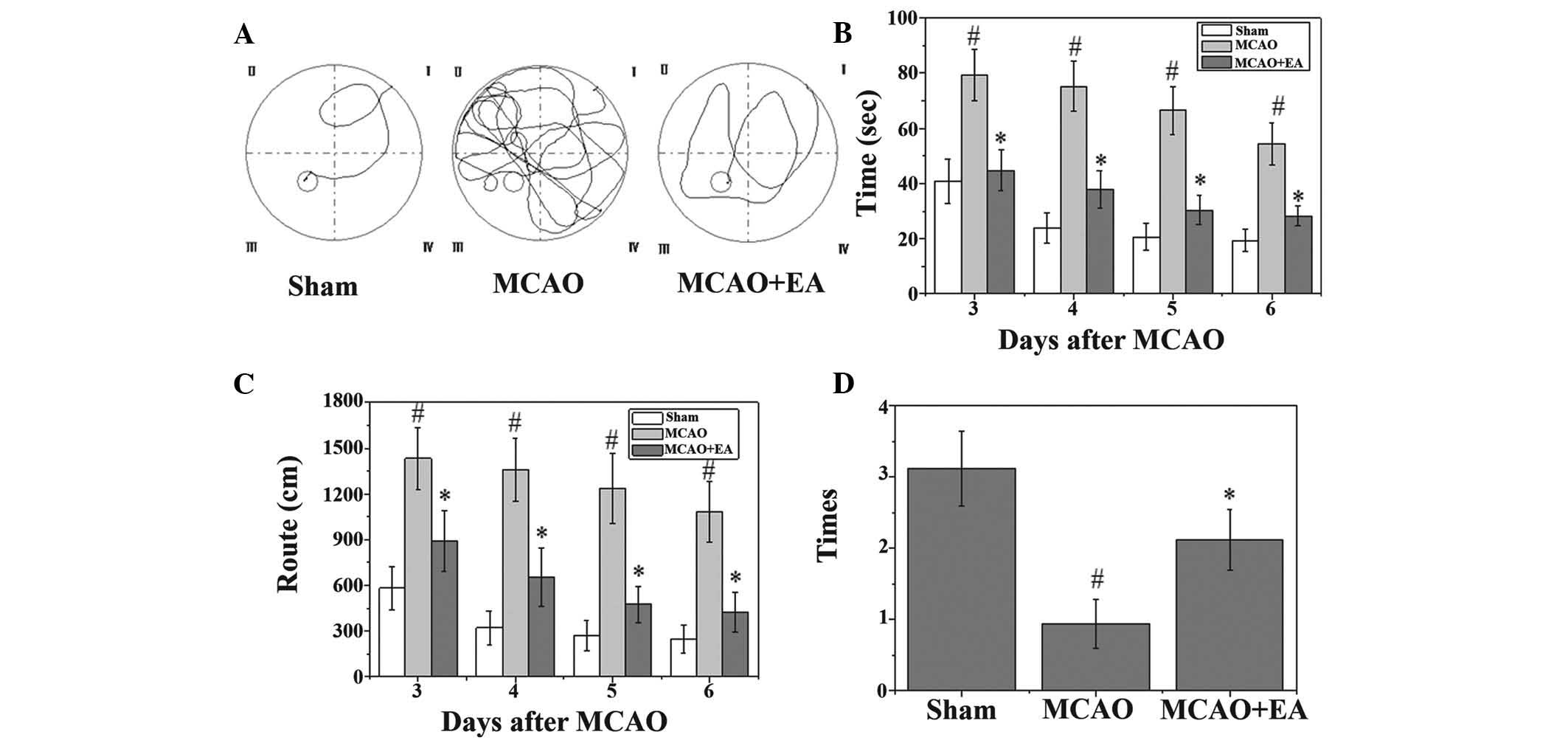
EA ameliorates cognitive impairment in
cerebral I/R-injured rats
A Morris water maze test was performed on the third
to seventh day following MCAO surgery. As shown in Fig. 3, the rats in the MCAO group had
longer latency periods and took longer routes to reach the hidden
platform. In addition, the number of times that the MCAO rats
crossed the location of the platform was significantly lower, as
compared with the rats in the sham group (P<0.05). However, the
rats in the EA group had a shorter latency and route length, and
the number of times they crossed the platform was higher, as
compared with the MCAO group (Fig.
