Introduction
Cerebrovascular diseases are one of the prominent
causes of mortality and permanent disability worldwide, the
occurrence of which is predicted to further increase due to
increasing life expectancies (1).
The pharmaceutical industry has developed numerous potential
neuroprotective drugs for the treatment of stroke, which have been
observed to decrease cerebral damage and enhance the recovery of
motor and cognitive abilities following ischemic insult in
preclinical studies using animal models. However, these results
have not been reproduced in phase III clinical trials and none of
the assessed compounds led to the successful post-ischemic
treatment of patients (2,3). Therefore, further investigation in
this field is required in order to examine novel therapeutic
strategies, which may ameliorate the recovery of post-ischemic
patients.
Stem cells, with self-renewal and pluripotent
differentiation potentials, are a promising source for tissue
regeneration, including neural regeneration (4). Neural stem cells (NSCs) have been
identified in the subventricular zone (SVZ) and dentate gyrus (DG)
of adult brain and these NSCs proliferate and generate neurons
throughout the adult life (5–9). It
is now generally considered that cerebral ischemia results in the
expansion of endogenous NSCs, as has been previously demonstrated
using various ischemia models, including transient global ischemia
(10), transient focal ischemia
(11,12) and permanent focal ischemia
(13). Stroke, induced by middle
cerebral artery occlusion (MCAO), leads to the increased
proliferation of NSCs in SVZ and the formation of neuroblasts,
which migrate towards the damaged striatum, where they
differentiate into mature striatal neurons (12). In addition to replacing lost
neurons, NSCs appear to contribute to regeneration following stroke
by providing neuroprotection and trophic support, which reduces
neuroinflammation and induces remodeling (14,15).
However, the spontaneous proliferation and differentiation of
endogenous NSCs stimulated by stroke is often insufficient to
overcome the neural damage (14).
Therefore, it is important to develop novel strategies to enhance
neurogenesis in order to improve rehabilitation following
stroke.
Acupuncture has been used in traditional Chinese
medicine for >3,000 years as a treatment for numerous diseases,
including stroke. The use of acupuncture has become more prevalent
in Western countries, and has been suggested to have potential
therapeutic benefits for the treatment of cerebral
ischemia-associated disorders (16,17).
A previous meta-analysis confirmed that acupuncture may be
effective in the treatment of post stroke rehabilitation (18). Electroacupuncture (EA) is a form of
acupuncture which involves a small electric current between pairs
of acupuncture needles. EA is considered to augment the use of
regular acupuncture (19) and
several clinical trials have demonstrated that EA treatment
improves limb function following stroke (20,21).
Studies involving murine models have demonstrated that EA treatment
promotes neurological functional recovery through a series of
mechanisms (22). Ke et al
(23) reported that EA treatment
upregulates the expression of brain-derived neurotrophic factor
(BDNF), an important peptide, which supports the growth and
maintenance of brain neurogenesis and facilitates motor recovery
following stroke (23). However,
whether EA treatment promotes neurogenesis through stimulating the
proliferation of NSCs remains to be elucidated.
The present study aimed to examine the effects of EA
treatment on the proliferation and differentiation of NSCs in the
DG area of the adult rat brain following stroke, and to assess the
impact of EA treatment on the expression of Notch1, an important
molecule maintaining the proliferation of stem cells.
Materials and methods
Animal care
The experimental procedure used in the present study
was approved by the Ethics Committee for Animal Experimentation of
Sun Yat-sen University (Guangzhou, China) and was performed
according to the Guidelines for Animal Experimentation of Sun
Yat-sen University. All efforts were made to minimize animal
suffering and the number of animals used. Male specific
pathogen-free (SPF) Sprague-Dawley rats (n=160), weighing 310±30 g,
were provided by the Experimental Animal Center of Sun Yat-sen
University and housed under controlled conditions with a 12 hour
light/dark cycle, temperature of 24±1°C and humidity of 50±5% for
at least 1 week prior to drug treatment or surgery. The rats were
allowed free access to a standard rodent diet and tap water.
