Introduction
Colorectal cancer (CRC) is a common malignancy
worldwide, associated with a high incidence and a high rate of
mortality (1). In particular, the
number of patients diagnosed with the disease continues to rise in
urban areas of China. Surgery is the most effective method for the
treatment of CRC, whereas chemotherapy is also essential for
achieving an improved prognosis (2). However, only a few drugs have been
identified to have an active effect on CRC. The resistance of CRC
to current chemotherapeutic strategies often leads to
unsatisfactory outcomes, therefore, there is an urgent requirement
for the development of effective drugs against CRC.
Abnormal apoptosis is a feature of cancer cells. It
provides them with a means to escape regulatory checks on cell
growth, and to mediate resistance to chemotherapy (3). B-cell leukemia/lymphoma 2 (Bcl-2)
family proteins exert important roles in the regulation of cell
apoptosis (4). Members of the
Bcl-2 protein family are divided into antiapoptotic proteins and
proapoptotic proteins. Their functions differ according to their
structural features. The Bcl-2 family proteins share the same four
conservative sequences, termed Bcl-2 homology domains (BH1-BH4).
Usually, the antiapoptotic Bcl-2 proteins feature a hydrophobic
groove formed by BH1-3. They can bind to the BH3 domain of a
proapoptotic member, and inhibit its proapoptotic function
(5). Previous studies have
identified that the antiapoptotic members of the protein family
(Bcl-2, Bcl-XL and Mcl-1) are widely overexpressed in a
number of cancer types (6–7). Therefore, the development of drugs
which mimic BH3 structure and inhibit the activity of antiapoptotic
proteins of the Bcl-2 family provides the basis of a feasible
strategy for cancer therapy.
Gossypol is a polyphenolic compound, which occurs
naturally in cotton seeds and roots. It was identified as having
antiproliferative activity in vitro and in vivo
against several cancer types (8,9). The
isolated active component of gossypol, (-)-gossypol, has been
assessed in phase II human clinical trials for a variety of cancer
types, including chronic lymphocytic leukemia and B-cell
malignancies (10–12). Apogossypolone (ApoG2) is a novel
derivative of gossypol, which is synthesized by removing two
aldehyde groups to achieve superior anticancer activity with less
toxicity (13). ApoG2 has been
reported to greatly suppress the growth of nasopharyngeal carcinoma
(14), breast cancer (15), gastric carcinoma (16), hepatocellular carcinoma cells
(17), prostate cancer cells
(18) and lymphoma cells (19); however, the activity of ApoG2
against CRC remains to be fully elucidated.
In the present study, the antiproliferative effects
of ApoG2 in CRC cells, and the ability of the compound to induce
cell apoptosis, were investigated. Furthermore, the possible
mechanism by which ApoG2 may induce apoptosis in CRC cells was also
investigated.
Materials and methods
Cells and reagents
The human HT29, HCT116 and SW480 CRC cell lines were
donated by the State Key Laboratory of Oncology in South China
(Guangzhou, China). The cells were cultured in RPMI-1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA), supplemented
with 10% fetal bovine serum (Invitrogen Life Technologies) and
incubated at 37°C with 5% CO2. ApoG2 was synthesized by
Professor Dajun Yang (Ascenta Therapeutics, Inc.,Malvern, PA, USA)
and dissolved in pure dimethyl sulfoxide (DMSO; MP Biomedicals,
Solon, OH, USA) with a stock concentration of 20 mmol/l, prior to
storage at −20°C. The
3-[4,5-dimethyl-thiazol-2-thiazolyl]-2,5-diphenyltetrazolium
bromide (MTT) reagent, for use in subsequent assays, was purchased
from MP Biomedicals.
MTT assay
The antiproliferative effects of ApoG2 on the three
CRC cell lines (HT29, HCT116 and SW480) were determined using an
MTT assay. A total of 104 cells were seeded into 96-well
plates and were incubated overnight. The cells were treated with
ApoG2 at concentrations of 1.25, 2.5 or 5 µM for durations
of 24, 48 or 72 h. Subsequently, 20 µl 5 mg/ml MTT was added
to each well and incubated for a further 4 h. The supernatant was
carefully discarded and 200 µl DMSO was added to dissolve
the formazan sediment. The absorbance was measured using a
microplate reader (V-530; JASCO International, Co., Ltd., Tokyo,
Japan) at a wavelength of 490 nm. All experiments were replicated
in triplicate. Tumor cells, which were cultured in the complete
medium with 0.1% DMSO, were used as a control, and wells containing
only complete medium with 0.1% DMSO served as a blank control. The
inhibition of cell growth was measured as the percentage of viable
cells relative to the control, and calculated as: [Optical density
(OD)treated − ODblank]/(ODcontrol
− ODblank)] × 100.
