Introduction
The poor regenerative capacity of injured central
nervous system (CNS) axons leads to permanent neurological deficits
following brain, spinal cord or optic nerve lesions. Recent studies
regarding the optic nerve revealed that stimulation of the cytokine
or mammalian target of rapamycin signaling pathways potently
enhances sprouting and regeneration of injured retinal ganglion
cell (RGC) axons in adult mice, however, does not allow the
majority of axons to reach their primary cerebral targets.
Furthermore, recent studies have revealed axon navigation defects
in the optic nerve and at the optic chiasm as a result of strong
growth stimulation (1).
As with other CNS tracts in higher vertebrates, the
optic nerve is unable to regenerate following traumatic injury.
Optic nerve crush (ONC) is a popular model for investigating this
phenomenon, and establishing novel regenerative strategies and
potential treatment methods. The adeno-associated virus, serotype 2
was shown to display specific tropism to, and a high rate of
infectivity for, RGCs; allowing researchers to target and
manipulate specific intracellular signaling molecules involved in
CNS neuron survival and axonal regeneration in vivo
(2). The weak intrinsic capacity
of adult neurons to reactivate a growth program following injury
was proposed to be a significant impediment to axonal regrowth
(3). Indeed, following birth,
intrinsic growth repressors, for example, those of the Kruppel-like
factor transcription factor family, are upregulated in the
developing retina, while growth-inducing transcription factors are
downregulated in RGCs and other CNS neurons (4). Similarly, the neuronal tissue
environment is growth enhancing during development, and switches to
being predominantly growth inhibitory in the adult brain and spinal
cord (5,6). The presence of growth-repressing
molecules in the CNS tissue and within the neurons, as well as the
lack of adequate stimulation and growth responses by adult neurons
are currently proposed to be the predominant causes of axon
regeneration failure (6).
SIRT1 belongs to a family of class III histone
deacetylases, originally identified as significant to calorie
restriction and longevity (7).
SIRT1 activity is dependent on NAD+ as a co-activator
(8), and SIRT1 generally localizes
to the nucleus, although it may translocate to the cytoplasm and
catalyzes deacetylation of various protein targets in addition to
histones (9). Numerous SIRT1
substrates are transcription factors, including peroxisome
proliferator-activated receptor γ coactivator 1α, forkhead box O3
(FOXO), and hypoxia-inducible factor, whose activities are altered
by SIRT1-mediated deacetylation, leading to altered expression
levels of target genes (9–11). SIRT1 has been associated with
various biologic processes, including oxidative stress, gene
silencing and DNA repair, in addition to senescence, neurogenesis,
circadian rhythms, neuroendocrine signals and dendritic branching,
all of which affect the NS during normal physiologic functioning,
as well as with aging and during pathologic processes (12). Within the brain, SIRT1 is crucial
for normal cognitive function (13), and its activation protects against
neurodegenerative diseases, including Parkinson's, Alzheimer's and
Huntington's disease, as observed in animal models (14–16).
In addition to its role in neurons, Sirt1 contributes to blood
vessel growth during development via regulation of Notch signaling
(17) and FOXO1 (18). Chen et al (19) observed that SIRT1 is upregulated in
RGCs in the vaso-obliteration zone of ischemic neuronal retinas,
and conditional depletion of SIRT1 in retinal neurons significantly
impaired vascular regrowth into the vascular zone and precipitated
pathologic neovascularization in retinopathy. These results
indicate that SIRT1 is a critical, stress-induced,
metabolically-dependent protective mechanism in retinopathy
(20).
In the current study the mechanism of optic nerve
injury, and the subsequent regeneration and repair associated with
SIRT1-regulated lipid metabolism was investigated. In addition, the
efficacy of resveratrol treatment on optic nerve injury was
assessed. It is hypothesized that a balanced growth stimulatory
treatment, combined with guidance factors and suppression of local
growth inhibitory factors, is required to obtain full regeneration
of the optic nerve following injury.
Materials and methods
Animals
All animal procedures adhered strictly to the
guidelines of the Association for Research in the Vision and
Ophthalmology Statement on the Use of Animals in Ophthalmic and
Visual Research, and were performed according to approved protocols
by the Board of Animal Welfare at the Chinese PLA General Hospital
(Beijing, China).
Adult male Sprague Dawley rats (n=70; weight,
280–320 g; (Sino-British SIPPR/BK Lab Animal Ltd., Shanghai, China)
were used in the present study, and the results of eye and fundus
examinations were normal. The rats were housed in a commercial
cage. They were maintained under a 12-h light/dark cycle at an
ambient temperature (22±1° C) and given free access to food. Using
the random number table method for grouping, 10 rats were assigned
to the control group, while 30 rats formed the model group [treated
with phosphate-buffered saline (PBS; Invitrogen Life Technologies,
Inc., Carlsbad, CA, USA)] and 30 rats formed the Res group (treated
with resveratrol; Sigma-Aldrich, St. Louis, MO, USA). Each of the
model and Res groups were comprised of three subgroups (n=10) of
rats that were treated for either seven, 14 or 21 days.