3).
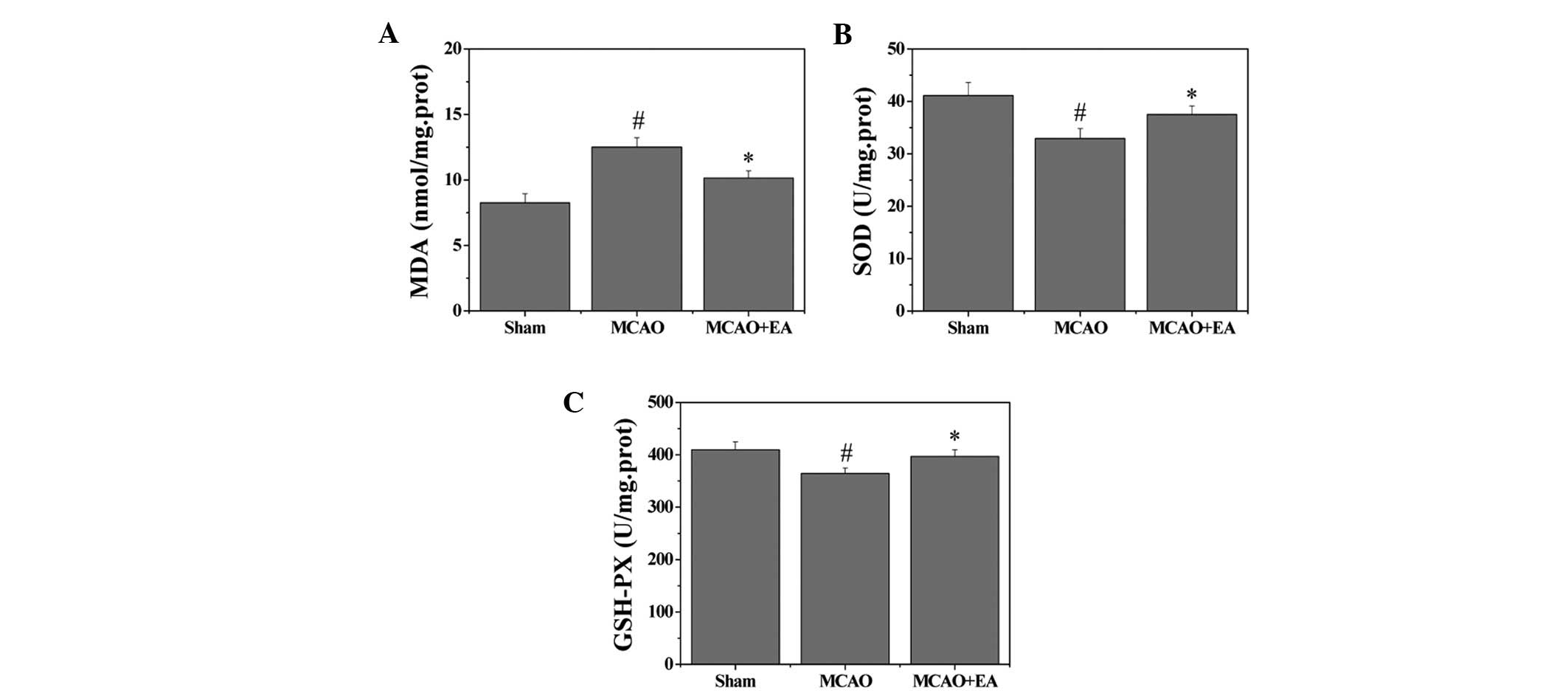
Effects of EA on MDA content and the
activity of antioxidant enzymes
To evaluate whether EA affects oxidative stress
damage, the MDA content and the activities of antioxidant enzymes
in the hippocampus were investigated. MDA content, an index of
lipid peroxidation was significantly increased following cerebral
I/R injury (P<0.05), whereas MDA content was significantly
decreased after EA treatment, as compared with the MCAO group
(P<0.05). Furthermore, the activities of the antioxidant enzymes
SOD and GSHPx were decreased in the MCAO group, as compared with
the sham group (P<0.05). However, EA treatment induced a
significant elevation of SOD and GSHPx activities as compared with
the MCAO group (P<0.05) (Fig.