Experimental stroke in rats
The rats were anesthetized with an intraperitoneal
injection of sodium pentobarbital (3%; Sigma-Aldrich, St. Louis,
MO, USA) at a dose of 30 mg/kg. Core body temperature was monitored
using a rectal probe (Zhengzhou Haorunqi Electronic Sci-Tech Co.,
Ltd., Henan, China) and were maintained at 37±0.5°C using a heating
lamp and a heating pad (Zhengzhou Haorunqi Electronic Sci-Tech Co.,
Ltd.). The arterial blood gases, pH, PaO2,
PaCO2 and blood pressure were closely monitored via
catheterizing the right femoral artery using a RM-6240BD
physiological minitoring system (Chengdu Instrument Factory,
Sichuan, China). MCAO was achieved using the Intraluminal Filament
method, as previously described (24). Following exposure of the external
carotid artery, the internal carotid artery (ICA) and the
pterygopalatine artery of the ICA, a piece of monofilament nylon
suture (diameter, 280 µm; Shadong Biotek, Beijing, China),
with its tip rounded by gentle heating (diameter, 380±20
µm), was introduced via the lumen of the left external
carotid artery stump and left ICA to embed into the left anterior
cerebral artery, resulting in the occlusion of the right middle
cerebral artery at its origin. Following surgery, the rats were
transferred to their cage, in which the temperature was maintained
at 37°C until the animals were completely conscious. Sham-operated
rats (S group) were manipulated in the same way, however the ICA
was not occluded.
Measurement of neurological deficits
Subsequent to the regaining consciousness,
neurological deficits were preliminarily determined using a
modified Bederson's scoring system (25,26)
as follows: 0, no observable deficit; 1, forelimb flexion; 2,
forelimb flexion with decreased resistance to lateral push; 3,
unidirectional circling; 4, unidirectional circling with decreased
level of consciousness. Rats with a score of 2–3 were selected for
use in the subsequent experiments.
Electroacupuncture treatment
A total of 128 eligible rats with successful MCAO
were randomly assigned into either the electroacupuncture treatment
group (EA group) or control group (M group). In the EA group rats,
stainless acupuncture needles (diameter of 0.3 mm) were inserted
into the acupuncture points Baihui (DU20) and Shuigou (DU26) at a
depth of 2–3 mm. Stimulation was then generated by the EA apparatus
(Model G6805; SMIF, Shanghai, China), and the stimulation
parameters were set as follows: Disperse wave, 4 and 20 Hz;
electric current, 1–2 mA; voltage, 2–4 mV; 15 min of each
treatment; once a day. EA treatment was performed at 72 h following
surgery and continued until the animals were sacrificed for tissue
preparation. The rats in the M group were subjected to the same
manipulation procedure without any electric stimulation.
Behavioral assessment
Behavioral assessments of the rats in the two groups
were performed by an investigator in a blinded-manner. The
assessments consisted of the modified Neurological Severity Score
(mNSS) and Morris water maze. All measurements were completed in a
dedicated behavioral investigation facility during this interval in
order to minimize the environmental impact associated with transfer
between home cage and the test arenas.
The mNSS test was performed at 3, 7, 14 and 21 days
of EA or control treatment. Table
I shows the mNSS scores (27).
Neurological function was graded on a scale of 0 to 18, with 0
indicating a normal score and 18 indicating the maximal deficit
score. The mNSS is a composite of motor, sensory, reflex and
balance tests. In the severity scores of injury, 1 score point is
awarded for the inability to perform the test or for the lack of a
reflex; thus, the higher the score, the more severe the injury
(27).
 | Table IModified Neurological Severity Score
tests and points. |
Table I
Modified Neurological Severity Score
tests and points.
| Test | Points |
|---|
| Motor | |
| Raising rat by
tail | 3 |
| Flexion of
forelimb | 1 |
| Flexion of
hindlimb | 1 |
| Head moved >10°
to vertical axis within 30 sec | 1 |
| Placing rat on
floor (normal=0; maximum=3) | 3 |
| Normal walk | 0 |
| Inability to walk
straight | 1 |
| Circling toward
paretic side | 2 |
| Falls down to
paretic side | 3 |
| Sensory | 2 |
| Placing test
(visual and tactile test) | 1 |
| Proprioceptive
test (deep sensation, pushing paw against table edge to stimulate
limb muscles) | 1 |
| Beam balance
(normal=0; maximum=6) | 6 |
| Balances with
steady posture | 0 |
| Grasps side of
beam | 1 |
| Hugs beam and 1
limb falls down from beam | 2 |
| Hugs beam and 2
limbs fall down from beam, or spins on beam (>60 sec) | 3 |
| Attempts to
balance on beam but falls off (>40 sec) | 4 |
| Attempts to
balance on beam but falls off (>20 sec) | 5 |
| Falls off; no
attempt to balance or hang on to beam (<20 sec) | 6 |
| Reflex absence and
abnormal movements | 4 |
| Pinna reflex (head
shake when auditory meatus is touched) | 1 |
| Corneal reflex
(eye blink when cornea is lightly touched with cotton) | 1 |
| Startle reflex
(motor response to a brief noise from snapping a clipboard
paper) | 1 |
| Seizures,
myoclonus, myodystony | 1 |
| Maximum points | 18 |
The Morris water maze test was used to measure
spatial learning and memory at days 7, 14 and 21 EA or control
treatment, as previously described (28). Each rat was placed individually in
a black water tank (136 cm in diameter and 60 cm in height) in a
well-lit room and filled with water (50 cm height at 27°C). The
tank was visually separated into four quadrants and, in the center
of one quadrant, a plexiglass platform (diameter, 10 cm; Zhengzhou
Haorunqi Electronic Sci-Tech Co., Ltd.) was hidden 2 cm below the
waterline. Numerous extra-maze cues surrounding the maze were fixed
at specific locations and were visible to the rats. Following a
single habituation trial, the rats were trained over 4 days and
received four trials each day. On each trial, the rat had 120 sec
to escape to the submerged platform; rats that failed to escape
were led to the platform and remained there for 30 sec prior to
being removed from the maze and dried off. On the fifth day, a
probe trial was performed following removal of the platform. The
latency of quadrant search, path length and swim speed were
measured by a TSE video tracking system (TSE Systems, Bad Homburg,
Germany) interfaced to a computer on days 3, 7, 14 and 21 of
treatment.