Western blot assay
The total cell lysates were harvested and
electrophoresed in 10% or 15% sodium dodecyl sulfate
(SDS)-polyacrylamide gels (Sigma-Aldrich, St. Louis, MO, USA). The
proteins were transferred onto polyvinylidene difluoride (PVDF)
membranes (Roche, Basel, Switzerland). The transferred PVDF
membranes were subsequently blocked with 5% non-fat milk for 1 h at
room temperature. Western blotting was performed with primary
antibodies raised against Bcl-2 (cat. no. #2870; Cell Signaling
Technology, Inc., Beverly, MA, USA), Bcl-XL (cat. no.
#2762; Cell Signaling Technology, Inc.), Mcl-1 (cat. no. #5354;
Cell Signaling Technology, Inc.), Bax (cat. no. sc-23959; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), Bak (cat. no.
#6947; Cell Signaling Technology, Inc.), cytochrome c (cat.
no. #4272; Cell Signaling Technology, Inc.) and
glyceraldehyde-3-phopsphate dehydrogenase (GAPDH; cat. no. #2118;
Cell Signaling Technology, Inc.), followed by incubation with
secondary antibodies conjugated to horseradish peroxidase (cat.
nos. #7074 and #7076; Cell Signaling Technology, Inc.). The
proteins were detected through film development using a
clarity-enhanced chemiluminescence reagent (cat. no. #170-5056;
Bio-Rad Laboratories, Hercules, CA, USA).
Immunoprecipitation analysis
Protein immunoprecipitation was performed to
determine protein interactions among the Bcl-2 family members. The
cell lysates were initially incubated with antibodies raised
against Bax (cat. no. sc-23959; Santa Cruz Biotechnology, Inc.) for
1 h at 4°C, prior to mixing with protein A/G agarose beads
(sc-2001, Santa Cruz Biotechnology, Inc.) and agitating the
solution overnight at 4°C. The proteins bound to the beads were
collected by centrifugation (2,000 × g, 10 sec, 4°C) and
subsequently eluted using denaturing loading buffer (Pierce 26149,
Co-Immunoprecipitation kit; Pierce Biotechnology, Rockford, IL,
USA). The antiapoptotic proteins, which had interacted with Bax
were identified by western blotting using antibodies against
antiapoptotic proteins, according to the method described
previously (14).
Detection of cells undergoing
apoptosis
A total of 105 cells were plated into
six-well plates and were cultured overnight. The cells were
subsequently incubated with 0.1% DMSO and 1.25, 2.5 or 5
µmol/l ApoG2 for 48 h. Following treatment, the cells were
fixed in mounting medium (P0126, Beyotime Institute of
Biotechnology, Haimen, China) for 10 min at room temperature,
rinsed twice with phosphate-buffered saline (PBS; 3 min each wash)
and subsequently stained with 5 µg/ml Hoechst 33258 for 5
min. The cells were rinsed twice with PBS and any nuclei changes
resulting from apoptosis were observed by fluorescence microscopy
(DFC480; Leica Microsystems CMS GmbH, Mannheim, Germany).
Flow cytometric analysis
Cell apoptosis was also assessed by flow cytometry
(Beckman Coulter, Fullerton, CA, USA) using annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide stain (PI) (cat. no.
556405, Apoptosis Detection kit; BD Biosciences, Franklin Lakes,
NJ, USA). ApoG2-treated cells were harvested, centrifuged at 800 ×
g for 5 min at room temper ature, washed with PBS, centrifuged as
before and resuspended in 500 µl binding buffer, containing
5 µl annexin V-FITC and 5 µl PI. The cells were
incubated in the dark in staining solution (cat. no. C0003,
Beyotime Institute of Biotechnology) for 15 min. The level of
apoptosis of the cells was analyzed using the flow cytometric
system with ModFit LT for windows Trial and Reader Version 3.2
(Verity Software House, Topsham, ME, USA).
