Introduction
Numerous studies have demonstrated that sympathetic
activity is enhanced in patients with essential (1) or secondary hypertension (2,3) in
addition to various hypertensive models including obesity (4), renovascular hypertensive rats
(5) and spontaneously hypertensive
rats (SHRs) (6). Several methods
have been used to evaluate sympathetic activity including the
cardiac sympathetic afferent reflex (7), the adipose afferent reflex (4), plasma norepinephrine levels (8) and blood pressure response to
ganglionic blockade (9).
Hexamethonium is a ganglionic blocker that is used
to treat hypertension (10,11).
It has been reported that hexamethonium produces a greater
reduction in blood pressure in angiotensin II-induced hypertensive
rats compared with saline-infused rats (12). Hexamethonium reduced bradycardia
and the pressor response in Wistar rats and SHRs, and hexamethonium
treatment in SHRs resulted in a greater reduction in blood pressure
(13). However, the effects of
hexamethonium on sympathetic nerve activity are not fully
understood.
Touw et al (14) evaluated sympathetic activity in
SHRs by measuring alterations in mean arterial pressure (MAP) in
response to hexamethonium intravenous injection to block
sympathetic nervous system (SNS) transmission, in order to evaluate
the sympathetic activity in the SHRs. Sato et al (15) used absolute blood pressure as an
index to evaluate peripheral SNS activity in deoxycorticosterone
acetate-treated rats injected with hexamethonium. However, the
precise association between sympathetic nerve activity and the
blood pressure response to hexamethonium remains unclear. The
current study aimed to examine renal sympathetic nerve activity
(RSNA), MAP and the association between RSNA and MAP in response to
intravenous injection of hexamethonium in normal Wistar rats and
SHRs.
Materials and methods
Animals
Experiments were conducted on male normotensive
Wistar rats and SHRs weighing 280–320 g obtained from Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). The
procedures were approved by the Experimental Animal Care and Use
Committee of Nanjing Medical University and complied with the Guide
for the Care and Use of Laboratory Animals (16). All efforts were made to minimize
the number of animals used and their suffering. The rats were
anesthetized using an intraperitoneal injection of sodium
pentobarbital (50 mg/kg−1) and ventilated with room air
using a Harvard 683 Small Animal Ventilator (Harvard Apparatus,
Holliston, MA, USA).
Systolic blood pressure (SBP)
measurements
The tail artery SBP was measured in conscious rats
using a Non-Invasive Blood Pressure monitor (ADInstruments, Bella
Vista, Australia). To minimize stress-induced SBP fluctuations, the
rats were trained by measuring SBP daily for a minimum of 10 days
prior to surgery. The rats were warmed for 10–20 min at 28°C prior
to the measurements to allow for detection of tail arterial
pulsations and to achieve a steady pulse. The SBP was obtained by
calculating the mean of 10 measurements.
RSNA recording
A retroperitoneal incision was made and the left
renal sympathetic nerve was isolated. The nerve was cut distally to
eliminate its afferent activity and was placed on a pair of silver
electrodes that were immersed in warm mineral oil. RSNA was
amplified using an AC/DC Differential Amplifier Model 3000 (A-M
Systems, Inc., Sequim, WA, USA) with a low frequency cut-off at 60
Hz and a high frequency cut-off at 3,000 Hz and integrated at a
time constant of 0.1 sec. The raw RSNA, integrated RSNA, MAP and HR
were simultaneously recorded on a PowerLab 8SP Data Acquisition
System (ADInstruments). The background noise level was determined
following sectioning of the central end of the nerve and was
subtracted from the RSNA value, as previously described (5).
Chemicals
Hexamethonium hydrochloride was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and dissolved in normal saline
(Shanghai Baxter Healthcare Co., Ltd., Shanghai, China). The
concentrations selected for the current study were 0.2, 1.0, 5.0 or
25.0 mg/kg body weight.
Intravenous injection
The femoral artery and vein were cannulated under
sodium pentobarbital anesthesia to prepare for MAP recordings and
the intravenous injection of hexamethonium. Saline or hexamethonium
hydrochloride (0.2, 1.0, 5.0 or 25 mg/kg body weight) was then
injected into the vein using a PM2000B Cell Microinjector
(MicroData Instrument, Inc., South Plainfield, NJ, USA). The
effects of hexamethonium on RSNA, MAP and HR were determined by
averaging 1 min of the maximal responses. The time course of the
maximal effects on RSNA, MAP and HR were determined from the end of
the injections to the maximum effect points. The recovery time from
the hexamethonium effects on RSNA, MAP and HR were determined from
the maximum effect points to the maximum recovery points. The
recovery of hexamethonium effects on RSNA, MAP and HR was
determined at the maximum recovery points by averaging the
parameters for 1 min.
