Introduction
Growing evidence has indicated that the tumor
microenvironment is largely regulated by orchestrated signaling of
chemokines and pro-inflammatory cytokines, which are produced by
inflammatory and cancer cells, and contribute to cancer progression
(1). The complex interactions
between the chemokine C-C chemokine receptor type 7 (CCR7) and the
inflammatory cytokinesmembrane-associated prostaglandin E synthase
1 (mPGES-1)/PGE2 were the subject of the present study.
CCR7 was reported to be a homing receptor regulating
the migration of immune cells to secondary lymphoid organs in
response to C-C ligand 21/secondary lymphoid organ chemokine
(2). Müller et al (3) also observed this mechanism in
CCR7-expressing cancer cells, which was associated with the
establishment of lymph node metastasis. Accumulating evidence
indicated that CCR7 is overexpressed in numerous cancer types and
is positively correlated with cancer metastasis and survival
(4,5). Previous studies by our group
demonstrated that CCR7 was frequently overexpressed in human colon
cancer and breast cancer tissues and was associated with poor
prognosis (6,7). Another study by our group showed that
CCR7 expression is regulated by cyclooxygenase (COX)-2/PGE2 through
prostaglandin EP4 receptor in colon as well as in breast cancer
cells (8). In this process,
inducible COX-2 and mPGES-1, which are frequently upregulated in
numerous cancer types, cooperatively synthesize PGE2, which
contributes to carcinogenesis and cancer progress (9,10).
These findings implied that overexpression of CCR7 in cancer cells
may proceed via mPGES-1/PGE2.
mPGES-1 is the terminal synthase responsible for the
synthesis of the most abundant pro-tumorigenic prostaglandin, PGE2,
which has a large number of biological actions via four types of
receptors, EP1-4. mPGES-1 is mainly an induced isomerase, is
overexpressed in a wide variety of cancer tissues and cells, and
mediates numerous critical processes involved in tumor progression,
including oncogene activation, DNA damage and tumor metastasis
(11). Although the pathological
implications of CCR7 and mPGES-1 have been revealed in a large
variety of malignant tumors, the role of mPGES-1 in CCR7 expression
in colon cancer as well as the underlying molecular mechanisms have
remained elusive. The present study investigated the role of
mPGES-1 in the induction of CCR7 expression as well as the possible
involvement of the EP4 receptor in SW620 colon cancer cells. The
involvement of the phosphoinositide-3 kinase (PI3K)/AKT/glycogen
synthase (GSK)-3β signaling pathway was also investigated.
Materials and methods
Chemicals and reagents
mPGES-1 recombinant protease was purchased from USCN
Life Sciences (Wuhan, China). PGE2, PGE2 ELISA kit, PI3K inhibitor
LY293002, EP4 inhibitor AH-23848 and GSK-3β inhibitor lithium
(LiCl) were obtained from Cayman Chemical (Ann Arbor, MI, USA).
Rabbit anti-human, monoclonal primary antibodies against
phosphorylated (p)-GSK-3β (cat. no. 9315L), p-Akt (Ser473; cat. no.
9271L), Akt (cat. no. 9088S), CCR7 (cat. no. 9412S) and GADPH (cat.
no. 9022L) were obtained from Cell Signaling Technology, Inc.
(Shanghai, China). Rabbit anti-human polyclonal primary antibody
against mPGES-1 (cat. no. 160140-1) were obtained from Cayman
Chemical. The plasmid pDC104 and the small interfering
(si)RNA-expressing pUC19-green fluorescence protein
(GFP)-siRNA-CCR7 vector (siRNA-CCR7) were purchased from Qiagen
China (Shanghai, China).
Cell culture
The SW620 colorectal carcinoma cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). SW620 cells were maintained in RPMI
1640 culture medium (Gibco-BRL, Invitrogen Life Technologies, Inc.,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco-BRL, Invitrogen Life Technologies) under a humidified 5%
CO2 atmosphere at 37°C in an incubator. In the present
study, the cells were harvested after treatment with PGE2 or
transfection with mPGES-1 Adv for 48 h. To further examine the
signal transduction, cells were treated with LY293002 (10
µmol/l), LiCl (10 µmol/l) or AH23848 (10
µmol/l) 2 h prior to administration of PGE2 (10
µmol/l) or transfection with mPGES-1 Adv.
siRNA transfection
One day prior to transfection, SW620 cells were
trypsinized and 3×105 cells were seeded into six-well
plates. Transient transfection of the pUC19-GFP-siRNA-CCR7 vector
was performed using Lipofectamine 2000 (Takara Bio Inc., Dalian,
China) in serum-reduced medium according to the manufacturer's
instructions. Cells were subjected to assays 48 h after
transfection.