Cytochrome c release assay
To assess the release of cytochrome c from
mitochondria into the cytoplasm, the cytosolic and mitochondrial
fractions were isolated using a mitochondria/cytosol fractionation
kit (cat. no. #K256-25; Biovision, Tuczon, AZ, USA), according to
the manufacturer's instructions. The cells were collected, washed
with PBS and suspended in 1 ml 5X dilution of cytosolic extraction
solution (K256-100; BioVision Inc., Milpitas, CA, USA) on ice for
10 min, prior to repeatedly beating the cells with a syringe to
destroy the cytomembranes. The cellular and nuclear debris were
removed by centrifuging the homogenates twice at 700 × g for 10 min
at 4°C. The supernatants were pelleted again at 10,000 × g for 30
min at 4°C, and the supernatants were subsequently collected as the
cytosolic fraction. The quantity of cytochrome c in the
cytosolic fraction was determined by western blotting, as described
above.
Detection of the cleavage of caspases 3
and 7
Western blotting was used to identify the cleavage
of the caspase family proteins, caspase 3 and 7. The total cell
proteins were extracted and separated by electrophoresis on 15%
SDS-polyacrylamide gels and transferred onto PVDF membranes.
Following blocking in 5% non-fat milk, the PVDF membranes were
incubated with primary antibodies raised against caspase 3 (cat.
no. #9665; Cell Signaling Technology, Inc.), caspase 7 (cat. no.
#9492; Cell Signaling Technology, Inc.) and GAPDH (cat. no. #2118;
Cell Signaling Technology, Inc.), prior to an incubation with
secondary antibodies conjugated to horseradish peroxidase. The
procaspase and cleaved caspase protein bands were detected using
the clarity-enhanced chemiluminescence reagent (Bio-Rad
Laboratories), as described previously.
Statistical analysis
All data are expressed as the mean ± standard error
of mean for three independent experiments. The mean was compared
with the one-way analysis of variance using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
ApoG2 inhibits the proliferation of the
different CRC cell lines
An MTT assay was performed to investigate the
growth-inhibitory effects of ApoG2 on the three CRC cell lines. As
shown in Fig. 1, ApoG2 inhibited
the growth of the HT29, SW480 and HCT116 cells in a time- and a
dose-dependent manner. ApoG2 exerted a more pronounced effect on
the SW480 and HCT116 cells compared with the HT29 cells. The
concentration of ApoG2 required for a half maximal inhibitory
concentration (IC50) of the HT29 cells at 48 and 72 h
was 3.12 and 2.57 µmol/l, respectively. The HT29 cells
failed to respond to ApoG2 treatment following a period of 24 h. By
contrast, the IC50 values of ApoG2 for the SW480 cells
at 24, 48 and 72 h were 4.04, 1.44 and 0.75 µmol/l,
respectively, and those for the HCT116 cells were 3.46, 0.77 and
0.57 µmol/l, respectively.
Protein expression levels for the Bcl-2
family members in the CRC cells
Since the Bcl-2 family proteins are possible targets
of ApoG2, the protein expression levels of the Bcl-2 family
proteins (Bcl-2, Bcl-XL, Mcl-1, Bax and Bak) in CRC
cells were investigated. The nasopharyngeal cancer cell line,
CNE-1, in which Bcl-2 family proteins are highly expressed, was
used as a positive control. As shown in Fig. 2, high protein expression levels of
the antiapoptotic proteins, Bcl-2 and Bcl-XL, were
obtained in the HT29, HCT116 and SW480 cell lines, whereas the
expression of the antiapoptotic protein, Mcl-1, differed among
these three CRC cell lines. The proapoptotic proteins, Bax and Bak,
were only highly expressed in HT29 cells compared with CNE-1.
ApoG2 affects the protein expression
levels of the Bcl-2 family
Western blot analysis was performed to observe the
changes in the protein expression levels of Bcl-2 family members
following treatment with serial concentrations of ApoG2 for 48 h.
As shown in Fig. 3A, the protein
expression levels of the antiapoptotic protein, Mcl-1, were
markedly decreased upon treatment with ApoG2, and the effect
observed was more pronounced in the SW480 and HCT116 cells compared
with the HT29 cells. The relative protein expression levels
compared with the control group following quantification are shown
in Fig. 3B.
 | Figure 3Effects of ApoG2 on the expression
levels and function of the Bcl-2 family proteins in human CRC cell
lines. (A) Western blot analysis was performed using specific
antibodies against Bcl-2, Bcl-XL, Mcl-1, Bax and Bak.
GAPDH was used as a control to normalize each lane as the loading
control. (B) Densitometric quantitative results of the protein
expression levels of the target proteins normalized against GAPDH.