Immunofluorescence confocal
microscopy
In order to track the fate of proliferating cells,
the rats were administered with intraperitoneal (i.p.) injections
of 75 mg/kg body weight bromodeoxyuridine (BrdU; Sigma-Aldrich)
dissolved in phosphate-buffered saline (PBS; Whiga Technology Co.,
Ltd., Guangzhou, Guangdong, China) twice a day for 5 days prior to
sacrifice. At days 3, 7, 14 and 21 of EA or control treatment, rats
(n=5 per experiment subgroup per time-point) were sacrificed
through deep anesthetization with 45 mg/kg sodium pentobarbital and
perfused intracardially with cold PBS and 4% buffered
paraformaldehyde. Brains were paraffin-embedded (Whiga Technology
Co., Ltd.) and sliced into 5-µm thick coronal sections,
starting at 3 mm posterior to the anterior pole. The sections were
then incubated with the mixture of monoclonal mouse antibodies
(1:400) for BrdU (cat. no. MCA2060T; Oxford Biotech, Oxford, UK)
with polyclonal mouse antibodies (1:400) for neuronal nuclei (NeuN;
cat. no. SAB4300883; Sigma-Aldrich) or polyclonal mouse antibodies
(1:10,000) for glial fibrillary acidic protein (GFAP; cat. no.
SAB1405864; Sigma-Aldrich) overnight at 4°C, followed by a mixture
of fluorescein isothiocyanate-conjugated goat anti-rabbit
immunoglobulin (Ig)G and cyanine dye (Cy)3-conjugated goat
anti-mouse IgG (1:100; Jackson Laboratory, Bar Harbor, ME, USA).
The sections were washed in Tris-buffered saline (TBS; 0.2% Triton
X-100 in PBS; Whiga Technology Co., Ltd.) and mounted with
Vectashield mounting medium (Vector Laboratories, Burlingame, CA,
USA). Confocal images were captured using an LSM710 confocal
spectral microscope (magnification, x400; ZEISS, Oberkochen,
Germany). Image analysis was performed using Leica QWin software
(Leica Microsystems, Weltzlar, Germany). The number of cells
showing double immunostaining was estimated by counting cells in
five random fields of the peri-necrotic cortex in five
non-continuous sections.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Following evaluation of neurological deficits, rats
(n=6 per experiment group per time-point) were sacrificed with an
overdose of sodium pentobarbital at days 3, 7, 14 and 21 of EA or
control treatment, as described above. Total RNA was extracted from
the perinecrotic cortex using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. RNA (2 µg) was then reverse transcribed into
first-strand cDNA using M-MLV Reverse Transcriptase (Promega Corp.,
Madison, WI, USA) according to the manufacturer's instructions.
Notch1, Hes1 and GAPDH were amplified by qPCR using the following
primers: Notch1, forward 5′-TCGTGCTCCTGTTCTTTGTG-3′ and reverse
5′-GGGTTCTCTCCGCTTCTTCT-3′; Hes1, forward
5′-GCTGGAGAAGGCAGACATTC-3′ and reverse 5′-GTCACCTCGTTCATGCACTC-3′;
and GAPDH, forward 5′-TGCCACTCAGAAGACTGTGG-3′ and reverse,
5′-TTCAGCTCTGGGATGACCTT-3′ (BGI Tech, Guangdong, China).