Statistical analysis
Data were analyzed using SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA). Comparisons between two
observations were assessed by Student's paired t-test. One-way
analysis of variance was used followed by the Bonferroni post hoc
test for multiple comparisons. All data were presented as the mean
± standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data
The SBP of the tail artery in the conscious state
and the baseline MAP of the femoral artery under anesthesia were
significantly greater in SHRs compared with Wistar rats. There were
no significant differences between Wistar rats and SHRs in body
weight or baseline HR (Table
I).
 | Table IBody weight, SBP, baseline MAP and
baseline HR in Wistar rats and SHRs. |
Table I
Body weight, SBP, baseline MAP and
baseline HR in Wistar rats and SHRs.
| Variable | Wistar | SHR |
|---|
| Number | 30 | 30 |
| Body weight (g) | 303.7±4.8 | 300.2±5.4 |
| SBP (mmHg) | 118.8±3.5 | 194.0±3.4a |
| Baseline MAP
(mmHg) | 91.1±1.5 | 132.2±2.2a |
| Baseline HR
(beats/min) | 350.7±7.4 | 357.2±7.7 |
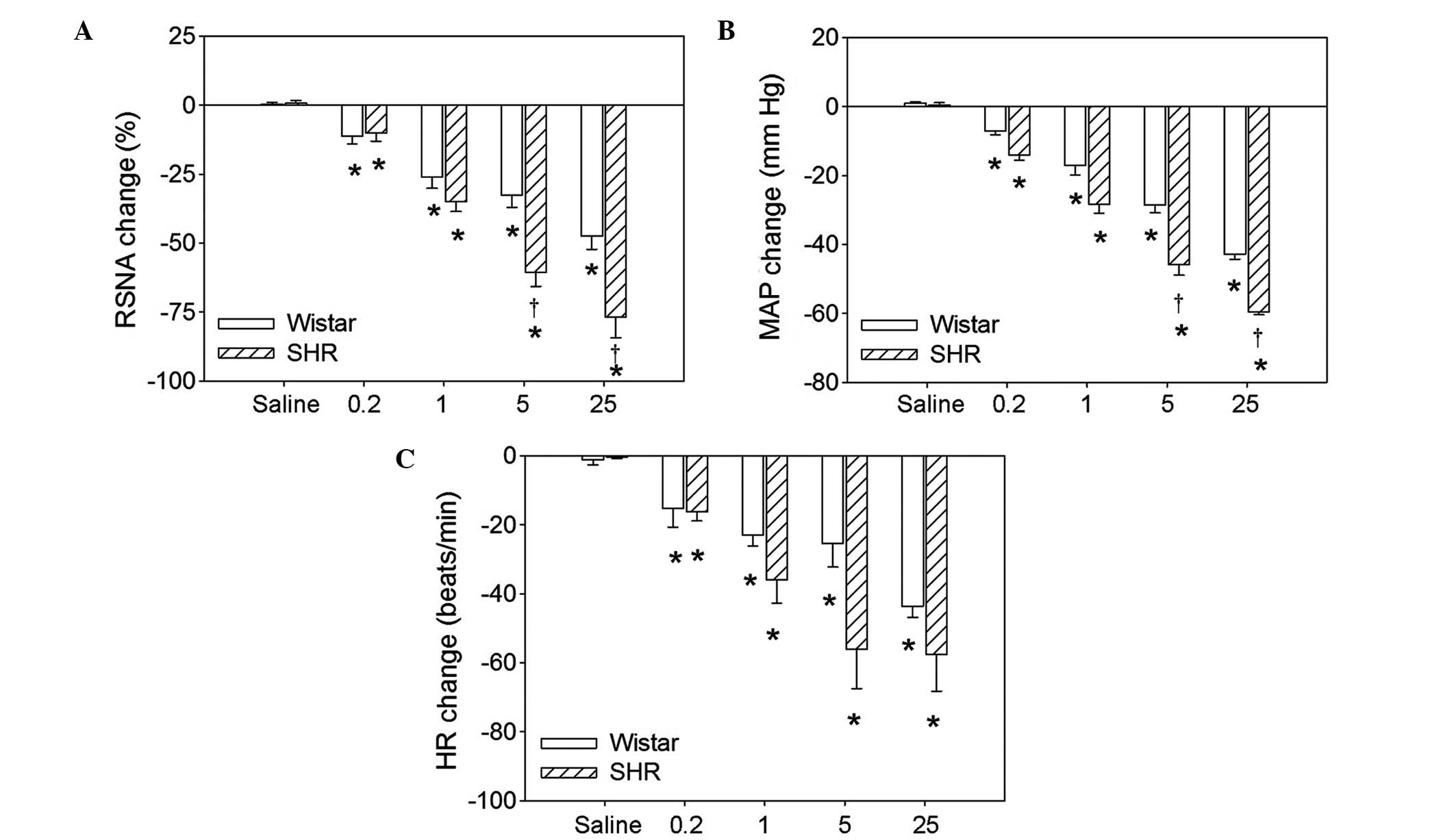
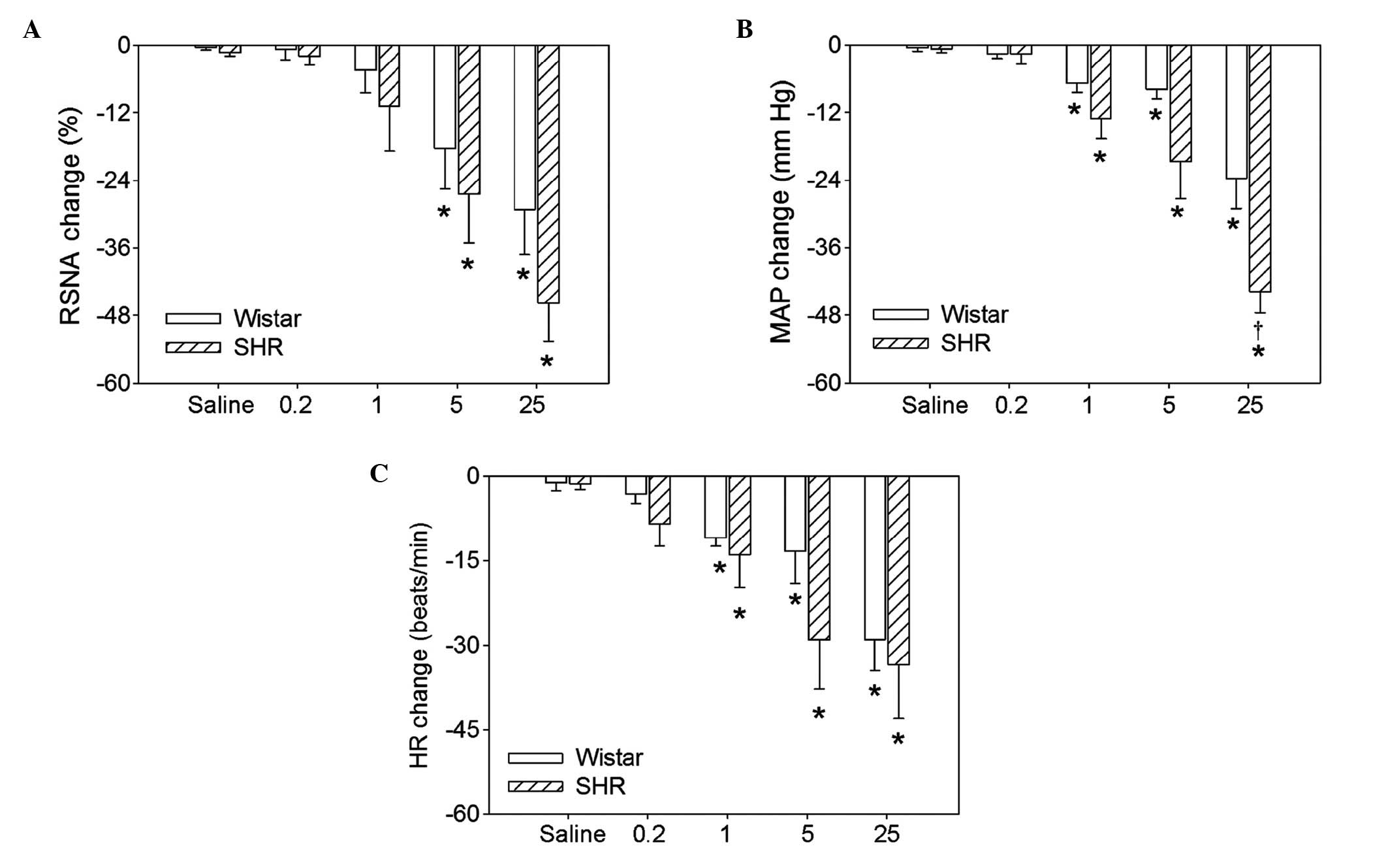
Dosage effects of hexamethonium
Intravenous injection of four doses (0.2, 1.0, 5.0
or 25.0 mg/kg body weight) of hexamethonium significantly reduced
the RSNA, MAP and HR in the Wistar rats and the SHRs. There were no