Construction of recombinant adenovirus
(Adv) encoding human mPGES-1
The recombinant replication-defective Adv expressing
full-length human mPGES-1 (Adv-mPGES-1) was constructed by
two-plasmid rescue methods as previously described (12). Human full-length mPGES-1 cDNA was
isolated and cloned into the EcoRI site of shuttle plasmid
pDC104 (Qiagen), and its sequence was confirmed. The sequence was
confirmed by DNA Sequencers HiSeq 2000 (Illumina, Inc., San Diego,
CA, USA) and a Genomic DNA Sample Prep kit (cat. no. FC-102-1001,
Illumina, Inc.). The purified shuttle plasmid was combined with
rescue plasmid pBHGlox-delE1/E3.cre2 (Qiagen) and co-transfected
into 293 cells (Type Culture Collection of the Chinese Academy of
Sciences) to rescue the Adv (12).
Large-scale virus production was performed by infection of 293T
cells for 72 h. The cells were maintained at −20°C for 15 min and
37°C for 15 min three times, centrifuged (2,500 × g, 10 min, 4°C),
then viruses were collected from cell lysates by cesium chloride
gradient ultracentrifugation (100,000 × g, 90 min, 4°C; Cayman
Chemical) and viral titers were detected using a plaque assay.
mPGES-1 transgene expression of Adv-mPGES-1-infected SW620 cells
was confirmed by reverse-transcription quantitative polymerase
chain reaction (RT-qPCR) and western blot analysis.
Transwell migration assay
A chemotaxis assay was performed in 24-well
Millicell cell culture inserts (Millipore Corp., Billerica, MA,
USA) with 8-µm pore membranes. SW620 cells transfected with
adenovirus (Adv)-mPGES-1, siRNA-CCR7 or treated with PGE2 (10
µmol/l, 24 h) were suspended in RPMI 1640 at
2×104/ml and added to the upper chamber. In parallel,
SW620 cells treated with mPGES-1 protein or PGE2 were suspended in
RPMI 1640 at 2×104/ml and added to the upper chamber.
600 µl medium containing 10% FBS was added to the lower
chamber and served as a chemotactic agent. After incubation for 24
h, cells which had migrated to the lower surface of the membrane
were stained with hematoxylin and eosin (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and counted under a
light microscope (IX83; Olympus, Tokyo, Japan) in five fields of
view at a magnification of ×400. Results were expressed as the mean
number of migrated cells/well in experiments performed in
triplicate.
RT-qPCR
Total RNA was isolated using TRIzol reagent (RNA
Extraction kit; cat no. 15596-026; Invitrogen Life Technologies,
Inc.) according to the manufacturer's instructions. cDNA was
synthesized from 500 ng total RNA using the SuperScript™ III
First-Strand Synthesis System (cat no. 18080-051; Invitrogen Life
Technologies, Inc.) according to the manufacturer's instructions.