Gene-specific amplification was performed in an ABI 7500 real-time
PCR system (Life Technologies, Carlsbad, CA, USA) using 15
µl PCR mix containing 0.5 µl cDNA, 7.5 µl 2x
SYBR Green master mix (Invitrogen Life Technologies) and 200 nM of
the appropriate primers. The mix was preheated at 95°C for 30 sec
and amplified in 45 cycles of 95°C for 5 sec and 60°C for 34 sec.
The resolution curve was measured at 95°C for 15 sec, 60°C for 15
sec and 95°C for 15 sec. The threshold cycle (Ct) value of each
sample was calculated and the relative expression of Notch1 and
Hes1 mRNA was normalized to the GAPDH value (2−ΔCt
method) (29).
Statistical analysis
All values are expressed as mean ± standard
deviation. A repeated measurement analysis of variance was
performed to analyze differences in the behavioral assessment data
and relative mRNA expression levels. Analysis of variance and the
Least Significant Difference test were used to further compare the
differences at each time-point. SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
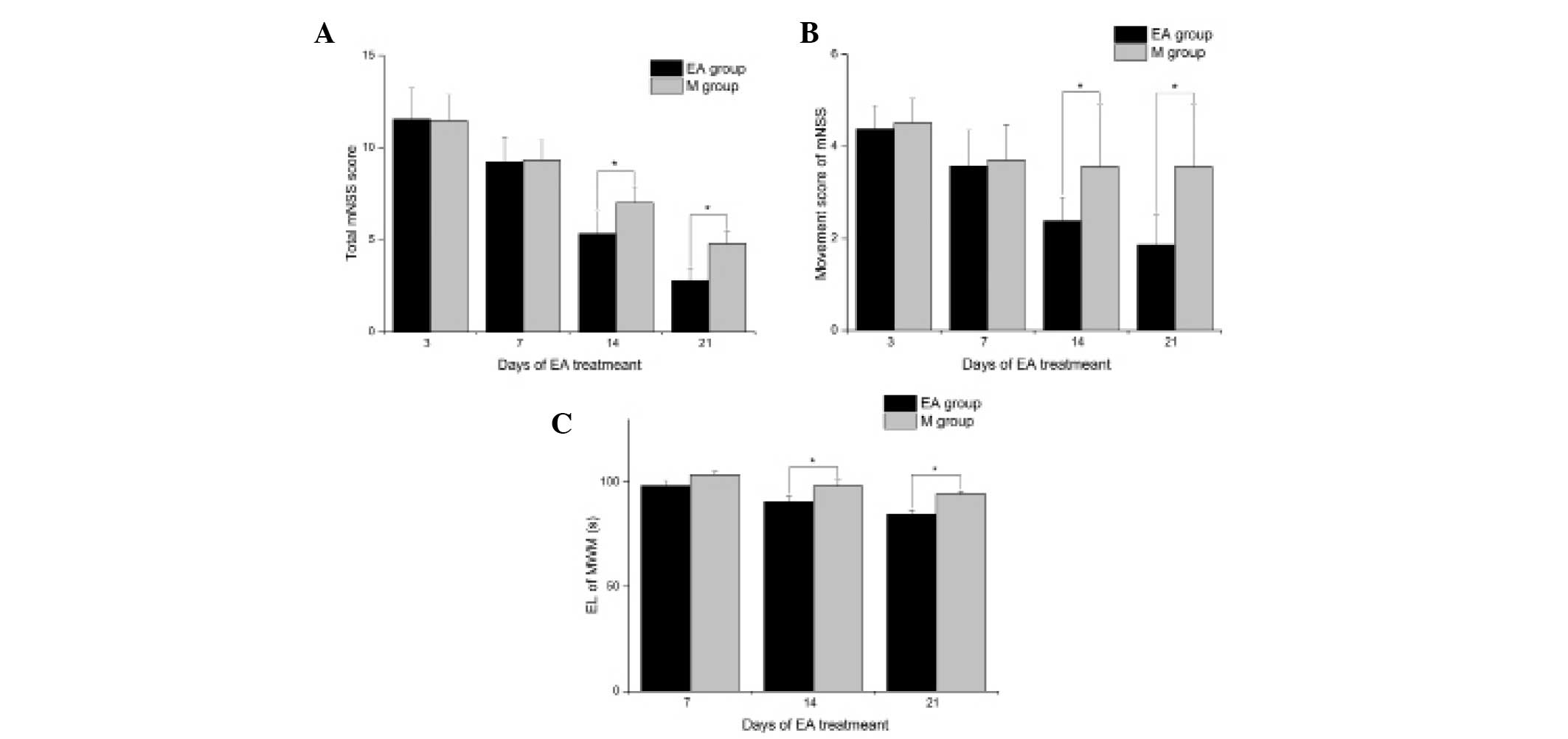
EA treatment improves the functional
recovery of rats following stroke
In order to examine the effects of EA treatment on
the functional recovery of MCAO rats, the mNSS was used to assess
neurological deficits. The mNSS of the rats in the S group were 0
at all time-points. As shown in Fig.