significant differences between Wistar rats and SHRs in the RSNA,
MAP or HR at the 0.2 or 1.0 mg/kg doses. However, 5.0 and 25.0
mg/kg hexamethonium resulted in greater reductions in the RSNA and
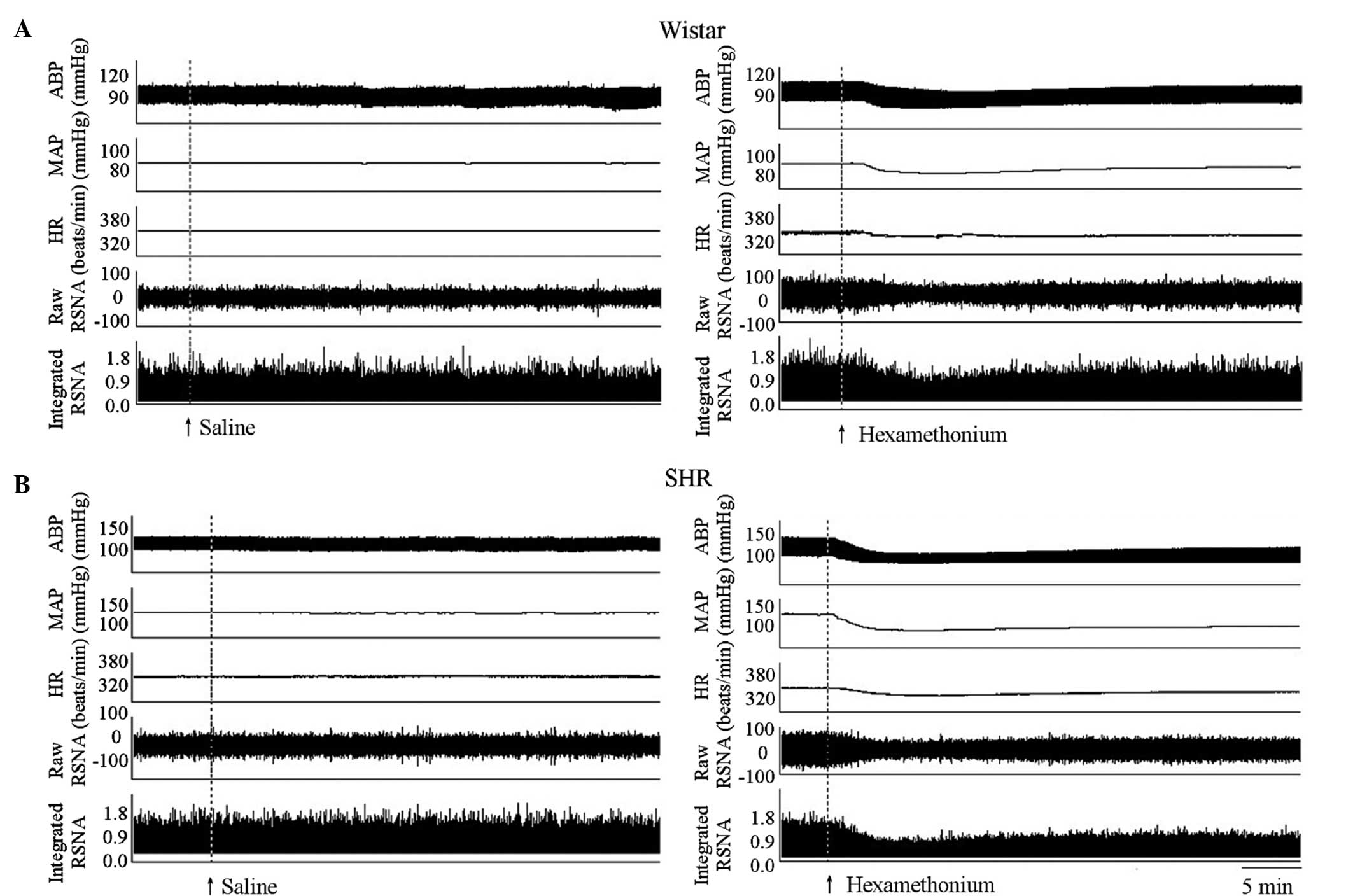
MAP in SHRs compared with Wistar rats (Fig. 1). Representative recordings
indicate that the intravenous injection of 5.0 mg/kg of
hexamethonium reduced the RSNA, MAP and HR (Fig. 2).
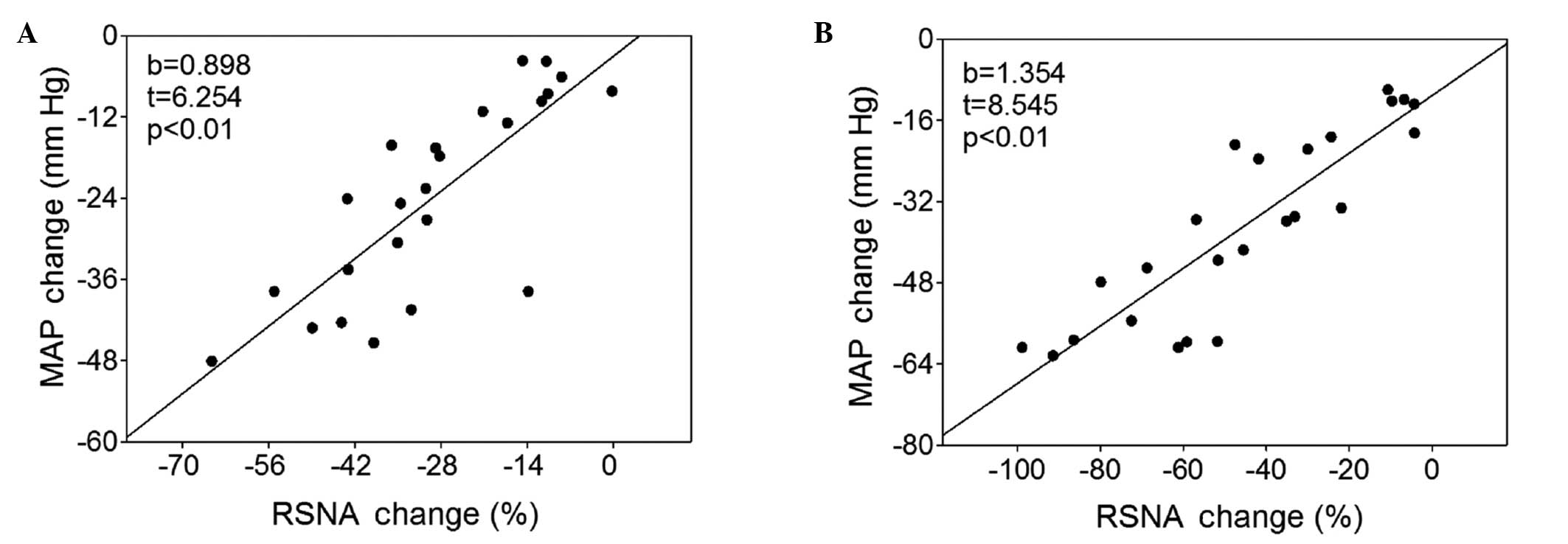
Association between RSNA and MAP
There was a significant positive correlation between
the alterations in RSNA and MAP response to intravenous injection
of hexamethonium in the Wistar rats and SHRs (Fig. 3).