Real-time PCR was performed in a 20-µl reaction mixture
containing 1 µl cDNA, 10 µl 2X SYBRH Premix Ex Taq™
(cat no. DRR420A; Takara Bio Inc.) and 300 nM of each paired primer
using the following thermocycling conditions: 35 cycles of 94°C for
3 min, 94°C for 30 sec, 62°C for 30 sec, 72°C for 10 sec and 72°C
for 2 min. Quantification of target and reference (GAPDH) genes was
performed in triplicate using an ABI7500 real-time fluorescent
quantitative PCR system (Applied Biosystems, Thermo Fisher
Scientific, Waltham, MA, USA). The primers used in each reaction
were as follows: CCR7 forward, 5′-GGT GGT GGC TCT CCT TGT CAT TTT
C3′ and reverse, 5′-AGT AGG CCC ACG AAA CAA ATG ATG G3′; mPGES-1
forward, 5′-TGC TCA GCC ACC ATCT GGA GTTTTA3′ and reverse, 5′-TTC
CAC CAT ACA GGA ACC CAA GACC3′; GAPDH forward, 5′-GCA CCG TCA AGG
CTG AGA AC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. After
normalization to GAPDH, expression levels for each target gene were
calculated using the comparative threshold cycle (CT) method using
ABI 7500 software v2.0.1 (Applied Biosystems). The ΔCt values were
calculated according to the formula ΔCt = Ct (gene of interest) -
Ct (GAPDH) in the correlation analysis. The mRNA levels of the
control group were used as the baseline, and ΔΔCt was calculated
using the formula ΔΔCt = ΔCt (target gene) - Ct (baseline). The
fold change of mRNA levels was calculated as fold =
2−ΔΔCt.
Determination of PGE2 levels by
ELISA
Approximately 2×106 SW620 cells were
cultured in 60-mm dishes for 15 h. One group of cells was
pre-incubated with MK886 for 1 h (10 µmol/l, Cayman
Chemical); cells were then transfected with Adv-mPGES-1 vector for
0–48 h. The culture medium was centrifuged at 14,000 × g for 10 min
at 4°C. The supernatant was collected and stored at −80°C until
analysis. PGE2 levels were measured using a Prostaglandin
E2 Enzyme Immunoassay kit (cat no. 500141; Cayman
Chemical) according to the manufacturer's instructions. Absorbance
values were determined using a Microplate Reader (model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Cells were lysed in lysis buffer (25 mmol/l
Tris-HCl, 300 mmol/l NaCl, 1 mmol/l CaCl2, 1% Triton
X-100, pH 7.4; Invitrogen Life Technologies, Inc.) containing
protease inhibitor (Complete Mini; Roche Diagnostics, Basel,
Switzerland). Whole-cell lysates were centrifuged at 10,000 ×g for
20 min and the supernatants were collected. The protein
concentration was determined using the bicinchoninic acid-based
Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL., USA).
Proteins (30–50 µg per sample) were separated using 10%
SDS-PAGE (Roche Diagnostics) and transferred onto polyvinylidene
difluoride membranes (Roche Diagnostics). After being blocked with
5% milk powder in Tris-buffered saline containing Tween 20 (TBST)
for 1 h at room temperature, the membranes were incubated with the
primary antibody and β-actin at 4°C overnight, followed by
incubation with the corresponding horseradish peroxidase-conjugated
secondary antibody at room temperature for 1 h. Detection was
performed using an enhanced chemiluminescence kit according to the
manufacturer's instructions and the protein bands were visualized
after exposure of the membranes to Kodak X-ray film. The results
were normalized to GAPDH expression. E-Gel® Imager
Software (Thermo Fisher Scientific) was used for grey value
analysis of the bands.
Statistical analysis
Values are expressed as the mean ± standard
deviation of three independent experiments unless otherwise
specified. Statistical analysis of data was performed using a
two-tailed unpaired Student's t-test between any two groups.