1A, the rats in the EA group had a significantly lower mNSS
compared with that in the M group at 14 (5.33±1.22 and 7.00±0.87,
respectively; P<0.05) and 21 (2.78±0.67 and 4.78±0.67,
respectively; P<0.05) days of treatment. Compared with those in
the M group, rats in EA group only had significant improvement in
the motor test score (P<0.05; Fig.
1B) but not in scores of other tests at 14 and 21 days of
treatment. The cognitive recovery of MCAO rats was further assessed
using the Morris water maze test. As shown in Fig. 1C, rats in the EA group had
significantly decreased escape latency compared with those in the M
group at 14 (90.63±2.62 and 98.13±2.90, respectively; P<0.05)
and 21 (84.63±1.92 and 94.13±1.46, respectively; P<0.05) days of
treatment. These results suggested that EA treatment effectively
improved the neurological dysfunction of cerebral ischemia in rats,
promoting nerve functional recovery.
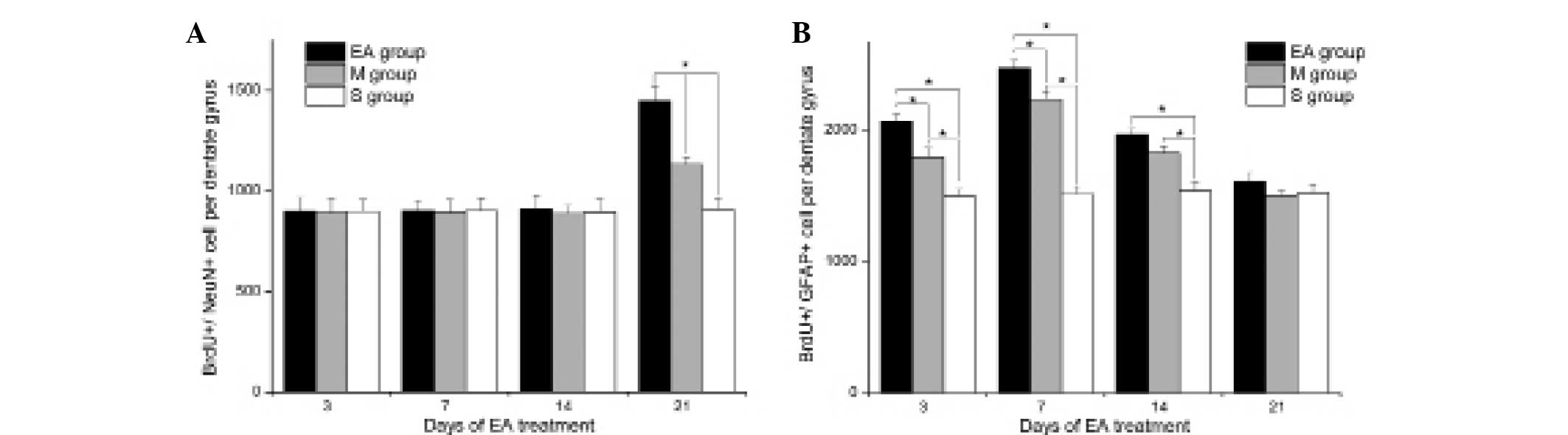
EA treatment promotes the proliferation
and differentiation of NSCs following stroke
BrdU/GFAP and BrdU/NeuN sdouble-labeling
immunofluorescence was performed to evaluate the proliferation and
differentiation of NSCs following MCAO in each group. As shown in
Figs. 2 and 3, MCAO rats had significantly higher
frequencies of BrdU+/GFAP+ and
BrdU+/NeuN+ cells in the DG area compared
with those in the S group (P<0.05), indicating that the
proliferation and differentiation of NSCs was stimulated by
ischemic stress. In addition, EA treatment significantly elevated
the frequencies of BrdU+/GFAP+ cells at 3, 7
and 14 days of treatment compared with the control treatment
(P<0.05), indicating the enhanced effects of EA treatment on the
differentiation of NSCs into glia cells. Furthermore, EA treatment
enhanced the neuronal differentiation of NSCs, as indicated by
increased frequencies of BrdU+/NeuN+ cells in
the EA group, compared with that of the M group at 21 days of
treatment (P<0.05).