Model preparation and treatment
process
Following intra-peritoneal injection of 50 mg/kg
ketamine (Sigma-Aldrich) to anesthetize the rats, the optic nerve
was fully exposed. Then reverse tweezers with an optic ball
(cross-locking tweezers, bent & straight reverse action,
stainless steel, 4–3/4″ 2pc; size, 0.5–1.0 mm) were used to clamp
the optic nerve and cause incomplete injury of rats. In the
resveratrol-treated group following postoperative injury, an
intraperitoneal injection of 30 mg/kg resveratrol was administered
every 12 h. The same protocol was followed in the control group,
however, the intraperitoneal injection of resveratrol was replaced
with PBS (2.5 µl/g). The rats were sacrificed seven, 14 or
21 days after treatment, and the optic nerves and retinas were
excised.
Retina dissection, staining and
imaging
The rats were anesthetized with Avertin (0.2 ml/10
g; Sigma-Aldrich) and sacrificed with CO2. The eyes were
enucleated, fixed in 4% paraformaldehyde (Novus Biologicals, LLC,
Littleton, CO, USA) in PBS for 1 h and dissected to isolate the
retina. The retinas without retina pigment epithelium were rapidly
(<5 min) dissected from the eyeball on ice, according to the
method of Glowinski and lverse (1). Each sample was immediately labeled
and then flash-frozen in liquid nitrogen and stored at −80° C for
further analysis. The retinas were subsequently stained overnight
with Isolectin GS-IB4 from Griffonia
simplicifolia, Alexa Fluor® 594 Conjugate (dilution,
1:100; Invitrogen Life Technologies) in PBS with 1 mM
CaCl2 to visualize the blood vessels. After 2-h washes
in PBS, the retinas were whole-mounted (photoreceptor side down)
onto Superfrost™ Plus microscope slides (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using SlowFade Antifade reagent (Invitrogen
Life Technologies). Whole-mounted retinas were imaged using an
AxioObserver.Z1 microscope (magnification, ×5; Carls Zeiss AG,
Oberkochen, Germany) and merged using AxioVision 4.6.3.0 software
(Carl Zeiss AG) to produce images of the entire retinal
vasculature. Estimates of RGC counts were obtained using a
previously published model (20),
which combines estimates of RGC numbers (from standard automated
perimetry sensitivity thresholds and retinal nerve fiber layer
thickness measurements) with spectral domain optical coherence
tomography.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared from retinal cells using the
RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) and subjected to
RT by SuperScriptH III reverse transcriptase (Invitrogen Life
Technologies) according to the manufacturer's instructions. A
hot-start at 95° C for 5 min was followed by 40 cycles of
denaturation at 95° C for 15 sec, annealing of the primers at 60° C
for 30 sec and elongation at 72° C for 30 sec, using an ABI 7500
Fast Real-Time PCR system (Invitrogen Life Technologies). Phire Hot
Start II DNA Polymerase (Thermo Fisher Scientific, Inc.)
incorporates a dsDNA-binding domain that allows short extension
times (10–15 sec/kb), helps improve yields, and can increase the
fidelity by two-fold compared to that of Taq DNA polymerase. In
addition, the unique hot start technology allows complete
re-activation of the enzyme in 'zero-time' at standard cycling
temperatures. This combination of features makes Phire Hot Start II
DNA Polymerase an ideal solution for routine and high-throughput
PCR applications. The primer sequences used were as follows:
Upstream, 5′-TGTACGACGACGAAGACGACGAC-3′ and downstream,
5′-GGTTATCTCGGTACCCAATCG-3′ for SIRT1; upstream,
5′-GCTGGCTCACCTTTCCAG-3′ and downstream,
5′-GGCCTCAGTTATTCTGTCTTGC-3′ for SREBP2; and upstream,
5′-CTTGAAGTTCATGGTGATCAGTG-3′ and downstream,
5′-CCATTTTCAACTGGCAGGA-3′ for HMGCR. Data were normalized to GAPDH
and represented as the average value of three independent duplicate
experiments.
Western blot analysis
Retinal cell lysates were prepared from cells using
a lysis buffer [50 mM Tris (pH 8), 150 mM NaCl, 0.02%
NaN3, 0.1% SDS, 1% NP-40 and 0.5% sodium deoxycholate;
Upstate Biotechnology, Lake Placid, NY, USA] containing 1 mM
phenylmethylsulfonyl fluoride (Sigma-Aldrich) and protease
inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The
protein concentration was determined by Bradford assay using the
Coomassie Plus Protein Reagent (Invitrogen Life Technologies) and
western blot analysis was performed using the Novex®
system (Invitrogen Life Technologies).
Blots were incubated with primary antibodies
anti-SIRT1 [cat no. sc-15404; H-300; rabbit polyclonal
immunoglobulin (Ig)G], anti-SREBP-2 (cat no. sc-13552; 1C6; mouse
monoclonal IgG1) or anti-HMGCR (cat no. sc-27578; C-18;
goat polyclonal IgG) (all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4° C overnight (dilution, 1:1,000) and washed
with PBS three times. Subsequently, blots were incubated with
horseradish peroxidase-conjugated secondary antibodies (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) at room temperature
for 1 h (dilution, 1:5,000) and washed with PBS three times.
Protein bands were detected using Enhanced Chemiluminescence
Western Blotting Detection Reagent (GE Healthcare
Bio-Sciences).