4).
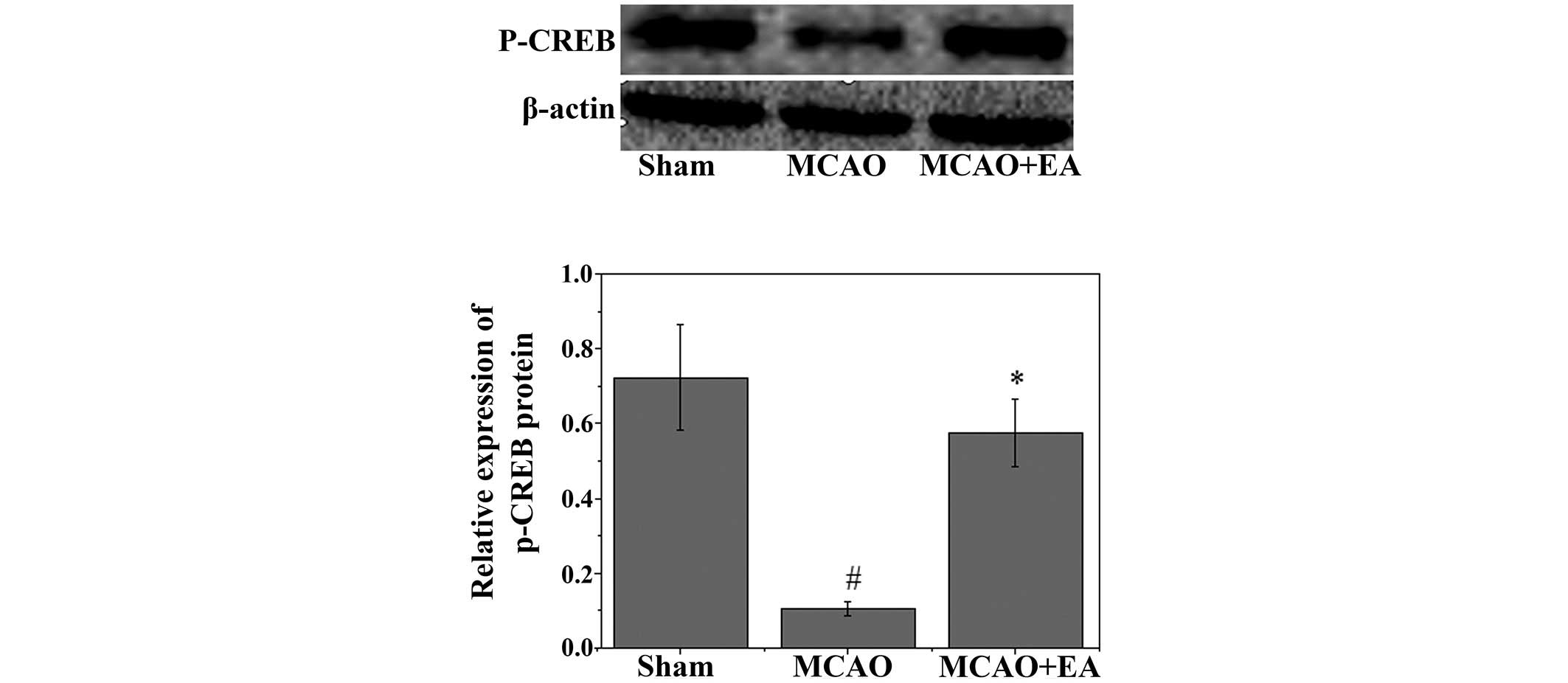
Effects of EA on p-CREB and
apoptosis-associated factors
To investigate the mechanism underlying the
anti-apoptotic effects of EA, western blot analysis was used to
examine the effects of EA on the immunoreactivity of p-CREB in the
hippocampus. As shown in Fig. 5, a
significant decrease in the immunoreactivity of p-CREB was observed
in the hippocampus following MCAO (P<0.05). Conversely, EA
significantly attenuated the decrease in the immunoreactivity of
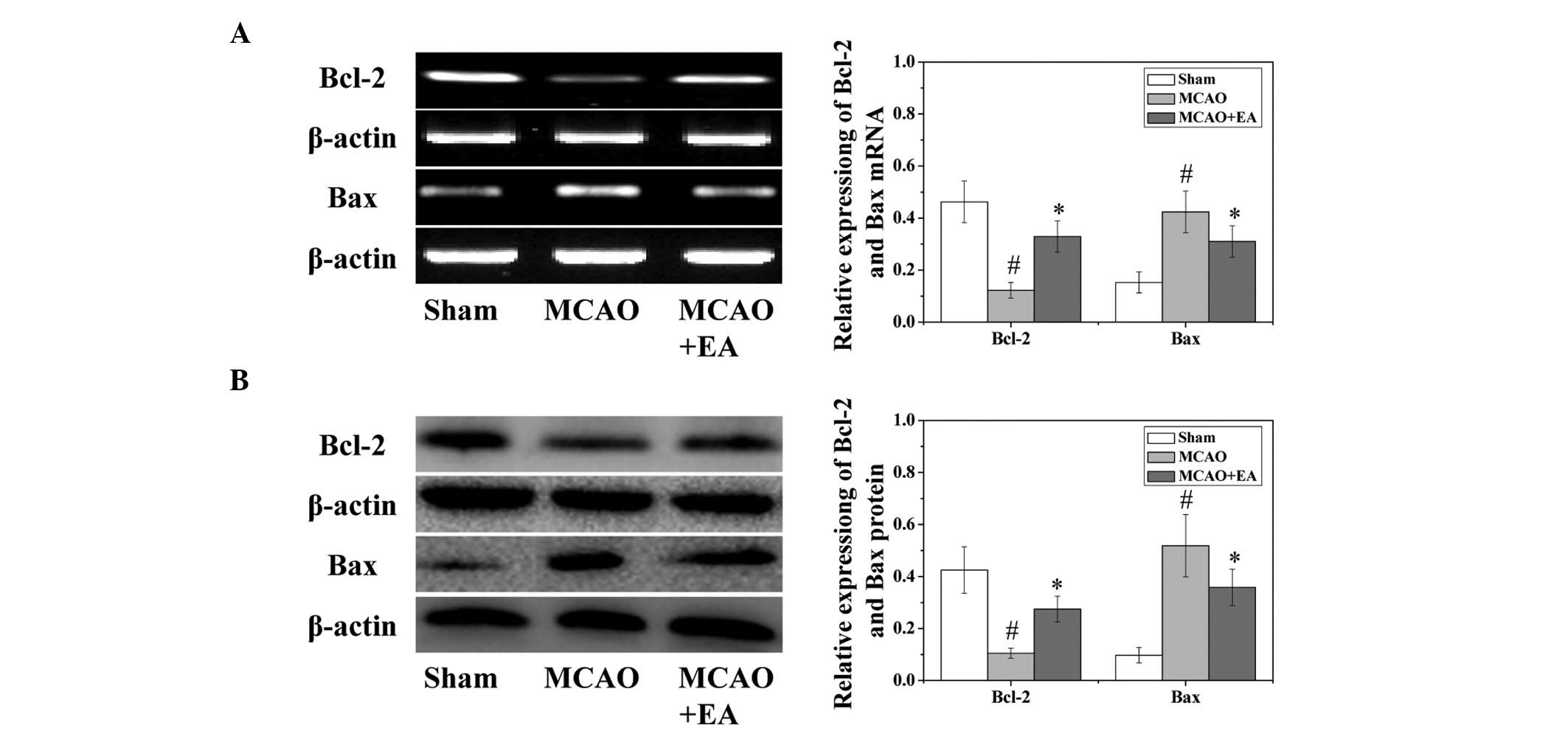
p-CREB (P<0.05). Western blotting and RT-qPCR were used to
evaluate both the protein and mRNA expression levels of the vital
target genes Bcl-2 and Bax. As shown in Fig. 6, EA treatment significantly
(P<0.05) increased the mRNA expression levels of Bcl-2 and
decreased the mRNA expression levels of Bax caused by the cerebral
I/R injury.
Discussion
EA is a core component of traditional Chinese
medicine, which is recognized as an effective treatment for
numerous chronic diseases, with no side effects. Numerous studies
have demonstrated the clinical efficacy of acupuncture in stroke
and cognitive impairment (28).