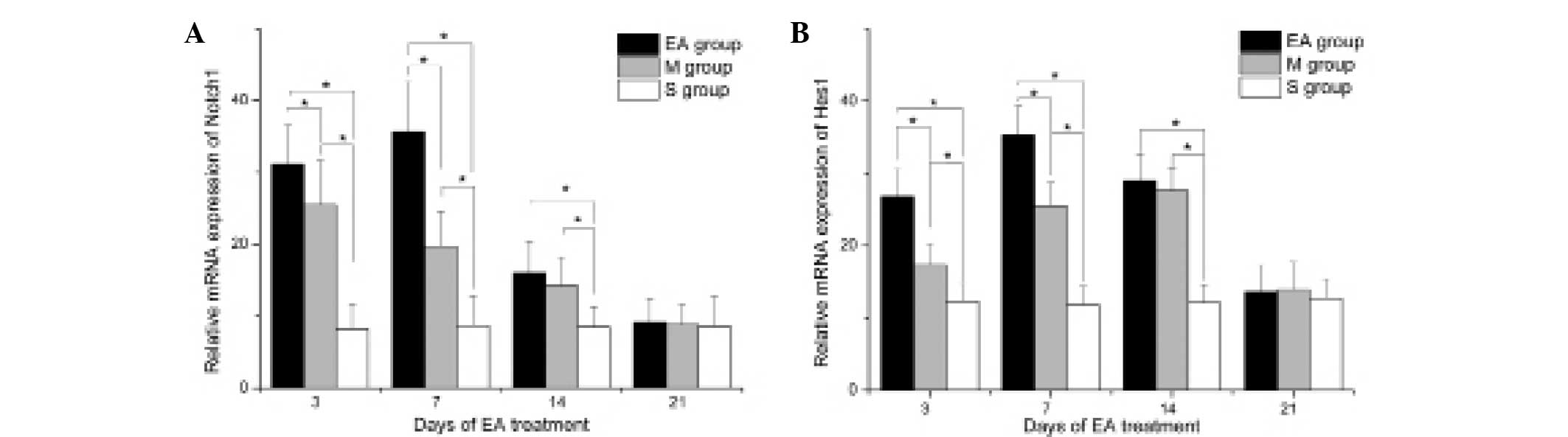
EA treatment enhances the expression of
Hotch1 and Hes1 following stroke
In order to examine the underlying molecular
mechanisms by which EA treatment promoted the proliferation and
differentiation of NSCs, the expression of Notch1 and its target
molecule, Hes1, were analyzed by RT-qPCR. The results revealed that
MCAO stress significantly stimulated the expression of Notch1 and
Hes1 in the DG area compared with that of the S group at 3, 7 and
14 days of treatment (P<0.05; Fig.
4).
Discussion
In the present study, the effects of EA treatment on
the recovery of neurological factions and on the proliferation and
differentiation of NSCs following stroke were investigated. The
results revealed that EA treatment significantly improved the
recovery of neural function in rats with MCAO. In addition, to the
best of our knowledge, the present study was the first to
demonstrate that EA treatment significantly promoted the
proliferation and differentiation of NSCs into glial cells and
neurons, possibly through the activation of Notch1 signaling
pathways.
Previous studies have demonstrated that acupuncture
or EA treatment promoted the recovery of neurological functions
following stroke (22). In
accordance with these findings, the present study confirmed that EA
treatment significantly improved the mNSS and the Morris water maze
performance of rats at 14 and 21 days of treatment following MCAO.
It has been reported that EA treatment exerted its neuroprotective
effects through a series of mechanisms, including reducing the
apoptosis of neural cells and inhibiting the inflammatory responses
(22). In addition, EA treatment
has been found to enhance neurogenesis via the upregulation of
neurotropic factors, including BDNF (30). However, the impact of EA treatment
on the proliferation and differentiation of NSCs remains to be
elucidated.
NSCs in the DG area of hippocampus are important in
neurogenesis (31). In normal
physiological conditions, these cells gradually generate neurons to
renew the granular layer of the cortex throughout adult life.
Pathological stress, including ischemic insult, markedly stimulates
the neurogenesis of NSCs in the DG area. These characteristics
suggest NSCs may offer a promising therapeutic strategy in stroke
treatment. However, difficulties in the availability of abundant
sources of NSCs and issues in legislation and ethics impede the
clinical application of NSC transplantation treatment for stroke
(32). Therefore, enhancing the
expansion and differentiation of endogenous NSCs offers a promising
alternative strategy for NSC treatment. In the present study, EA
treatment was found to significantly promote the proliferation and
differentiation of NSCs into glial cells and neurons, as indicated
by increased BrdU+/GFAP+ and
BrdU+/NeuN+ cell frequencies, respectively.