Cholesterol level quantification in the
optic nerve
The cholesterol level was determined in the optic
nerve tissues using the Cholesterol/Cholesteryl Ester Detection kit
(Abcam, Cambridge, MA, USA) by oxidase-peroxidase enzymatic assay
according to the manufacturer's instructions. Briefly, 10 µl
suspension liquid of optic nerve tissues and 1 ml working fluid
(oxidase peroxidase enzyme in cholesterol assay buffer) were
incubated at 37° C in a water bath for 10 min, and then the
absorbance value of cholesterol standard pipe (A) and samples (B)
were determined under the spectrophotometer (Mithras LB 940;
Berthold Technologies, Bad Wildbad, Germany)at a wavelength of 500
nm. The following formula was used to calculate the content of
cholesterol in every 10 g of tissue protein: Cholesterol level
(mg/10 g tissue protein) = [(A/B) × concentration of standard fluid
x 150/weight of sample] × 10.
Statistical analysis
Continuous normally distributed variables were
represented graphically as the mean ± standard deviation. For
statistical comparison of quantitative data between groups,
analysis of variance (ANOVA) or a two-tailed Student's t-test was
performed. To determine differences between groups that were not
normally distributed, medians were compared using Kruskal-Wallis
ANOVA. The χ2 test was used when necessary for
qualitative data. The degree of association between variables was
assessed using Spearman's non-parametric correlation. All
statistical analyses were conducted using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Effective establishment of the rat model
of optic nerve injury
Following excision, the rats' wounds healed well and
there was no inflammatory response. The corneas were transparent
and there were no cases of traumatic cataract. Furthermore, no
vitreous inflammatory reaction was observed and there was no blood
in the vitreous cavity, retinal bleeding or detachment of the
retina as a result of the surgery. Mydriasis was apparent following
surgery (diameter, of 2–4 mm), indicated by a direct (−) and
indirect light reflex (+), which indicated relative afferent
pupillary defect. These evaluation indexes indicated the successful
creation of the rat model of optic nerve injury.
Morphological changes of the retina
The ganglion cell layer, and inner and outer nuclear
layers of the retina of the normal control rats, from outside to
inside, were dense and arranged in parallel. Seven days after optic
nerve injury, the cell nuclei of the ganglion cell layer exhibited
scattered distribution, shrinkage of the nuclei volume, and
chromatin margination and condensation. The inner and outer nuclear
layers were thinning and the cell arrangement was disordered.
However, no marked changes were observed in the rats treated with
resveratrol. At 14 days after optic nerve injury, the cell nuclei
count in the ganglion cell layer decreased markedly, and
demonstrated a sparse arrangement and increased nuclear
condensation. The inner and outer nuclear layers had become
thinner. In the rats treated with resveratrol, the number of large
and stained nuclei of the gangliocytes decreased, while the nuclei
of the gangliocytes exhibited slight changes in size or number.
After 21 days, the cell count in each layer decreased and few
nuclei were observed in the gangliocytes. Chromatin margination,
cavitation of the RGCs and glial cell infiltration were observed.