One-way analysis of variance was used to assess difference between
mean values among groups. SPSS 10.0 (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
mPGES-1/PGE2 mediated migration of SW620
cells is partly blocked by siRNA-CCR7
Migration and invasion are among the hallmarks of
tumor cells and are correlated with their aggressiveness and
patient prognosis. Tumor cells with an aggressive phenotype acquire
migratory and invasive capabilities. This involves the upregulation
of biochemical mechanisms which promote the dissemination of tumors
cells to distant organs. The effects of mPGES-1/PGE2 on the
migratory ability of SW620 cells was measured using a Transwell
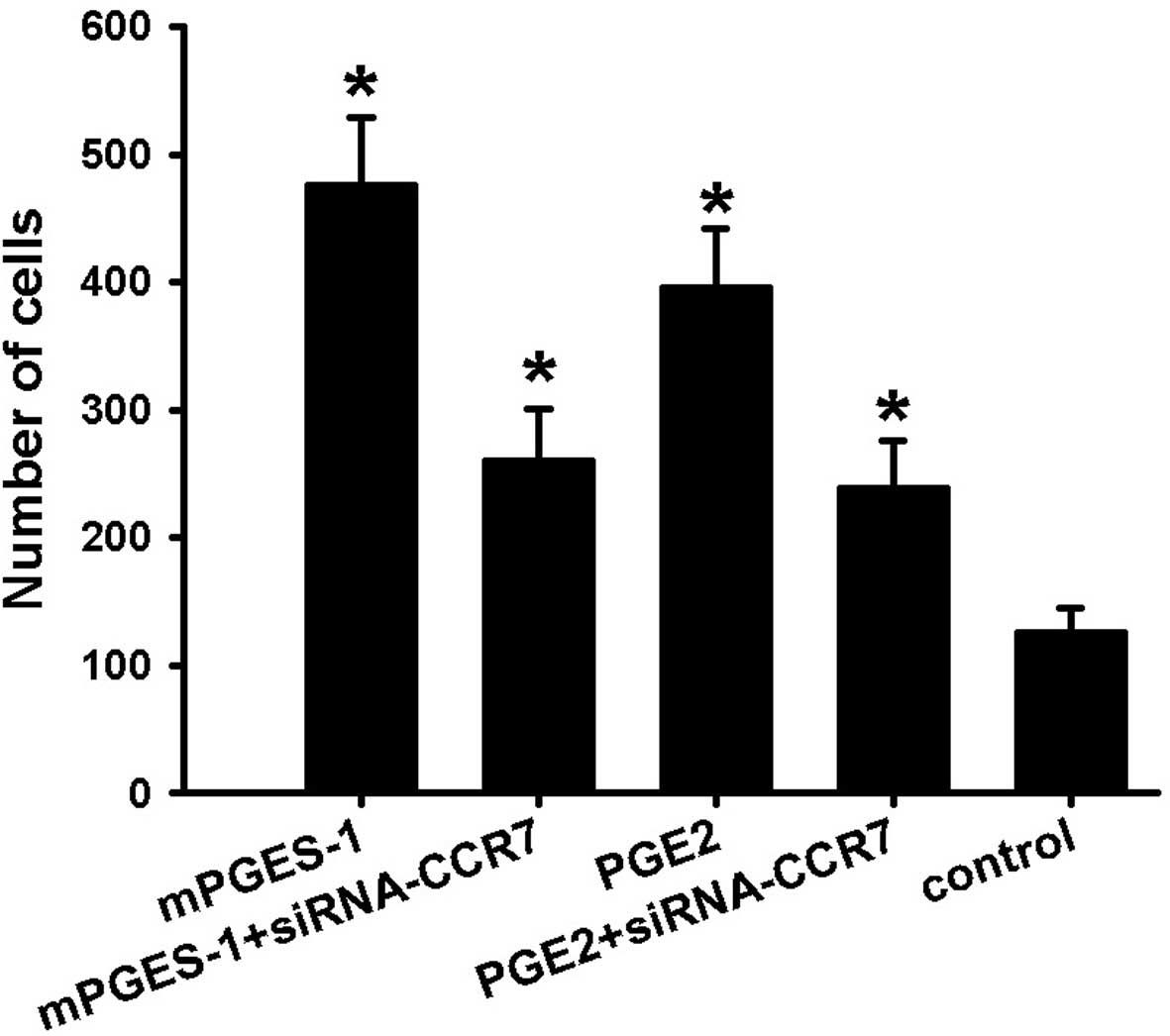
migration assay. As shown in Fig.
1, treatment of cells with mPGES-1 recombinant protease or PGE2
(1 µM) for 48 h resulted in a significant increase in cell
migration, and compared with the control, the number of migrated
cells increased by ~4-fold, which was significantly inhibited by
siRNA-mediated knockout of CCR7. However, in the groups in which
CCR7 knockout was performed, treatment with mPGES-1 or PGE2
increased the number of migrated cells by 2-fold compared with that
in the control group. As the enhancement of the migratory ability
by mPGES-1 or PGE2 was partly blocked by siRNA-CCR7, it is
indicated that mPGES-1 and PGE2 contribute to the migration of
SW620 cells and that CCR7 is crucial in mPGES-1/PGE2-mediated
migration of SW620 cells.