However, in line with previous studies, the proliferation and
differentiation of NSCs in the DG area in the M or EA groups only
lasted for 3 weeks. These data highlight the requirement for the
development of novel strategies to extend the duration and
intensity of neurogenesis following stroke.
Notch1 is an essential regulator of NSC maintenance
and self-renewal during development. In addition, components of the
Notch signaling pathway are expressed in neuroproliferative regions
of the postnatal brain (33).
Previous studies have reported that Notch signaling may augment the
expansion and differentiation of adult NSCs following stroke.
Activated Notch1 (NICD) and its downstream transcriptional targets,
including Hes1, have been identified in SVZ cells following
ischemic injury (34–36). The expression levels of NICD and
Hes1 are significantly upregulated in the SVZ at 4 h following
focal cerebral ischemia. In addition, Notch1 signaling activation
enhances SVZ cell proliferation, whereas inhibition of Notch1
signaling results in the reduced proliferation of cells in the SVZ.
It has also been reported that, during differentiation, the
expression levels of Notch and Hes1 are downregulated in neural
progenitor cells following ischemia, which is associated with a
significant increase in the size of the neuronal population. This
suggests that Notch signaling is involved in mediating adult SVZ
neural progenitor cell proliferation and differentiation following
stroke (37). In line with these
findings, the present study confirmed that stroke significantly
increased the expression levels of Notch1 and Hes1. In addition, EA
treatment significantly enhanced the expression levels of Notch1
and Hes1 stimulated by ischemia insult, which may, at least in
part, account for the higher frequencies of
BrdU+/GFAP+ and
BrdU+/NeuN+ cells in the DG area of rats in
the EA group.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that EA treatment
promoted the neurogenesis of NSCs through upregulation of the
expression of Notch1 following stroke. These findings contribute to
the current understanding of the mechanisms by which acupuncture
promotes the recovery of neurological functions following stroke
and may provide support for novel integrative therapeutic
strategies for stroke treatment.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81171863).
References
|
1
|
Kavanagh S, Knapp M and Patel A: Costs and
disability among stroke patients. J Public Health Med. 21:385–394.
1999. View Article : Google Scholar
|
|
2
|
De Keyser J, Sulter G and Luiten PG:
Clinical trials with neuro-protective drugs in acute ischaemic
stroke: are we doing the right thing? Trends Neurosci. 22:535–540.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liebeskind DS and Kasner SE:
Neuroprotection for ischaemic stroke: an unattainable goal? CNS
Drugs. 15:165–174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gage FH and Temple S: Neural stem cells:
generating and regenerating the brain. Neuron. 80:588–601. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiss S, Reynolds BA, Vescovi AL, Morshead
C, Craig CG and van der Kooy D: Is there a neural stem cell in the
mammalian forebrain? Trends Neurosci. 19:387–393. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McKay R: Stem cells in the central nervous
system. Science. 276:66–71. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Solway K, Messing RO and Sharp FR:
Increased neuro-genesis in the dentate gyrus after transient global
ischemia in gerbils. J Neurosci. 18:7768–7778. 1998.PubMed/NCBI
|
|
11
|
Jin K, Minami M, Lan JQ, et al:
Neurogenesis in dentate subgranular zone and rostral subventricular
zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci
USA. 98:4710–4715. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arvidsson A, Collin T, Kirik D, Kokaia Z
and Lindvall O: Neuronal replacement from endogenous precursors in
the adult brain after stroke. Nat Med. 8:963–970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takasawa K, Kitagawa K, Yagita Y, et al:
Increased proliferation of neural progenitor cells but reduced
survival of newborn cells in the contralateral hippocampus after
focal cerebral ischemia in rats. J Cereb Blood Flow Metab.
22:299–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lie DC, Song H, Colamarino SA, Ming GL and
Gage FH: Neurogenesis in the adult brain: new strategies for
central nervous system diseases. Annu Rev Pharmacol Toxicol.
44:399–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ceniceros S and Brown GR: Acupuncture: a
review of its history, theories and indications. South Med J.