The cell count in the inner and outer nuclear layer decreased.
However, the extent of damage was relatively small in the
resveratrol-treated rats (Fig.
1).
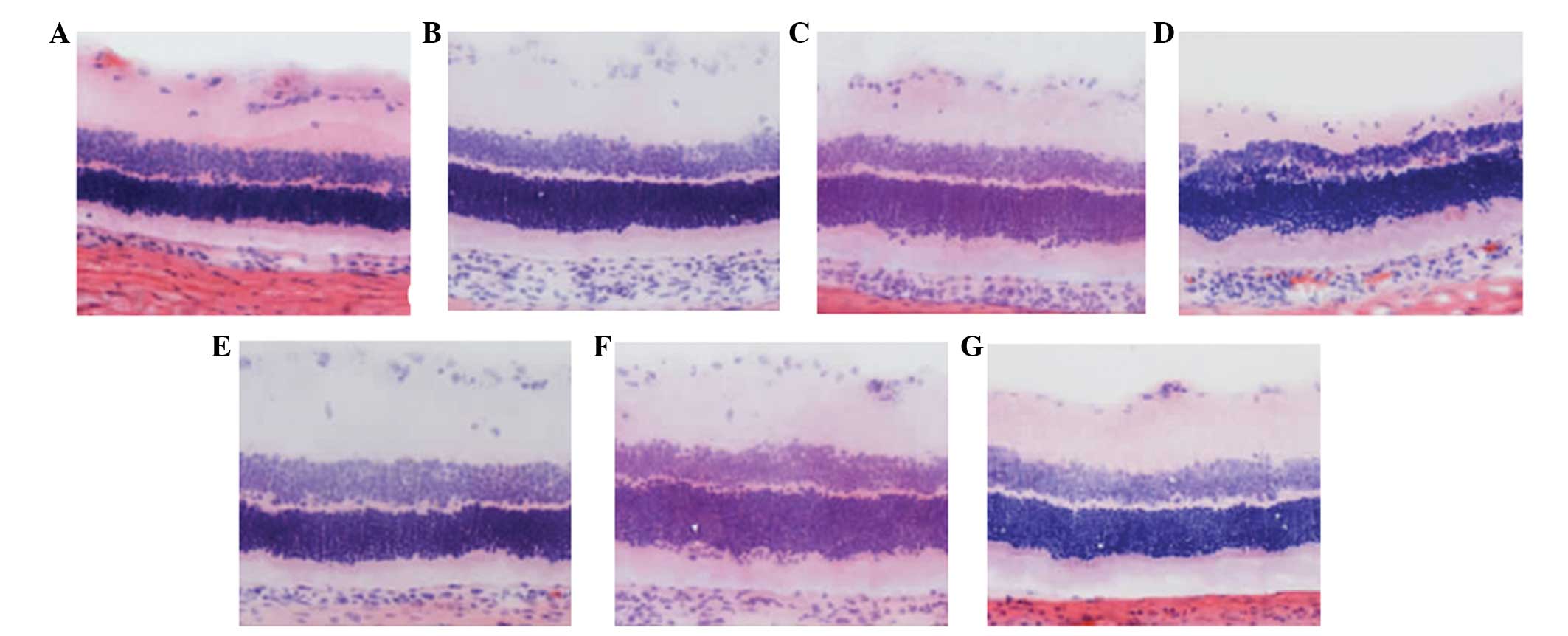 | Figure 1Morphological changes in the retina
(magnification, ×400; hematoxylin and eosin staining). (A) In the
normal control rats, the ganglial cell layer as well as the inner
or outer nuclear layers of the retinas were dense and arranged in
parallel from the exterior to the interior. (B) Seven days after
optic nerve injury, the cell nuclei of the ganglion cell layer
exhibited a scattered distribution, volume shrinkage, and patterns
of chromatin margination and condensation were observed. The inner
and outer nuclear layers were thinning. (C) At 14 days after optic
nerve injury, the cell nuclei count in the ganglion cell layer
decreased markedly and demonstrated a sparse arrangement. A greater
number of condensed nuclei was observed. (D) After 21 days, the
cell count in each layer had decreased and ganglial cells rarely
contained nuclei. In addition, chromatin margination, cavitation of
ganglion cells and infiltration of glial cells was apparent. The
cell count of the inner and outer nuclear layer decreased. The
inner and outer nuclear layers were thinning, and the cell
arrangement was disordered. (E) No marked changes were apparent in
the rats that were treated with resveratrol. (F) The quantity of
large and stained gangliocytic nuclei decreased and chromatin
margination was observed in the rats treated with resveratrol. (G)
The extent of damage in rats treated with resveratrol was
relatively small. |
Resveratrol restores RGC number
The number of surviving RGCs in the rat models
significantly reduced, in a time-dependent manner, when compared
with those in the control group at each time point (P<0.01). In
the resveratrol-treated group, the number of surviving RGCs
decreased marginally at each time point, in a time-dependent
manner, when compared with the control group (P<0.01). The
differences between the resveratrol group and the control group
were statistically significant at each time point (P<0.01;
Table I). This indicated that the
number of surviving RGCs in rats with optic nerve damage was
restored by treatment with resveratrol.
 | Table INumber of RGCs at each time point. |
Table I
Number of RGCs at each time point.
| Group | n | Number of RGCs
|
|---|
| 7 days | 14 days | 21 days |
|---|
| Control | 10 | 33.18±5.02 | 32.47±4.38 | 34.65±5.18 |
| Resveratrol | 30 | 29.46±3.28a | 25.19±2.54a | 22.66±1.87a |
| Model | 30 | 17.31±3.18 | 14.55±2.28 | 13.62±2.88 |
Cholesterol levels in RGCs are restored
by resveratrol treatment
The levels of cholesterol in the RGCs of the model
rats reduced significantly and in a time-dependent manner when
compared with those of the control group at each time point
(P<0.01). The cholesterol levels of the rats in the resveratrol
group decreased marginally at each time point and in a
time-dependent manner compared with the control group (P<0.05).
The differences between the resveratrol group and the model group
were statistically significant at each time point (P<0.01).
Thus, cholesterol levels in the RGCs of rat models of optic nerve
damage were restored by treatment with resveratrol (Table II).
 | Table IICholesterol levels in RGCs. |
Table II
Cholesterol levels in RGCs.