The Baihui (DU20) and Shenting (DU24) acupoints are situated on the
Du meridian, which is considered to be beneficial to human health,
good spirits, and memory function. The results of the present study
demonstrated that EA on the Baihui and Shenting acupoints could
significantly ameliorate neurological deficits and reduce cerebral
infarct volume. Consistent with previous reports (27,36,37),
a Morris water maze test revealed that EA improved the learning and
memory ability of rats with cerebral I/R injury, demonstrating the
therapeutic efficacy of EA against post-stroke cognitive
impairment.
The most effective treatment for acute ischemic
stroke is reperfusion of the ischemic penumbra. However, I/R injury
often leads to secondary damage. Therefore, anti-reperfusion injury
and neuroprotection are critical for stroke management.
Furthermore, oxidative stress has been well-established as the main
mechanism underlying I/R injury, and reactive oxygen species (ROS)
produced in the mitochondria have an important role in regulating
the neurocyte apoptotic pathway during I/R (38).
Under physiological conditions, ROS are generated at
low levels, controlled by endogenous antioxidants, such as SOD and
GSHPx (39). However, the sudden
overproduction of ROS during cerebral I/R leads to oxidative
stress, which results in cell damage in nervous tissue. This may
lead to the induction of chain reactions, such as membrane lipid
peroxidation (17). ROS produce
MDA, a toxic end-product of lipid peroxidation, and MDA levels
directly reflect the rate and extent of lipid peroxidation
(40). SOD and GSHPx enzymes are
thought to act as free radical scavengers that may prevent the
deleterious stroke-induced ROS generation (41); therefore, their expression levels
may directly reflect the capacity of the brain tissue to eliminate
free radicals. The results of the present study demonstrated that
EA could protect the brain tissue from damage by stimulating SOD
and GSHPx activity and by decreasing the levels of MDA.
Apoptosis is one of the predominant types of
neurocyte death in the ischemic penumbra during the progression of
an ischemic stroke. A previous study (42) demonstrated that memory impairment
in MCAO rats was associated with neuronal apoptosis in the
hippocampus. In addition, apoptosis was reportedly suppressed by
the enhanced expression of Bcl-2 (43,44).