The effect of ApoG2 on target protein levels is presented as the
fold of protein expression compared with the control
vehicle-treated cells, which are expressed as 1-fold. The height of
the bars indicate the mean of three independent experiments, while
the error bars indicate the standard error of mean
(*P<0.05, compared with the control). (C) Cell
lysates were immunoprecipitated with primary anti-Bax antibodies.
Western blots were performed to detect the quantity of Mcl-1
protein binding to Bax. GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; ApoG2, apogossypolone; IP, immunoprecipitate; IB,
immunoblot; CRC, colorectal cancer. |
ApoG2 disrupts the interactions between
the Bcl-2 family proteins
To further assess whether the binding of the Mcl-1
protein to Bax was altered by treatment with ApoG,
immunoprecipitation assays were performed. As shown in Fig. 3C, in the untreated CRC cells, Mcl-1
and Bax, were bound to each other (lane 1); however, when the cells
were treated with 2.5 µmol/l ApoG2 for 48 h, the Mcl-1/Bax
binding in all three CRC cells was completely disrupted (lane 2).
Lane 3 was loaded with homologous immunoglobulin G and the anti-Bax
antibody as a negative control.
ApoG2 induces apoptosis in the CRC
cells
Hoechst 33258 staining and flow cytometry were used
to assess ApoG2-induced apoptosis in the CRC cells. The nuclear
morphological changes of the cells exposed to ApoG2, as revealed by
Hoechst 33258 staining and observed by fluorescence microscopy, is
shown in Fig. 4A. ApoG2-treated
cells exhibited clear apoptotic characteristics, including cell
shrinkage and nuclear fragmentation. The results of the flow
cytometric analysis shown in Fig.
4B also indicated that ApoG2 promoted cell apoptosis at 48 h.
The percentages of apoptotic cells increased with the concentration
of ApoG2. The level of apoptosis induced by ApoG2 was also assessed
over time. As shown in Fig. 4C,
apoptosis occurred as early as 24 h following the treatment with
ApoG2, and the apoptotic rate increased concomitantly with the
duration of the treatment. The percentages of the cells undergoing
apoptosis for the three cell lines, and at the different time
points following treatment with ApoG2, are shown in Table I.
 | Table IPercentage of apoptotic cells induced
by treatment with ApoG2. |
Table I
Percentage of apoptotic cells induced
by treatment with ApoG2.
A, Cells treated
with ApoG2 at different concentrations
|
|---|
| Cell line | Control | Concentration of
ApoG2 (µM)
|
|---|
| 1.25 | 2.5 | 5 |
|---|
| HT29 | 14.80±1.33 | 15.27±0.50 | 25.06±1.16a | 67.97±4.54b |