| Group | n | Cholesterol levels in
RGCs (mg/10 g tissue protein)
|
|---|
| 7 days | 14 days | 21 days |
|---|
| Control | 10 | 102.26±4.23 | 108.35±7.33 | 110.79±6.25 |
| Resveratrol | 30 | 87.54±8.63a | 72.15±6.38a | 64.38±5.14a |
| Model | 30 | 54.21±4.67b,c | 43.59±3.96b,c | 32.65±3.12b,c |
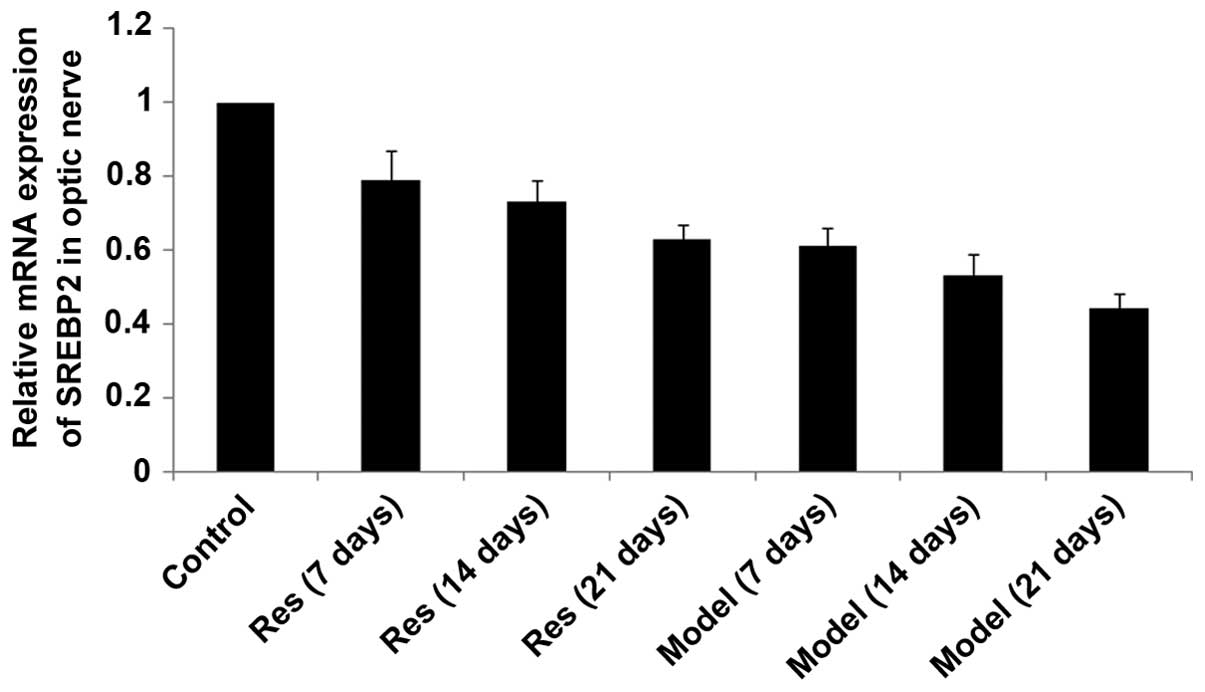
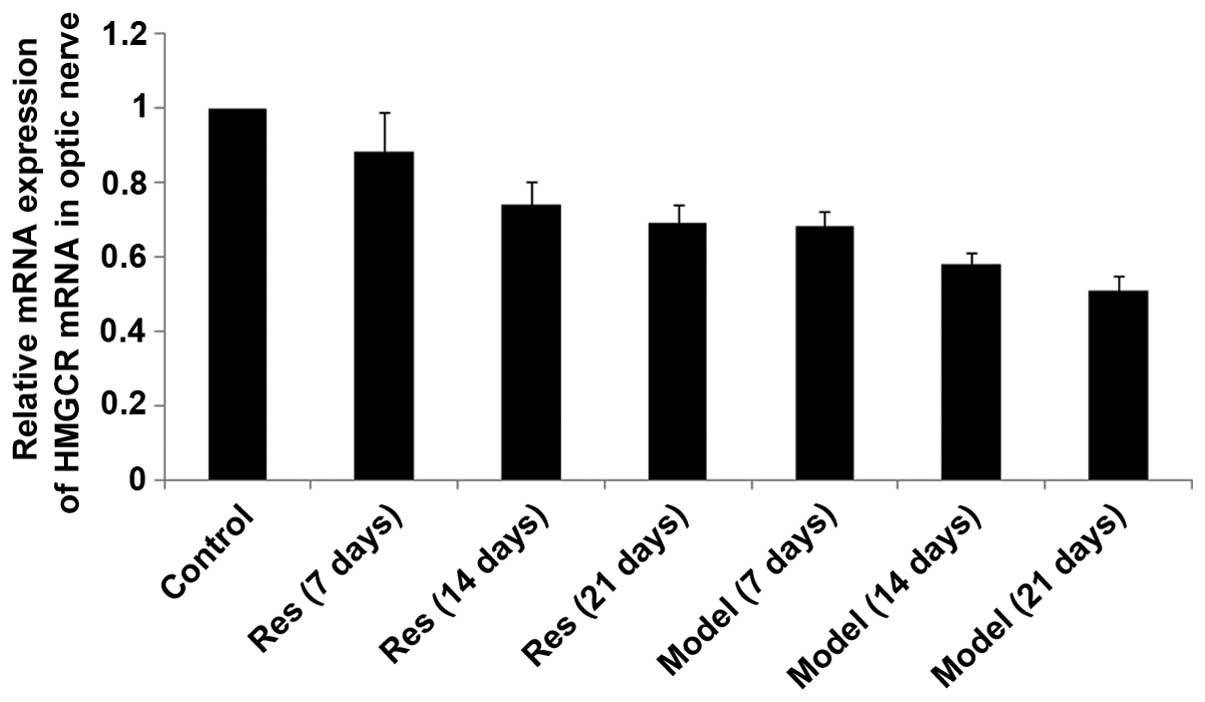
SIRT1, SREBP-2 and HMGCR mRNA levels
decrease in rat models of optic nerve injury
The expression levels of SIRT1, SREBP-2 and HMGCR
mRNA (Figs. 2Figure 3–4, respectively) in the rat models of
optic nerve injury reduced significantly, and in a time-dependent
manner, at each time point when compared with the control group
(P<0.01). The expression levels of SIRT1, SREBP-2 and HMGCR mRNA
(Figs. 2Figure 3–4, respectively) in the resveratrol group
decreased marginally, and in a time-dependent manner, at each time
point when compared with the normal group (P<0.05). The
differences between the resveratrol group and the model group were
statistically significant at each time point (P<0.01).