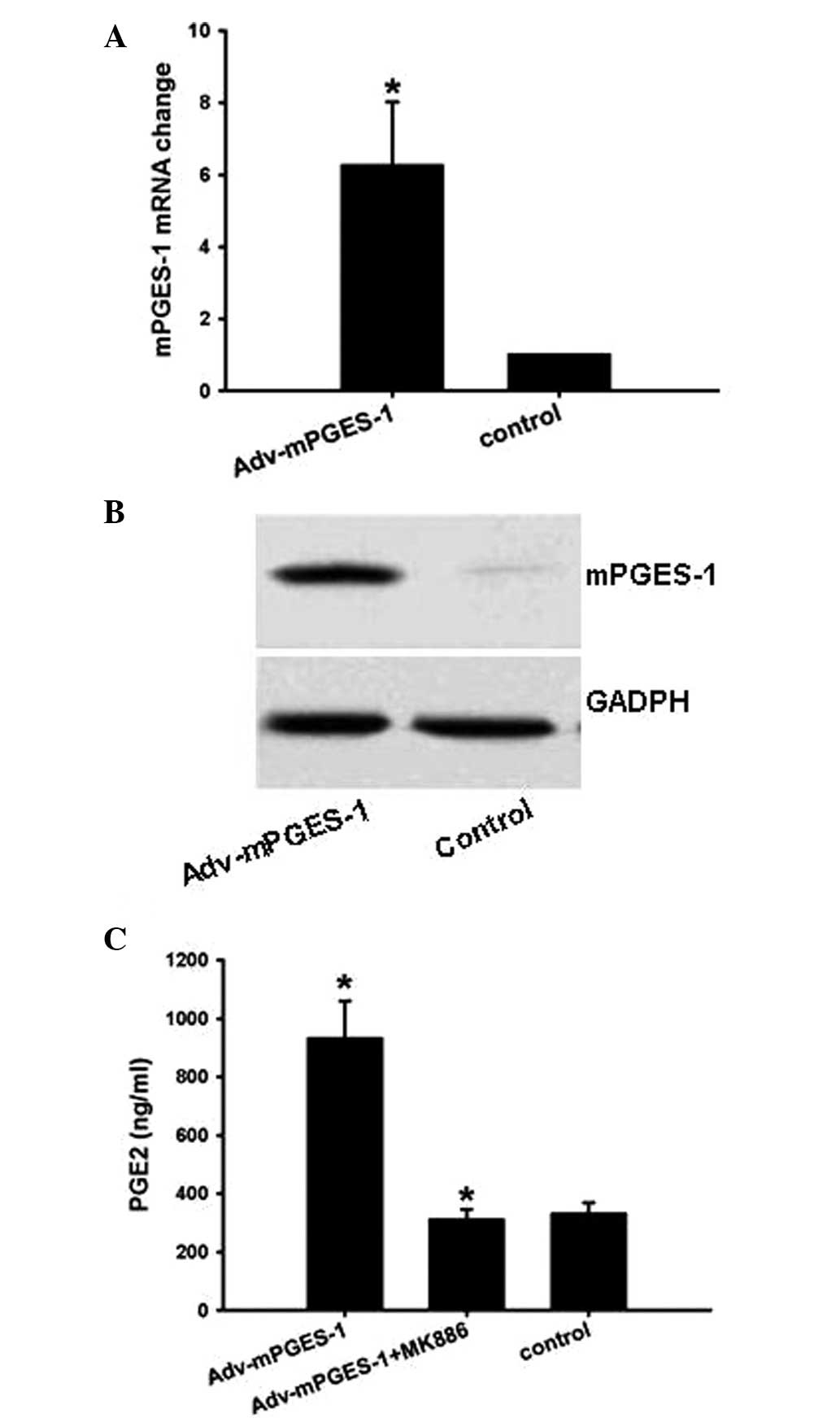
Detection of Adv-mPGES-1 vector
efficiency
As PGE2-mediated cell migration was partly blocked
by siRNA-CCR7, the present study next constructed the
overexpression vector Adv-mPGES-1, which was used to investigate
whether CCR7 expression can be upregulated by mPGES-1-derived PGE2.