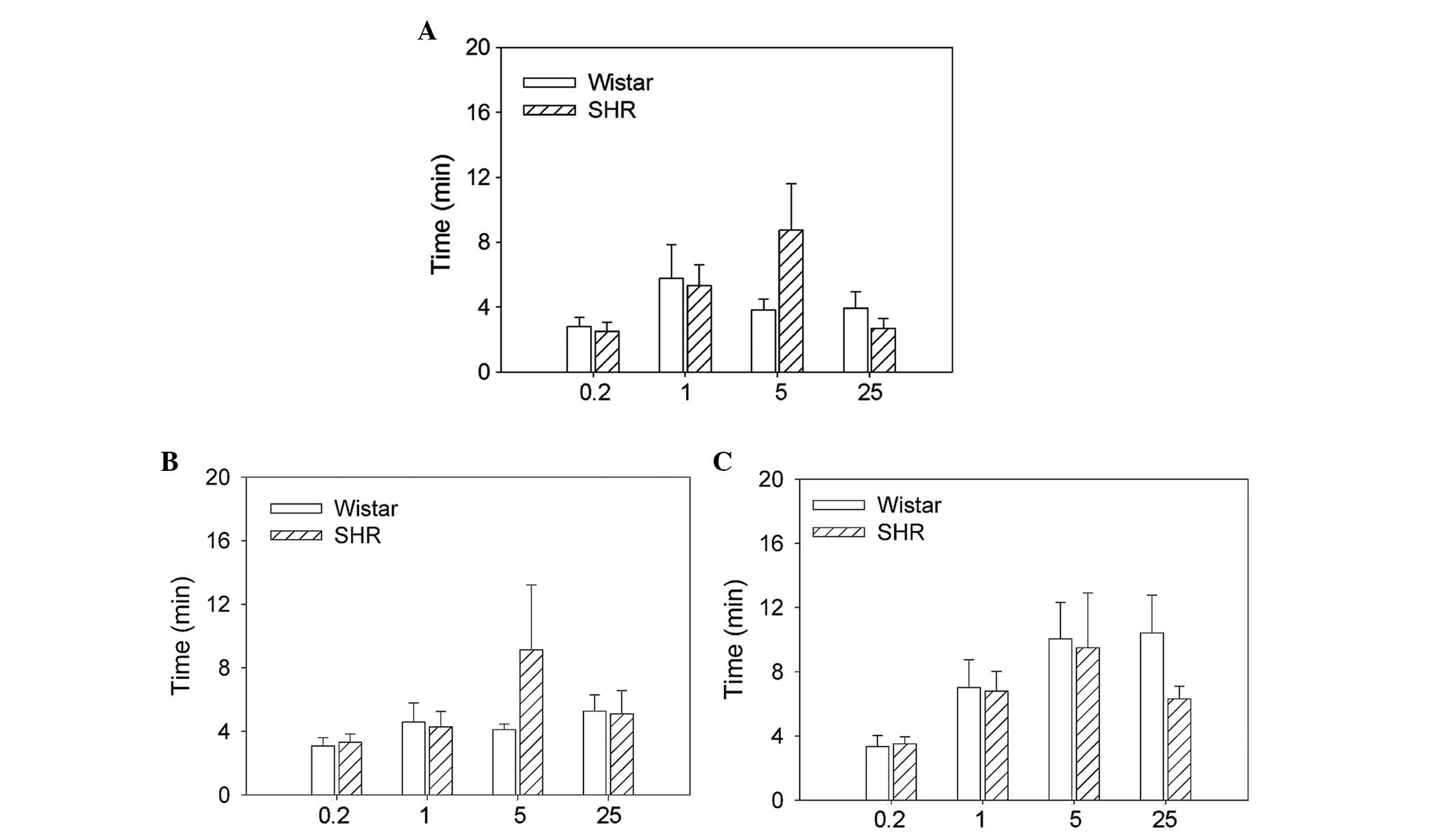
Time at which hexamethonium reached its
maximum effects
There was no significant difference between the SHRs
and Wistar rats in the time course of the maximal effects of the
four doses of hexamethonium on the RSNA, MAP or HR (Fig. 4).