| SW480 | 11.31±0.52 | 17.41±0.80 | 22.65±1.34a | 55.88±3.01b |
| HCT116 | 7.13±0.93 | 7.46±0.56 | 24.65±2.27b | 32.14±0.93b |
B, Cells treated
with ApoG2 for different durations
|
|---|
| Cell line | Control | Duration of
treatment (h)
|
|---|
| 24 | 48 | 72 |
|---|
| HT29 | 6.40±1.13 | 10.67±0.92 | 16.83±1.11a | 18.27±1.36b |
| SW480 | 8.26±0.92 | 18.58±0.99 | 20.63±1.34a | 56.38±2.93b |
| HCT116 | 13.72±1.94 | 21.95±5.58 | 31.05±2.31a | 57.11±2.18b |
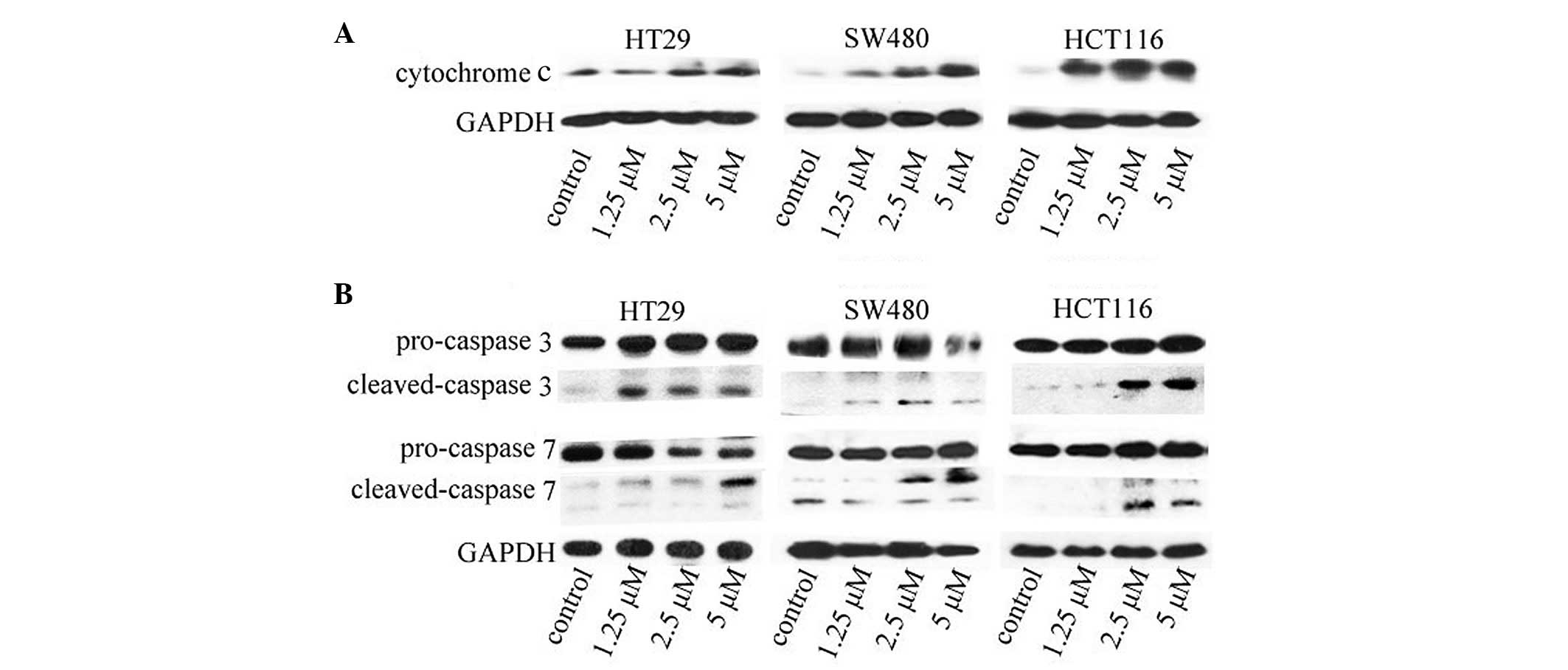
ApoG2 promotes cytochrome c release
Since the release of cytochrome c from the
mitochondria into the cytosol is usually indicative of apoptosis,
whether ApoG2-induced apoptosis of the CRC cells involved the
release of cytochrome c was investigated. As shown in
Fig. 5A, an investigation of the
levels of cytochrome c in the cytosol revealed that
cytochrome c was almost undetectable in the cytosol of
untreated CRC cells (leftmost lane in each gel). Following
treatment of the cells with ApoG2 at the indicated concentrations
for 48 h, cytochrome c was translocated into the cytosol in
a concentration-dependent manner (lanes 2–4 of each gel).
ApoG2 induces the activation of caspase 3
and 7
Caspase 3 is one of the executioners of apoptosis,
and it is the first caspase to be activated by initiator cleavage.
The activation of caspase 3 provides a marker of cell apoptosis. To
confirm that ApoG2-promoted apoptosis signaling was occurring in
CRC cells, the activation of caspase 3 and caspase 7 was
investigated. Caspase 7 is normally activated subsequently to
caspase 3. As shown in Fig. 5B,
the levels of the cleaved caspases 3 and 7 increased significantly
following treatment of ApoG2 at the indicated concentrations at 48
h.
Discussion
The absence of effective chemotherapeutic drugs
often leads to a poor prognosis of CRC. It is a major concern that
CRC is becoming resistant to treatment with the drugs currently
used, including 5-fluorouracil and oxaliplatin. Members of the
Bcl-2 protein family are considered to be associated with
tumorigenesis and the resistance of CRC to chemotherapy.
Prabhudesai et al (20)
revealed that increased expression levels of the antiapoptotic
proteins, and reduced expression levels of Bax, are associated with
poor responses to chemotherapy and shorter overall survival rates
in CRC, indicating that the Bcl-2 protein family is involved in
tumorigenesis and the resistance of CRC to chemotherapies.
ApoG2 is a non-peptide molecular inhibitor of the
antiapoptotic Bcl-2 family of proteins, derived from gossypol.