The expression of Bcl-2 is mediated by CREB (45), and p-CREB decreases Bax expression
(46). In brain tissue, CREB is
associated with learning, memory, and dendritic transmission. When
hippocampal CREB activity decreases due to cerebral ischemia,
impairment of learning and memory ability occurs. Furthermore, CREB
knockout mice were reported to exhibit memory impairment (47), with the relative level of CREB
activity at the time of learning being a key factor in determining
whether a neuron was recruited into the memory trace. The present
study demonstrated that compared with the MCAO group, treatment
with EA could increase the immunoreactivity of p-CREB and Bcl-2,
and decrease the immunoreactivity of Bax. These results suggested
that EA is associated with pro-apoptotic activity and the
amelioration of learning and memory ability, which may be mediated
via activation of CREB phosphorylation.
In conclusion, the present study demonstrated that
EA on the Baihui (DU20) and Shenting (DU24) acupoints was able to
improve cognitive impairment following cerebral ischemia. The
protective effects of EA were associated with an anti-apoptotic
mechanism, via activation of CREB, and inhibition of oxidative
stress. These results indicate that EA may have therapeutic
potential for the treatment of post-stroke cognitive
impairment.
Acknowledgments
The present study was supported by the Mechanism of
Acupuncture to Improve Cognitive Function (grant no. X2012
004-collaborative), the National Natural Science Foundation of
China (grant no. 81273835), and the National Natural Science
Foundation of China (grant no. 81373778). The authors thank Clarity
Manuscript Consultants, LLC for editorial assistance with the
manuscript.
References
|
1
|
Lindeboom J and Weinstein H:
Neuropsychology of cognitive ageing, minimal cognitive impairment,
Alzheimer's disease, and vascular cognitive impairment. Eur J
Pharmacol. 490:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nyenhuis Dl, Gorelick PB, Geenen EJ, et
al: The pattern of neuropsychological deficits in Vascular
Cognitive Impairment-No Dementia (Vascular CIND). Clin
Neuropsychol. 18:41–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sachdev PS, Brodaty H, Valenzuela MJ, et
al: The neuropsychological profile of vascular cognitive impairment
in stroke and TIA patients. Neurology. 62:912–919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savva GM and Stephan BC; Alzheimer's
Society Vascular Dementia Systematic Review Group: Epidemiological
studies of the effect of stroke on incident dementia: A systematic
review. Stroke. 41:e41–e46. 2010. View Article : Google Scholar
|
|
5
|
Liu F, Li ZM, Jiang YJ and Chen LD: A
meta-analysis of acupuncture use in the treatment of cognitive
impairment after stroke. J Altern Complement Med. 20:535–544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Cheng YH and Yu XG: Observation on
therapeutic effect of acupuncture combined with medicine on mild
cognition disorders in patients with post-stroke. Zhongguo Zhen
Jiu. 32:3–7. 2012.In Chinese. PubMed/NCBI
|
|
7
|
Fang Z, Ning J, Xiong C and Shulin Y:
Effects of electroacu-puncture at head points on the function of
cerebral motor areas in stroke patients: A PET study. Evid Based
Complement Alternat Med. 2012:9024132012. View Article : Google Scholar
|
|
8
|
Lloyd-Jones D, Adams R, Carnethon M, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2009
update: A report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
119:e21–e181. 2009. View Article : Google Scholar
|
|
9
|
Donovan NJ, Kendall DL, Heaton SC, Kwon S,
Velozo CA and Duncan PW: Conceptualizing functional cognition in
stroke. Neurorehabil Neural Repair. 22:122–135. 2008. View Article : Google Scholar
|
|
10
|
Petersen RC, Doody R, Kurz A, et al:
Current concepts in mild cognitive impairment. Arch Neurol.
58:1985–1992. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Busse A, Bischkopf J, Riedel-Heller SG and
Angermeyer MC: Mild cognitive impairment: Prevalence and incidence
according to different diagnostic criteria. Results of the Leipzig
Longitudinal Study of the Aged (LEILA75+). Br J Psychiatry.