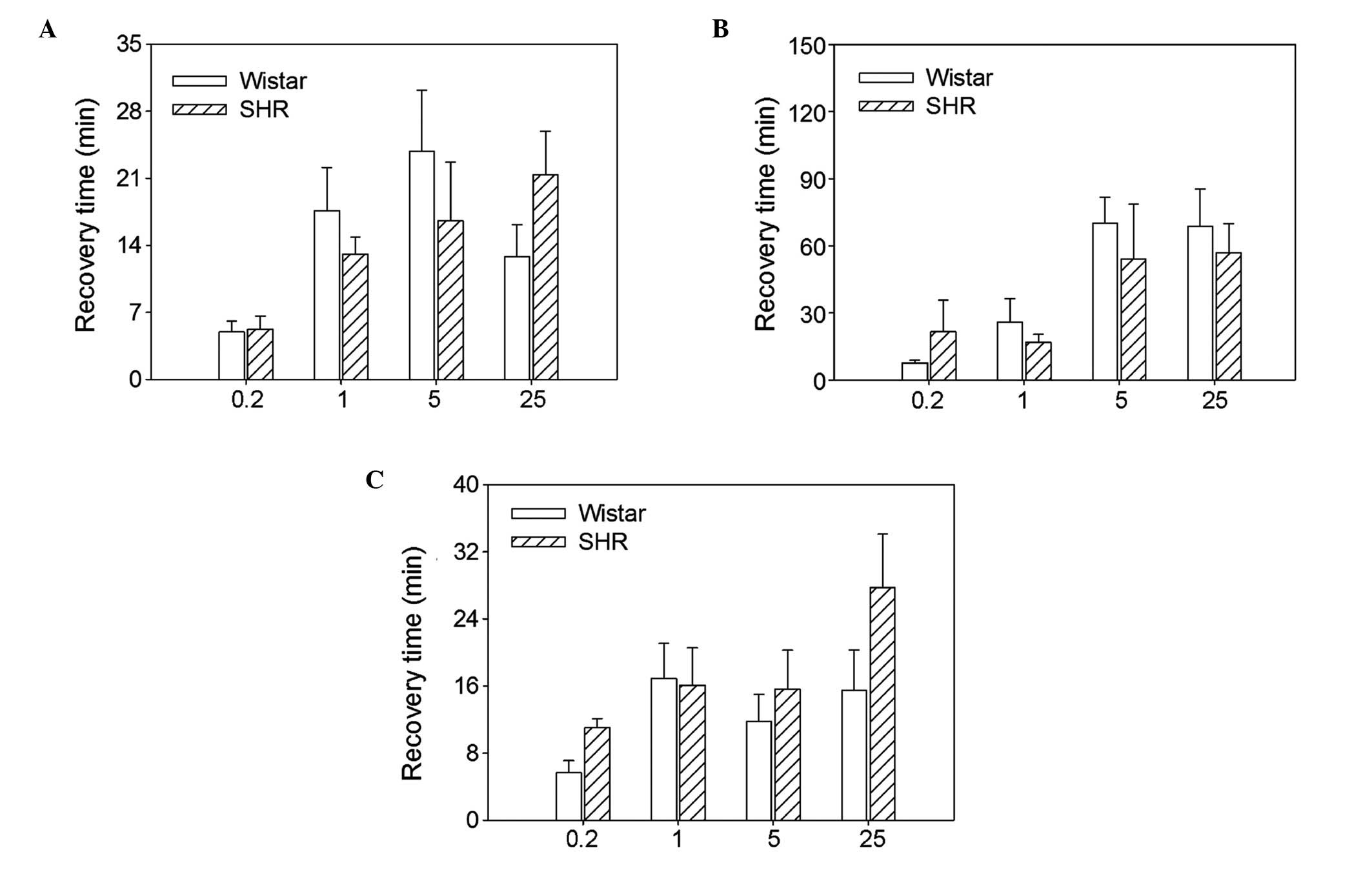
Recovery time of hexamethonium
There were no significant differences between the
SHRs and Wistar rats in the recovery time of the RSNA, MAP or HR at
the four doses of hexamethonium investigated (Fig. 5).
Recovery from hexamethonium
RSNA fully recovered at the hexamethonium doses of
0.2 and 1.0 mg/kg and partially recov-ered at doses of 5.0 and 25.0
mg/kg (Fig. 5). MAP and HR were
fully recovered at a dose of 0.2 mg/kg and partially
recovered at doses of 1.0, 5.0 and 25.0 mg/kg. There were no
significant differences between the Wistar rats and SHRs in the
recovery time of RSNA at the four doses of hexame-thonium
investigated (0.2 mg/kg, 5.0±1.1 vs. 5.2±1.4 min; 1.0 mg/kg,
17.6±4.5 vs. 13.1±1.8 min; 5.0 mg/kg, 23.8±6.4 vs. 16.6±6.1 min;
and 25.0 mg/kg, 12.8±3.4 vs. 21.4±4.5 min), MAP (0.2 mg/kg, 7.6±1.1
vs. 21.6±14.1 min; 1.0 mg/kg, 25.9±10.5 vs. 16.9±3.5 min; 5.0
mg/kg, 70.1±11.5 vs. 54.0±24.5 min; and 25.0 mg/kg, 68.6±16.8 vs.
57.0±12.9 min) and HR (0.2 mg/kg, 5.7±1.4 vs. 11.0±1.1 min; 1.0
mg/kg, 16.9 ± 4.2 vs. 16.1±4.5 min; 5.0 mg/kg, 11.8±3.2 vs.
15.6±4.6 min; and 25.0 mg/kg, 15.5±4.8 vs. 27.7±10.4 min) (Fig. 6).
Discussion
Sympathetic activity is enhanced in patients with
essential (1) or secondary
hypertension (2,3) and in various hypertensive models
(9,17,18).