Compared with gossypol, ApoG2 retains the identical antitumor
effects, while having less toxicity. As previously reported, ApoG2
was well tolerated by healthy mice, and caused no toxic
side-effects when administered to normal tissues, to gastric
xenografts or to hepatocellular carcinoma xenografts at antitumor
doses (16,21). ApoG2 exhibits a similar action to
cis-platinum in cancer cells in nasopharyngeal carcinoma
(14), or to CHOP chemotherapy
(cyclophosphamide, hydoxydaunorubicin, Oncovin® and
prednisone in combination) in large-cell lymphoma and prednisolone
in combination] (22). Numerous
previous studies have confirmed the antitumor effects of ApoG2 and
its safe administration in a variety of cancer models (14–19).
It was reported that gossypol exerts a cytotoxic
effect on CRC cells (23).
Furthermore, CRC cells were more sensitive to gossypol compared
with other human cancer cell lines, including erythroleukemia and
mammary adenocarcinoma (24).
Consequently, it may be surmised that anti-Bcl-2 agents may provide
an alternative to well-established CRC therapies. As an inhibitor
of the Bcl-2 family proteins, ApoG2 is therefore a promising
candidate.
Three CRC cell lines, which exhibit different
degrees of differentiation (HT29, HCT116 and SW480), were selected
as models to investigate CRC in vitro. The results of the
MTT assay revealed that the poorly differentiated cell lines,
HCT116 and SW480, were markedly more sensitive to ApoG2 compared
with the highly differentiated cell line, HT29, suggesting that
ApoG2 may be more effective as a therapy for poorly differentiated
cancers. As observed in the western blot analysis, the HT29 cells
expressed higher levels of the antiapoptotic proteins, Bcl-2,
Bcl-XL and Mcl-1, compared with the HCT116 and SW480
cell lines, consistent with a previous study, which reported the
association of the protein expression of Bcl-2 with the degree of
tumor differentiation (25).
ApoG2 is reported to be a pan-inhibitor of the Bcl-2
family of antiapoptotic proteins, including Bcl-2,
Bcl-XL and Mcl-1 (26).
ApoG2 binds to recombinant Bcl-2, Bcl-XL and Mcl-1
proteins with Ki values of 35, 660 and 25 nmol/l,
respectively, suggesting that ApoG2 has the highest affinity for
Mcl-1 among the anti-apoptotic Bcl-2 family proteins (22). Antiapoptotic proteins of the Bcl-2
family are therefore potential targets of ApoG2, as demonstrated in
other cancer models. In the present study, the effects of ApoG2 on
the expression and interaction of Bcl-2 protein family members in
CRC cells was investigated. ApoG2 disrupted the binding of Mcl-1 to
Bax in all three CRC cell lines investigated. The sensitivity of
the CRC cell lines to ApoG2 correlated with the extent to which
Mcl-1 was inhibited, indicating that the anti-CRC effect of ApoG2
may be associated with its antagonism to Mcl-1. ApoG2 treatment
also led to a reduction in the protein expression level of Mcl-1,
concomitantly with an increased protein expression of Bax,
suggesting that the antagonistic effects of ApoG2 on Mcl-1 released
Bax protein from the suppressive action of Mcl-1. Differently from
the effects of ApoG2 reported previously in other types of cancer
cells, Mcl-1 protein, and not Bcl-2 or Bcl-XL, appears
to be the predominant target of ApoG2 in CRC cells.
The apoptosis-inducing effects of ApoG2 in CRC cells
were investigated in the present study. As expected, CRC cells
treated with ApoG2 exhibited clear apoptotic characteristics,
including cell shrinkage and nuclear fragmentation. The flow
cytometric analysis also revealed higher percentages of cells
undergoing apoptosis upon treatment with ApoG2. In addition, the
ApoG2-inducing apoptosis effects were investigated over time. As
determined from the viability of the cells using an MTT assay, the
number of apoptotic cells increased upon treatment with higher
concentrations of ApoG2 or for a prolonged time period, suggesting
that ApoG2 inhibited the proliferation of CRC cells by inducing
cell apoptosis.
In the present study, ApoG2 inhibited Mcl-1/Bax
binding and led to an increase in the protein expression of Bax.