182:449–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Modrego PJ, Fayed N and Pina MA:
Conversion from mild cognitive impairment to probable Alzheimer's
disease predicted by brain magnetic resonance spectroscopy. Am J
Psychiatry. 162:667–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geslani DM, Tierney MC, Herrmann N and
Szalai JP: Mild cognitive impairment: An operational definition and
its conversion rate to Alzheimer's disease. Dement Geriatr Cogn
Disord. 19:383–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ravaglia G, Forti P, Maioli F, et al:
Conversion of mild cognitive impairment to dementia: Predictive
role of mild cognitive impairment subtypes and vascular risk
factors. Dement Geriatr Cogn Disord. 21:51–58. 2006. View Article : Google Scholar
|
|
15
|
Nakka VP, Gusain A, Mehta SL and Raghubir
R: Molecular mechanisms of apoptosis in cerebral ischemia: Multiple
neuro-protective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar
|
|
16
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manzanero S, Santro T and Arumugam TV:
Neuronal oxidative stress in acute ischemic stroke: Sources and
contribution to cell injury. Neurochem Int. 62:712–718. 2013.
View Article : Google Scholar
|
|
18
|
Finkbeiner S: CREB couples neurotrophin
signals to survival messages. Neuron. 25:11–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakamoto K, Karelina K and Obrietan K:
CREB: A multifaceted regulator of neuronal plasticity and
protection. J Neurochem. 116:1–9. 2011. View Article : Google Scholar
|
|
20
|
Meller R, Minami M, Cameron JA, et al:
CREB-mediated Bcl-2 protein expression after ischemic
preconditioning. J Cereb Blood Flow Metab. 25:234–246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu LB, Chan WC, Lo KC, Yum TP and Li L:
Wrist-ankle acupuncture for the treatment of pain symptoms: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2014:2617092014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie YH, Chai XQ, Wang YL, Gao YC and Ma J:
Effect of electro-acupuncture stimulation of Ximen (PC4) and
Neiguan (PC6) on remifentanil-induced breakthrough pain following
thoracal esophagectomy. J Huazhong Univ Sci Technology Med Sci.
34:569–574. 2014. View Article : Google Scholar
|
|
23
|
Hyun SH, Im JW, Jung WS, et al: Effect of
ST36 acupuncture on hyperventilation-induced CO2
reactivity of the basilar and middle cerebral arteries and heart
rate variability in normal subjects. Evid Based Complement Alternat
Med. 2014:5749862014. View Article : Google Scholar
|
|
24
|
Zhao L, Zhang FW, Zhang H, et al: Mild
cognitive impairment disease treated with electroacupuncture: A
multi-center randomized controlled trial. Zhongguo Zhen Jiu.
32:779–784. 2012.In Chinese. PubMed/NCBI
|
|
25
|
Zhou L, Zhang YL, Cao HJ and Hu H:
Treating vascular mild cognitive impairment by acupuncture: A
systematic review of randomized controlled trials. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 33:1626–1630. 2013.In Chinese.
|
|
26
|
Zhang H, Zhao L, Yang S, et al: Clinical
observation on effect of scalp electroacupuncture for mild
cognitive impairment. J Tradit Chin Med. 33:46–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Guo F, Zhang Q, et al:
Electroacupuncture decreases cognitive impairment and promotes
neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern
Med. 14:372014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Zhang H, Zheng Z and Huang J:
Electroacupuncture on the head points for improving gnosia in
patients with vascular dementia. J Tradit Chin Med. 29:29–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LP, Wang FW, Zuo F, Jia JJ and Jiao
WG: Clinical research on comprehensive treatment of senile vascular
dementia. J Tradit Chin Med. 31:178–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou F, Guo J, Cheng J, Wu G and Xia Y:
Electroacupuncture increased cerebral blood flow and reduced
ischemic brain injury: Dependence on stimulation intensity and
frequency. J Appl Physiol. 111:1877–1887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen A, Lin Z, Lan L, et al:
Electroacupuncture at the Quchi and Zusanli acupoints exerts
neuroprotective role in cerebral ischemia-reperfusion injured rats
via activation of the PI3K/Akt pathway. Int J Mol Med. 30:791–796.