Therefore, treatment with resveratrol restored the expression
levels of SIRT1, SREBP-2 and HMGCR mRNA (Figs. 2Figure 3–4, respectively) in rat models of optic
nerve damage.
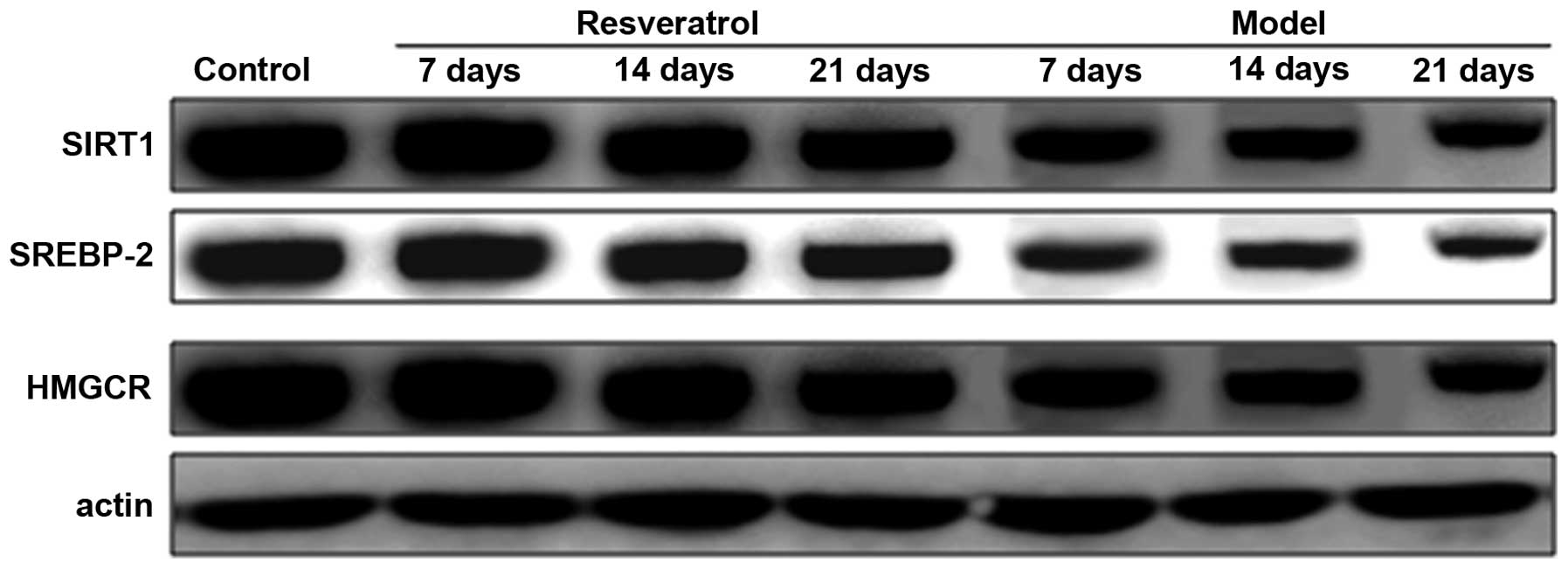
Levels of SIRT1, SREBP-2 and HMGCR
protein decrease in rat models of optic nerve injury
The expression levels of SIRT1, SREBP-2 and HMGCR
proteins in the rat models of optic nerve injury reduced
significantly, and in a time-dependent manner, at each time point
when compared with the control group (P<0.01). The expression
levels of SIRT1, SREBP-2 and HMGCR proteins in the resveratrol
group decreased marginally at each time point and in a
time-dependent manner compared with the normal group (P<0.05).
The differences between the resveratrol group and the model group
at each time point were statistically significant (P<0.01). The
findings demonstrate that the expression level of SIRT1, SREBP-2
and HMGCR proteins in rat models of optic nerve damage were
restored by treatment with resveratrol (Fig. 5).
Discussion
The retina and optic nerve form part of the CNS.
Since CNS damage is irreversible, injuries to the optic nerve, such
as those caused by trauma, inflammation and ocular hypertension,
lead to RGC loss, which results in permanent loss of vision or
blindness. Currently, and to the best of our knowledge, no
therapeutic agent has been developed for the effective treatment of
optic nerve injury. Therefore, a neuroprotective remedy that delays
or prevents RGC loss following optic nerve injury is required
(21).