91:1121–1125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang R, Huang ZN and Cheng JS:
Anticonvulsion effect of acupuncture might be related to the
decrease of neuronal and inducible nitric oxide synthases. Acupunct
Electrother Res. 24:161–167. 1999. View Article : Google Scholar
|
|
18
|
Junhua Z, Menniti-Ippolito F, Xiumei G, et
al: Complex traditional Chinese medicine for poststroke motor
dysfunction: a systematic review. Stroke. 40:2797–2804. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ulett GA, Han S and Han JS:
Electroacupuncture: mechanisms and clinical application. Biol
Psychiatry. 44:129–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong RK, Ng MF and Li LS: Effectiveness of
gait training using an electromechanical gait trainer, with and
without functional electric stimulation, in subacute stroke: a
randomized controlled trial. Arch Phys Med Rehabil. 87:1298–1304.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan T, Hui-Chan CW and Li LS: Functional
electrical stimulation improves motor recovery of the lower
extremity and walking ability of subjects with first acute stroke:
a randomized placebo-controlled trial. Stroke. 36:80–85. 2005.
View Article : Google Scholar
|
|
22
|
Ho TJ, Chan TM, Ho LI, Lai CY, Lin CH,
Macdonald I, Harn HJ, Lin JG, Lin SZ and Chen YH: The possible role
of stem cells in acupuncture treatment for neurodegenerative
diseases: a literature review of basic studies. Cell Transplant.
23:559–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke Z, Yip SP, Li L, Zheng XX and Tong KY:
The effects of voluntary, involuntary and forced exercises on
brain-derived neurotrophic factor and motor function recovery: a
rat brain ischemia model. PLoS One. 6:e166432011. View Article : Google Scholar
|
|
24
|
Xi GM, Wang HQ, He GH, Huang CF and Wei
GY: Evaluation of murine models of permanent focal cerebral
ischemia. Chin Med J (Engl). 117:389–394. 2004.
|
|
25
|
Yang Y, Li Q, Miyashita H, Howlett W,
Siddiqui M and Shuaib A: Usefulness of postischemic thrombolysis
with or without neuroprotection in a focal embolic model of
cerebral ischemia. J Neurosurg. 92:841–847. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Li Q and Shuaib A: Neuroprotection
by 2-h postischemia administration of two free radical scavengers,
alpha-phenyl-n-tert-butyl-nitrone (PBN) and
N-tert-butyl-(2-sulfophenyl)-nitrone (S-PBN), in rats subjected to
focal embolic cerebral ischemia. Exp Neurol. 163:39–45. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Sanberg PR, Li Y, et al:
Intravenous administration of human umbilical cord blood reduces
behavioral deficits after stroke in rats. Stroke. 32:2682–2688.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohapel P, Mundt-Petersen K, Brundin P and
Frielingsdorf H: Working memory training decreases hippocampal
neurogenesis. Neuroscience. 142:609–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Pan K, Li S, Xia J, Wang W, Chen
J, Zhao J, Lü L, Wang D, Pan Q, et al: Decreased expression of
V-set and immunoglobulin domain containing 1 (VSIG1) is associated
with poor prognosis in primary gastric cancer. J Surg Oncol.
106:286–293. 2012. View Article : Google Scholar
|
|
30
|
Kim MW, Chung YC, Jung HC, et al:
Electroacupuncture enhances motor recovery performance with
brain-derived neurotrophic factor expression in rats with cerebral
infarction. Acupunct Med. 30:222–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu Y, Janoschka S and Ge S: Neurogenesis
and hippocampal plasticity in adult brain. Curr Top Behav Neurosci.
15:31–48. 2013. View Article : Google Scholar
|
|
32
|
Grisolía JS: CNS stem cell
transplantation: clinical and ethical perspectives. Brain Res Bull.
57:823–826. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piccin D, Yu F and Morshead CM: Notch
signaling imparts and preserves neural stem characteristics in the
adult brain. Stem Cells Dev. 22:1541–1550. 2013. View Article : Google Scholar
|
|
34
|
Pujia F, Serrao M, Brienza M, et al:
Effects of a selective serotonin reuptake inhibitor escitalopram on
the cutaneous silent period: A randomized controlled study in
healthy volunteers. Neurosci Lett. 566:17–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo YJ, Zhang ZJ, Wang SH, Sui YX and Sun
Y: Notch1 signaling, hippocampal neurogenesis and behavioral
responses to chronic unpredicted mild stress in adult ischemic
rats. Prog Neuropsychopharmacol Biol Psychiatry. 33:688–694. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Zacharek A, Li A, et al:
Atorvastatin promotes presenilin-1 expression and Notch1 activity
and increases neural progenitor cell proliferation after stroke.
Stroke. 39:220–226. 2008. View Article : Google Scholar
|
|
37
|
Wang L, Chopp M, Zhang RL, et al: The
Notch pathway mediates expansion of a progenitor pool and neuronal
differentiation in adult neural progenitor cells after stroke.
Neuroscience. 158:1356–1363. 2009. View Article : Google Scholar :
|