2012.PubMed/NCBI
|
|
32
|
Lan L, Tao J, Chen A, et al:
Electroacupuncture exerts anti-inflammatory effects in cerebral
ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB
pathway. Int J Mol Med. 31:75–80. 2013.
|
|
33
|
Pouzet B, Zhang WN, Feldon J and Rawlins
JN: Hippocampal lesioned rats are able to learn a spatial position
using non-spatial strategies. Behav Brain Res. 133:279–291. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Veng LM, Granholm AC and Rose GM:
Age-related sex differences in spatial learning and basal forebrain
cholinergic neurons in F344 rats. Physiol Behav. 80:27–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng X, Yang S, Liu J, et al:
Electroacupuncture ameliorates cognitive impairment through
inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral
ischemia-reperfusion injured rats. Mol Med Rep. 7:1516–1522.
2013.PubMed/NCBI
|
|
36
|
Cheng H, Yu J, Jiang Z, et al: Acupuncture
improves cognitive deficits and regulates the brain cell
proliferation of SAMP8 mice. Neurosci Lett. 432:111–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu B, Ma Z, Cheng F, et al: Effects of
electroacupuncture on ethanol-induced impairments of spatial
learning and memory and Fos expression in the hippocampus of rats.
Neurosci Lett. 576:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun L, Jin Y, Dong L, Sumi R, Jahan R and
Li Z: The neuroprotective effects of Coccomyxa gloeobotrydiformis
on the ischemic stroke in a rat model. Int J Biol Sci. 9:811–817.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loh KP, Huang SH, De Silva R, Tan BK and
Zhu YZ: Oxidative stress: Apoptosis in neuronal injury. Curr
Alzheimer Res. 3:327–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Springer JE, Azbill RD, Mark RJ, Begley
JG, Waeg G and Mattson MP: 4-hydroxynonenal, a lipid peroxidation
product, rapidly accumulates following traumatic spinal cord injury
and inhibits glutamate uptake. J Neurochem. 68:2469–2476. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niizuma K, Yoshioka H, Chen H, et al:
Mitochondrial and apoptotic neuronal death signaling pathways in
cerebral ischemia. Biochim Biophys Acta. 1802:92–99. 2010.
View Article : Google Scholar
|
|
42
|
Li X, Han F, Liu D and Shi Y: Changes of
Bax, Bcl-2 and apoptosis in hippocampus in the rat model of
post-traumatic stress disorder. Neurol Res. 32:579–586. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Franke C, Noldner M, Abdel-Kader R, et al:
Bcl-2 upregulation and neuroprotection in guinea pig brain
following chronic simvastatin treatment. Neurobiol Dis. 25:438–445.
2007. View Article : Google Scholar
|
|
44
|
Sriraksa N, Wattanathorn J, Muchimapura S,
Tiamkao S, Brown K and Chaisiwamongkol K: Cognitive-enhancing
effect of quercetin in a rat model of Parkinson's disease induced
by 6-hydroxydopamine. Evid Based Complement Alternat Med.
2012:8232062012. View Article : Google Scholar
|
|
45
|
Kim SS, Jang SA and Seo SR: CREB-mediated
Bcl-2 expression contributes to RCAN1 protection from hydrogen
peroxide-induced neuronal death. J Cell Biochem. 114:1115–1123.
2013. View Article : Google Scholar
|
|
46
|
Royer C, Lucas TF, Lazari MF and Porto CS:
17Beta-estradiol signaling and regulation of proliferation and
apoptosis of rat Sertoli cells. Biol Reprod. 86:1082012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han JH, Kushner SA, Yiu AP, et al:
Neuronal competition and selection during memory formation.
Science. 316:457–460. 2007. View Article : Google Scholar : PubMed/NCBI
|