Excessive sympathetic output contributes to the pathogenesis of
hypertension and the progression of organ damage (19–21).
The therapeutic targeting of sympathetic activation is considered
to be an effective antihypertensive strategy (22,23).
The MAP response to ganglionic blockade has been
used to evaluate basal sympathetic nerve activity in conscious rats
(8). In the present study, the
four doses of hexamethonium investigated were demonstrated to
significantly reduce the RSNA, MAP and HR in the Wistar rats and
the SHRs. The highest doses of hexamethonium (5.0 and 25.0 mg/kg)
resulted in a greater reduction in the RSNA and MAP in SHRs
compared with Wistar rats. There was a significant positive
correlation between the alterations in the RSNA and MAP response to
hexamethonium in the Wistar rats and SHRs.
It has been demonstrated that the sympathetic
activity of obese rats (4,24), renovascular hypertensive rats
(5,25,26)
and SHRs (6,27,28)
is enhanced compared with normotensive rats. Hexamethonium is a
ganglionic blocker (10,11) and reduces blood pressure in SHR and
Wistar rats (8). In the present
study, the intravenous injection of four doses (0.2, 1.0, 5.0 or
25.0 mg/kg body weight) of hexamethonium was demonstrated to
significantly reduce the RSNA, MAP and HR in the Wistar rats and
the SHRs. The highest doses of hexamethonium (5.0 and 25.0 mg/kg)
resulted in a greater reduction in the RSNA and MAP in SHRs
compared with Wistar rats. There was a significant positive
correlation between the alterations in RSNA and MAP in response to
the intravenous injection of hexamethonium in the Wistar rats and
SHRs. These data suggest that the MAP response to hexamethonium may
be used to evaluate basal sympathetic nerve activity.
The current study investigated whether the time
point at which hexamethonium reaches its maximum effect, the time
course of recovery from hexamethonium or the extent of recovery
from hexamethonium may be used to evaluate sympathetic activity.
There were no significant differences observed in the timing of the
maximal effects on RSNA, MAP and HR or in recovery following
hexamethonium treatment, the RSNA was fully recovered at doses of
0.2 and 1 mg/kg and partially recovered at doses of 5.0 and 25.0
mg/kg hexamethonium. MAP and HR were fully recovered at a dose of
0.2 mg/kg and partially recovered at the doses of 1.0, 5.0 and 25.0
mg/kg hexamethonium. Additionally, there were no significant
differences between SHRs and Wistar rats in the recovery of RSNA,
MAP and HR. These data suggest that the times at which
hexamethonium reached its maximal effect, the recovery time course
and the extent of recovery from hexamethonium are not suitable for
use in the evaluation of sympathetic nerve activity.
In conclusion, 5.0 and 25.0 mg/kg hexamethonium
resulted in a greater reduction in the RSNA and MAP in SHRs
compared with Wistar rats. There was a significant positive
correlation between the alterations in RSNA and MAP in response to
the intravenous injection of hexamethonium in the Wistar rats and
SHRs. The MAP response to ganglionic blockade by hexamethonium may
be used to evaluate basal sympathetic nerve activity.
Acknowledgments
The authors would like to thank the Collaborative
Innovation Center for Cardiovascular Disease Translational Medicine
for their support, as well as the National Natural Science
Foundation of China (grant no. 81400315).
References
|
1
|
Hogarth AJ, Mackintosh AF and Mary DA: The
effect of gender on the sympathetic nerve hyperactivity of
essential hypertension. J Hum Hypertens. 21:239–245. 2007.
|
|
2
|
Remuzzi G: Sympathetic overactivity in
hypertensive patients with chronic renal disease. N Engl J Med.
340:1360–1361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neumann J, Ligtenberg G, Klein IH, Boer P,
Oey PL, Koomans HA and Blankestijn PJ: Sympathetic hyperactivity in
hypertensive chronic kidney disease patients is reduced during
standard treatment. Hypertension. 49:506–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang
F, Gao XY and Zhu GQ: Enhanced adipose afferent reflex contributes
to sympathetic activation in diet-induced obesity hypertension.
Hypertension. 60:1280–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li P, Sun HJ, Cui BP, Zhou YB and Han Y:
Angiotensin-(1–7) in the rostral ventrolateral medulla modulates
enhanced cardiac sympathetic afferent reflex and sympathetic
activation in reno-vascular hypertensive rats. Hypertension.
61:820–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye ZY, Li DP and Pan HL: Regulation of
Hypothalamic Presympathetic Neurons and Sympathetic Outflow by
Group II Metabotropic Glutamate Receptors in Spontaneously
Hypertensive Rats. Hypertension. 62:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun HJ, Li P, Chen WW, Xiong XQ and Han Y:
Angiotensin II and angiotensin-(1-7) in paraventricular nucleus
modulate cardiac sympathetic afferent reflex in renovascular
hypertensive rats. PLoS One. 7:e525572012. View Article : Google Scholar
|
|
8
|
Yuan N, Zhang F, Zhang LL, Gao J, Zhou YB,
Han Y and Zhu GQ: SOD1 gene transfer into paraventricular nucleus
attenuates hypertension and sympathetic activity in spontaneously
hypertensive rats. Pflugers Arch. 465:261–270. 2013. View Article : Google Scholar
|
|
9
|
Fujita M, Ando K, Nagae A and Fujita T:
Sympathoexcitation by oxidative stress in the brain mediates
arterial pressure elevation in salt-sensitive hypertension.