Cell apoptosis was also observed following ApoG2 treatment. It is
well established that the Bcl-2 protein family regulates cell
apoptosis predominantly through the mitochondrial pathway. The
antagonistic action of antiapoptotic proteins of the Bcl-2 family
facilitates the penetration of proapoptotic proteins into the
mitochondrial membrane, and the subsequent release of cytochrome
c into the cytoplasm, in order to activate caspase family
proteins and to initiate cell apoptosis (5). Since ApoG2 disrupted Mcl-1/Bax
binding in the CRC cells, it was surmised that ApoG2 may stimulate
the release of cytochrome c to initiate cell apoptosis. As
expected, higher levels of cytochrome c in the cytoplasm
were observed following ApoG2 treatment. The release of cytochrome
c into the cytoplasm is a common feature of
mitochondrial-dependent apoptosis. The results in the present study
indicated that ApoG2 may induced apoptosis in CRC cells through the
mitochondrial signaling pathway. Subsequently, the activation of
the caspase proteins was assessed. Caspase 3 is one of the key
executioners of apoptosis activated by upstream apoptotic signaling
molecules, including cytochrome c. Caspase 7 is another
executioner of apoptosis, activated after caspase 3. The activation
of caspases 3 and 7 by ApoG2 in confirmed that cell apoptosis was
induced by ApoG2. In addition to the disruption of the activity of
Mcl-1, the protein expression levels of Mcl-1 were decreased upon
ApoG2 treatment in CRC cells. A previous study reported that the
Mcl-1 protein was downregulated by caspase 3 in response to tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in leukemic T cells (27). Since
the present study revealed that ApoG2 activated caspase 3 in CRC
cells, this may offer an explanation for the decreased expression
of Mcl-1 following ApoG2 treatment. An accumulating body of
evidence supports that Mcl-1 is associated with colorectal
carcinogenesis (28-29), whereas the role of Bcl-2 in
colorectal carcinoma remains controversial, and remains to be fully
elucidated (30). Since ApoG2
inhibited the protein expression and the function of Mcl-1, it may
potentially be of use in colorectal cancer therapy.
In conclusion, the present study identified that
ApoG2 exerts marked antiproliferative effects on human colorectal
cancer cells, which is at least partly dependent on preventing the
Bcl-2 family protein member, Mcl-1, from binding to the
proapoptotic protein, Bax, resulting in the induction of
mitochondrial signaling pathway-dependent apoptosis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar
|
|
3
|
Reed JC: Dysregulation of apoptosis in
cancer. Cancer J Sci Am. 4(Suppl 1): S8–S14. 1998.PubMed/NCBI
|
|
4
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
6
|
Joensuu H, Pylkkanen L and Toikkanen S:
Bcl-2 protein expression and long-term survival in breast cancer.
Am J Pathol. 145:1191–1198. 1994.PubMed/NCBI
|
|
7
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
8
|
Ligueros M, Jeoung D, Tang B, Hochhauser
D, Reidenberg MM and Sonenberg M: Gossypol inhibition of mitosis,
cyclin D1 and Rb protein in human mammary cancer cells and
cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer.
76:21–28. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Kulp SK, Sugimoto Y, Liu S and
Lin YC: The effects of gossypol on the invasiveness of MAT-LyLu
cells and MAT-LyLu cells from the metastasized lungs of
MAT-LyLu-bearing Copenhagen rats. Anticancer Res. 20:4591–4597.
2000.
|
|
10
|
Ready N, Karaseva NA, Orlov SV, Luft AV,
Popovych O, Holmlund JT, Wood BA and Leopold L: Double-blind,
placebo-controlled, randomized phase 2 study of the proapoptotic
agent AT-101 plus docetaxel, in second-line non-small cell lung
cancer. J Thorac Oncol. 6:781–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sonpavde G, Matveev V, Burke JM, Caton JR,
Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT,
et al: Randomized phase II trial of docetaxel plus prednisone in
combination with placebo or AT-101, an oral small molecule Bcl-2
family antagonist, as first-line therapy for metastatic
castration-resistant prostate cancer. Ann Oncol. 23:1803–1808.
2012. View Article : Google Scholar
|
|
12
|
Baggstrom MQ, Qi Y, Koczywas M, Argiris A,
Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan
R, et al: A phase II study of AT-101 (Gossypol) in
chemotherapy-sensitive recurrent extensive-stage small cell lung
cancer. J Thorac Oncol. 6:1757–1760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitada S, Kress CL, Krajewska M, Jia L,
Pellecchia M and Reed JC: Bcl-2 antagonist apogossypol (NSC736630)
displays single-agent activity in Bcl-2-transgenic mice and has
superior efficacy with less toxicity compared with gossypol
(NSC19048). Blood. 111:3211–3219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu ZY, Zhu XF, Zhong ZD, Sun J, Wang J,
Yang D and Zeng YX: ApoG2, a novel inhibitor of antiapoptotic Bcl-2
family proteins, induces apoptosis and suppresses tumor growth in
nasopha-ryngeal carcinoma xenografts. Int J Cancer. 123:2418–2429.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu X, Li S, Wei F, Huang J, Wu G, Xu L,
Xu D and Wang S: Apogossypolone induces autophagy and apoptosis in
breast cancer MCF-7 cells in vitro and in vivo. Breast Cancer.