Lipidomics, a subgroup within the field of
metabolomics, presents two areas of interest. Architecture/membrane
lipidomics describes the comprehensive and quantitative nature of
membrane lipid constituents, while mediator lipidomics includes the
structural characterization and quantification of bioactive lipid
species (8). The lipid composition
of the membranes of numerous tissues and cell types has been well
established (13), however, little
is known regarding the distribution of lipids within these
membranes. One specific class of lipid molecules, the docosanoids,
appears to be significant in retinal structure and function, as
well as being involved in the orchestration of homeostasis,
anti-inflammation, inflammatory resolution, and cell survival
bioactivity (22,23). Previous studies have described the
following observations: (i) Docosahexaenoic acid (DHA) from the
liver is targeted to the brain and retina, where it is actively
retained and protected from peroxidation (23–25).
When lipid peroxidation is triggered in animal models of retinal
degeneration, alterations in photoreceptor function, damage and
cell death are observed. (ii) The blood concentration of DHA is
decreased in various forms of retinitis pigmentosa and in Usher
syndrome, indicating that decreased DHA supply may cause
photoreceptor impairment (22,26)
and when animal models overexpress rhodopsin mutations that are
homologous to human retinitis pigmentosa, the photoreceptors
contain a reduced quantity of DHA (27). However, while these observations
may suggest that reduced retinal DHA is associated with
photoreceptor dysfunction, a reduction in DHA may, instead, be an
adaptive response to metabolic stress caused by the mutation
(27,28). Furthermore, the association between
circulating DHA, retinal DHA and pathology has not been
conclusively demonstrated, and human data has been obtained that
does not reveal a positive association between circulating and
retinal DHA (29,30) or between circulating DHA and
age-related macular degeneration (31). Therefore, it is important to note
that the association between decreased DHA availability, disease
initiation and progression of retinal degeneration remains unclear.
(iii) Finally, in constant light-mediated retinal degeneration,
photoreceptor DHA levels are reduced, and rats reared in bright
cyclic light were shown to be protected from DHA loss and
degeneration, which indicates a mechanism of adaptation and/or a
plasticity response (31). DHA, in
conjunction with pigment epithelial-derived factor, triggers early
synthesis of neuroprotectin D1, which is neuroprotective, and
promotes regeneration of corneal nerves and restores corneal
sensitivity.
In the present study, the neuroprotective effect of
the agonist for SIRT1 was examined, in addition, the efficacy of
resveratrol treatment on RGCs was analyzed and the role of
cholesterol synthesis was investigated following ONC injury in
rats. In the control group, the ganglion cell layers of the
retinas, including inner and outer nuclear layers, were dense and
arranged in parallel. After optic nerve injury, they exhibited a
scattered distribution; the nuclei changed, the inner and outer
nuclear layer were thinner and a disordered arrangement of cells
was observed. Of note, there was significant improvement in rats
treated with resveratrol, with the degree of damage being markedly
attenuated by resveratrol in a concentration-dependent manner. The
retinas were damaged in model rats with optic nerve injury, while
the degree of damage in rats treated with resveratrol was
relatively low. The number of surviving RGCs and the content of
cholesterol in RGCs of model rats was restored by resveratrol.
Resveratrol treatment also restored the mRNA and protein expression
levels of SIRT1, SREBP2 and HMGCR.
In conclusion, treatment with resveratrol represents
a novel approach for regenerating the optic nerve, as well as a
potential treatment for prevention of severe injury following
surgery or disease of the ocular surface. Notably, mediator
lipidomic characterization of signaling mechanisms, triggered by
stress and cellular damage that upregulate neuroprotective events
leading to nerve regeneration, may provide unique pharmacological
targets for numerous neuronal and ocular degenerative diseases.
Acknowledgments
The present study was supported the National Natural
Science Foundation of China (grant no. 30600524).
References
|
1
|
Pernet V and Schwab ME: Lost in the
jungle: New hurdles for optic nerve axon regeneration. Trends
Neurosci. 37:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pernet V, Hauswirth WW and Di Polo A:
Extracellular signal-regulated kinase 1/2 mediates survival, but
not axon regeneration, of adult injured central nervous system
neurons in vivo. J Neurochem. 93:72–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldberg JL, Klassen MP, Hua Y and Barres
BA: Amacrine-signaled loss of intrinsic axon growth ability by
retinal ganglion cells. Science. 296:1860–1864. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore DL, Blackmore MG, Hu Y, Kaestner KH,
Bixby JL, Lemmon VP and Goldberg JL: KLF family members regulate
intrinsic axon regeneration ability. Science. 326:298–301. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwab ME: Functions of Nogo proteins and
their receptors in the nervous system. Nat Rev Neurosci.
11:799–811. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guarente L and Franklin H: Epstein
lecture: Sirtuins, aging and medicine. N Engl J Med. 364:2235–2244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canto C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+ metabolism
and SIRT1 activity. Nature. 458:1056–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stressdependent regulation of FOXO transcription factors by the
SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodgers JT, Lerin C, Haas W, Gygi SP,
Spiegelman BM and Puigserver P: Nutrient control of glucose
homeostasis through a complex of PGC-1alpha and SIRT1. Nature.