Hypertension. 50:360–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burt CC and Graham AJ: Pentamethonium and
hexamethonium iodide in investigation of peripheral vascular
disease and hypertension. BMJ. 1:455–460. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bechgaard P, Iversen T and Nielsen AL:
Hexamethonium therapy of essential hypertension. Ugeskr Laeger.
113:1335–1340. 1951.In Danish. PubMed/NCBI
|
|
12
|
Nunes FC and Braga VA: Chronic angiotensin
II infusion modulates angiotensin II type I receptor expression in
the subfornical organ and the rostral ventrolateral medulla in
hypertensive rats. J Renin Angiotensin Aldosterone Syst.
12:440–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
França-Silva MS, Monteiro MM, Queiroz TM,
Santos AF, Athayde-Filho PF and Braga VA: The new nitric oxide
donor 2-nitrate-1,3-dibuthoxypropan alters autonomic function in
spontaneously hypertensive rats. Auton Neurosci. 171:28–35. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Touw KB, Haywood JR, Shaffer RA and Brody
MJ: Contribution of the sympathetic nervous system to vascular
resistance in conscious young and adult spontaneously hypertensive
rats. Hypertension. 2:408–418. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato Y, Ando K and Fujita T: Role of
sympathetic nervous system in hypotensive action of taurine in
DOCA-salt rats. Hypertension. 9:81–87. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guide for the Care and Use of Laboratory
Animals. 6th edition. National Research Council (US) Institute for
Laboratory Animal Research; Washington, DC, USA: 1996
|
|
17
|
Li DP and Pan HL: Role of
gamma-aminobutyric acid (GABA) A and GABAB receptors in
paraventricular nucleus in control of sympathetic vasomotor tone in
hypertension. J Pharmacol Exp Ther. 320:615–626. 2007. View Article : Google Scholar
|
|
18
|
Katholi RE, Whitlow PL, Winternitz SR and
Oparil S: Importance of the renal nerves in established two-kidney,
one clip Goldblatt hypertension. Hypertension. 4:166–174.
1982.PubMed/NCBI
|
|
19
|
Rahn KH, Barenbrock M and Hausberg M: The
sympathetic nervous system in the pathogenesis of hypertension. J
Hypertens Suppl. 17:S11–S14. 1999.PubMed/NCBI
|
|
20
|
Mancia G, Grassi G, Giannattasio C and
Seravalle G: Sympathetic activation in the pathogenesis of
hypertension and progression of organ damage. Hypertension.
34:724–728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morise T, Horita M, Kitagawa I, Shinzato
R, Hoshiba Y, Masuya H, Suzuki M and Takekoshi N: The potent role
of increased sympathetic tone in pathogenesis of essential
hypertension with neurovascular compression. J Hum Hypertens.
14:807–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del Colle S, Morello F, Rabbia F, Milan A,
Naso D, Puglisi E, Mulatero P and Veglio F: Antihypertensive drugs
and the sympathetic nervous system. J Cardiovasc Pharmacol.
50:487–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fisher JP and Fadel PJ: Therapeutic
strategies for targeting excessive central sympathetic activation
in human hypertension. Exp Physiol. 95:572–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balasubramanian P, Sirivelu MP, Weiss KA,
Wagner JG, Harkema JR, Morishita M, Mohankumar PS and Mohankumar
SM: Differential effects of inhalation exposure to PM2.5 on
hypothalamic monoamines and corticotrophin releasing hormone in
lean and obese rats. Neurotoxicology. 36:106–111. 2013. View Article : Google Scholar
|
|
25
|
Zhou H, Sun HJ, Chang JR, Ding L, Gao Q,
Tang CS, Zhu GQ and Zhou YB: Cardiac sympathetic afferent reflex
response to intermedin microinjection into paraventricular nucleus
is mediated by nitric oxide and γ-amino butyric acid in
hypertensive rats. Exp Biol Med (Maywood). 239:1352–1359. 2014.
View Article : Google Scholar
|
|
26
|
Zhang LL, Ding L, Zhang F, Gao R, Chen Q,
Li YH, Kang YM and Zhu GQ: Salusin-β in rostral ventrolateral
medulla increases sympathetic outflow and blood pressure via
superoxide anions in hypertensive rats. J Hypertens. 32:1059–1067.
2014. View Article : Google Scholar
|
|
27
|
Maranon RO, Lima R, Mathbout M, do Carmo
JM, Hall JE, Roman RJ and Reckelhoff JF: Postmenopausal
hypertension: Role of the sympathetic nervous system in an animal
model. Am J Physiol Regul Integr Comp Physiol. 306:R248–R256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shanks J, Manou-Stathopoulou S, Lu CJ, Li
D, Paterson DJ and Herring N: Cardiac sympathetic dysfunction in
the prehypertensive spontaneously hypertensive rat. Am J Physiol
Heart Circ Physiol. 305:H980–H986. 2013. View Article : Google Scholar : PubMed/NCBI
|