21:223–230. 2014. View Article : Google Scholar
|
|
16
|
Xin J, Zhan Y, Liu M, Hu H, Xia L, Nie Y,
Wu K, Liang J and Tian J: ApoG2 induces ER stress-dependent
apoptosis in gastric cancer cells in vitro and its real-time
evaluation by bioluminescence imaging in vivo. Cancer Lett.
336:260–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng P, Ni Z, Dai X, Wang B, Ding W, Rae
Smith A, Xu L, Wu D, He F and Lian J: The novel BH-3 mimetic
apogossypolone induces Beclin-1- and ROS-mediated autophagy in
human hepatocellular carcinoma [corrected] cells. Cell Death Dis.
4:e4892013. View Article : Google Scholar
|
|
18
|
Zhang X, Hu X, Mu S, Zhan Y, An Q, Liu Z
and Huang X: Apogossypolone inhibits the proliferation of LNCaP
cells in vitro and in vivo. Mol Med Rep. 10:1184–1194.
2014.PubMed/NCBI
|
|
19
|
Sun J, Li ZM, Hu ZY, Zeng ZL, Yang DJ and
Jiang WQ: Apogossypolone inhibits cell growth by inducing cell
cycle arrest in U937 cells. Oncol Rep. 22:193–198. 2009.PubMed/NCBI
|
|
20
|
Prabhudesai SG, Rekhraj S, Roberts G,
Darzi AW and Ziprin P: Apoptosis and chemo-resistance in colorectal
cancer. J Surg Oncol. 96:77–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mi JX, Wang GF, Wang HB, Sun XQ, Ni XY,
Zhang XW, Tang JM and Yang DJ: Synergistic antitumoral activity and
induction of apoptosis by novel pan Bcl-2 proteins inhibitor
apogossypolone with adriamycin in human hepatocellular carcinoma.
Acta Pharmacol Sin. 29:1467–1477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Wu J, Aboukameel A, Banerjee S,
Arnold AA, Chen J, Nikolovska-Coleska Z, Lin Y, Ling X, Yang D, et
al: Apogossypolone, a nonpeptidic small molecule inhibitor
targeting Bcl-2 family proteins, effectively inhibits growth of
diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol
Ther. 7:1418–1426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Wang J, Wong SC, Chow LS, Nicholls
JM, Wong YC, Liu Y, Kwong DL, Sham JS and Tsa SW: Cytotoxic effect
of gossypol on colon carcinoma cells. Life Sci. 67:2663–2671. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuszynski GP and Cossu G: Differential
cytotoxic effect of gossypol on human melanoma, colon carcinoma and
other tissue culture cell lines. Cancer Res. 44:768–771.
1984.PubMed/NCBI
|
|
25
|
Lombardi L, Frigerio S, Collini P and
Pilotti S: Immunocytochemical and immunoelectron microscopical
analysis of BCL2 expression in thyroid oxyphilic tumors.
Ultrastruct Pathol. 21:33–39. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei J, Stebbins JL, Kitada S, Dash R, Zhai
D, Placzek WJ, Wu B, Rega MF, Zhang Z, Barile E, et al: An
optically pure apogossypolone derivative as potent pan-active
inhibitor of antiapoptotic bcl-2 family proteins. Front Oncol.
1:282011. View Article : Google Scholar
|
|
27
|
Weng C, Li Y, Xu D, Shi Y and Tang H:
Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in Jurkat leukemia T cells. J Biol Chem. 280:10491–10500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernandez JM, Farma JM, Coppola D, Hakam
A, Fulp WJ, Chen DT, Siegel EM, Yeatman TJ and Shibata D:
Expression of the antiapoptotic protein survivin in colon cancer.
Clin Colorectal Cancer. 10:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Chen Y and Chen Z: MiR-125a/b
regulates the activation of cancer stem cells in
paclitaxel-resistant colon cancer. Cancer Invest. 31:17–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poincloux L, Durando X, Seitz JF, Thivat
E, Bardou VJ, Giovannini MH, Parriaux D, Barriere N, Giovannini M,
Delpero JR and Monges G: Loss of Bcl-2 expression in colon cancer:
A prognostic factor for recurrence in stage II colon cancer. Surg
Oncol. 18:357–365. 2009. View Article : Google Scholar
|