434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dioum EM, Chen R, Alexander MS, Zhang Q,
Hogg RT, Gerard RD and Garcia JA: Regulation of hypoxia-inducible
factor 2alpha signaling by the stress-responsive deacetylase
sirtuin 1. Science. 324:1289–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michan S: Acetylome regulation by sirtuins
in the brain: From normal physiology to aging and pathology. Curr
Pharm Des. 19:6823–6838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michan S, Li Y, Chou MM, Parrella E, Ge H,
Long JM, Allard JS, Lewis K, Miller M, Xu W, et al: SIRT1 is
essential for normal cognitive function and synaptic plasticity. J
Neurosci. 30:9695–9707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donmez G, Wang D, Cohen DE and Guarente L:
SIRT1 suppresses beta-amyloid production by activating the
alpha-secretase gene ADAM10. Cell. 142:320–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang M, Wang J, Fu J, Du L, Jeong H, West
T, Xiang L, Peng Q, Hou Z, Cai H, et al: Neuroprotective role of
Sirt1 in mammalian models of Huntington's disease through
activation of multiple Sirt1 targets. Nat Med. 18:153–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donmez G, Arun A, Chung CY, McLean PJ,
Lindquist S and Guarente L: SIRT1 protects against α-synuclein
aggregation by activating molecular chaperones. J Neurosci.
32:124–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guarani V, Deflorian G, Franco CA, Krüger
M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R,
Schmidt MH, et al: Acetylation-dependent regulation of endothelial
Notch signalling by the SIRT1 deacetylase. Nature. 473:234–238.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Potente M, Ghaeni L, Baldessari D,
Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana
E, Alt FW, et al: SIRT1 controls endothelial angiogenic functions
during vascular growth. Genes Dev. 21:2644–2658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Michan S, Juan AM, Hurst CG,
Hatton CJ, Pei DT, Joyal JS, Evans LP, Cui Z, Stahl A, et al:
Neuronal sirtuin1 mediates retinal vascular regeneration in
oxygen-induced ischemic retinopathy. Angiogenesis. 16:985–992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michan S, Juan AM, Hurst CG, Cui Z, Evans
LP, Hatton CJ, Pei DT, Ju M, Sinclair DA, Smith LE and Chen J:
Sirtuin1 over-expression does not impact retinal vascular and
neuronal degeneration in a mouse model of oxygen-induced
retinopathy. PLoS One. 9:e850312014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiao X, Peng Y and Yang L: Minocycline
protects retinal ganglion cells after optic nerve crush injury in
mice by delaying autophagy and upregulating nuclear factor-κB2.
Chin Med J (Engl). 127:1749–1754. 2014.
|
|
22
|
Bazan NG: Homeostatic regulation of
photoreceptor cell integrity: Significance of the potent mediator
neuroprotectin D1 biosynthesized from docosahexaenoic acid: The
proctor lecture. Invest Ophthalmol Vis Sci. 48:4866–4881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bazan NG, Birkle DL and Reddy TS:
Biochemical and nutritional aspects of the metabolism of
polyunsaturated fatty acids and phospholipids in experimental
models of retinal degeneration. Retinal Degeneration: Experimental
and Clinical Studies. LaVail MM, Hollyfield JG and Anderson RE: 2.
A.R. Liss; New York, NY: pp. 159–187. 1985
|
|
24
|
Scott BL and Bazan NG: Membrane
docosahexaenoate is supplied to the developing brain and retina by
the liver. Proc Natl Acad Sci USA. 86:2903–2907. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon WC, Rodriguez de Turco EB and Bazan
NG: Retinal pigment epithelial cells play a central role in the
conservation of docosahexaenoic acid by photoreceptor cells after
shedding and phagocytosis. Curr Eye Res. 11:73–83. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffman DR, Boettcher JA and
Diersen-Schade DA: Toward optimizing vision and cognition in term
infants by dietary docosahexaenoic and arachidonic acid
supplementation: A review of randomized controlled trials.
Prostaglandins Leukot Essent Fatty Acids. 81:151–158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anderson RE, Maude MB and Bok D: Low
docosahexaenoic acid levels in rod outer segment membranes of mice
with rds/peripherin and P216L peripherin mutations. Invest
Ophthalmol Vis Sci. 42:1715–1720. 2001.PubMed/NCBI
|
|
28
|
Gordon WC and Bazan NG: Mediator
lipidomics in ophthalmology: Targets for modulation in
inflammation, neuro-protection and nerve regeneration. Curr Eye
Res. 38:995–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bretillon L, Thuret G, Grégoire S, Acar N,
Joffre C, Bron AM, Gain P and Creuzot-Garcher CP: Lipid and fatty
acid profile of the retina, retinal pigment epithelium/choroid and
the lacrimal gland and associations with adipose tissue fatty acids
in human subjects. Exp Eye Res. 87:521–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Acar N, Berdeaux O, Grégoire S, Cabaret S,
Martine L, Gain P, Thuret G, Creuzot-Garcher CP, Bron AM and
Bretillon L: Lipid composition of the human eye: Are red blood
cells a good mirror of retinal and optic nerve fatty acids? PLoS
One. 7:e351022012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kabasawa S, Mori K, Horie-Inoue K,
Gehlbach PL, Inoue S, Awata T, Katayama S and Yoneya S:
Associations of cigarette smoking but not serum fatty acids with
age-related macular degeneration in a Japanese population.
Ophthalmology. 118:1082–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|