The results showed that Adv-mPGES-1 vector enhanced mPGES-1 mRNA
and protein levels (Fig. 2A and
B), which was most efficient following transfection with the
vector for 48 h (data not shown). Following transfection with
Adv-mPGES-1 vector, PGE2 secretion in the cell culture supernatant
was markedly enhanced by 3-fold, which, however, was conpletely
blocked by mPGES-1 inhibitor MK886 (Fig. 2C).
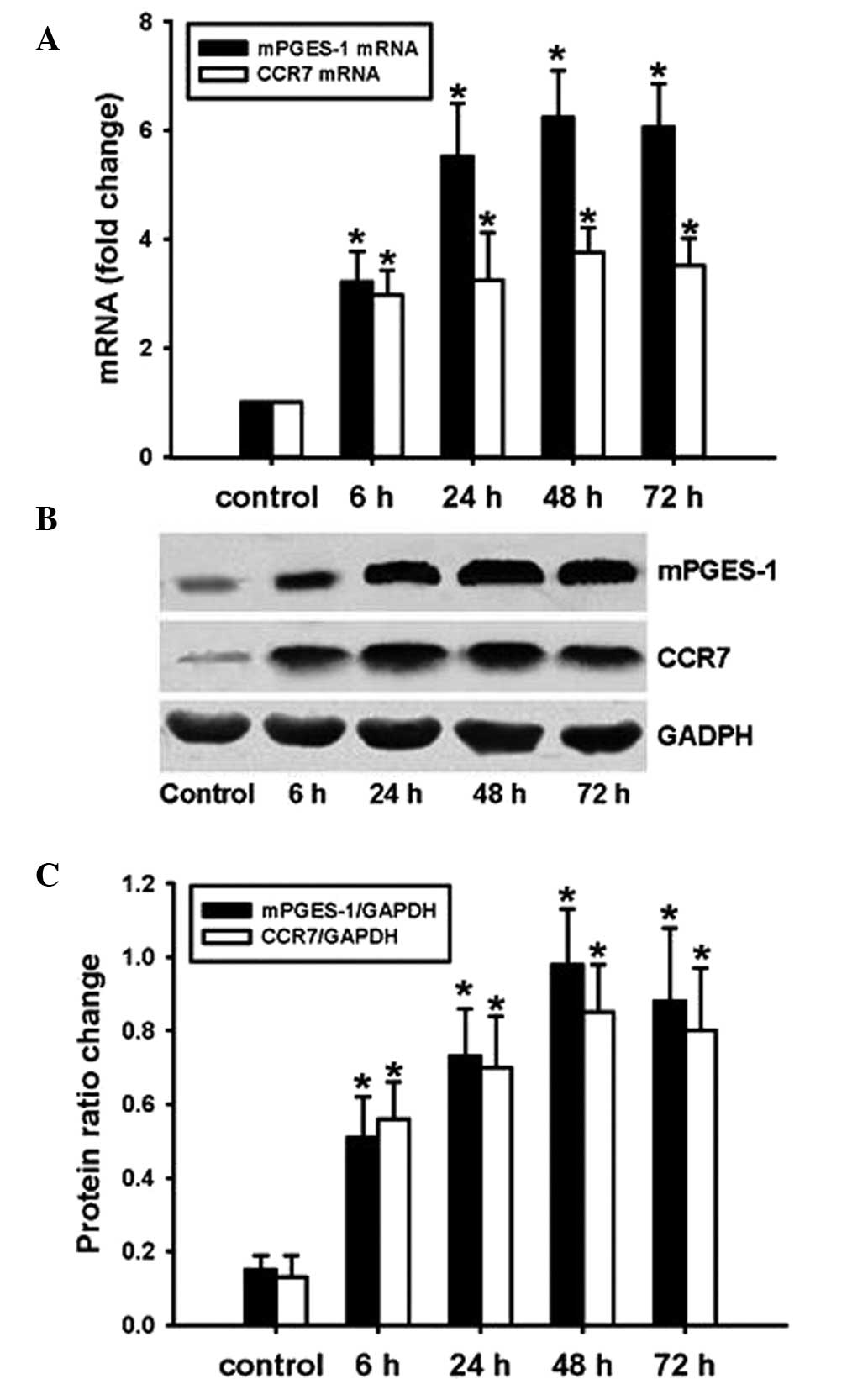
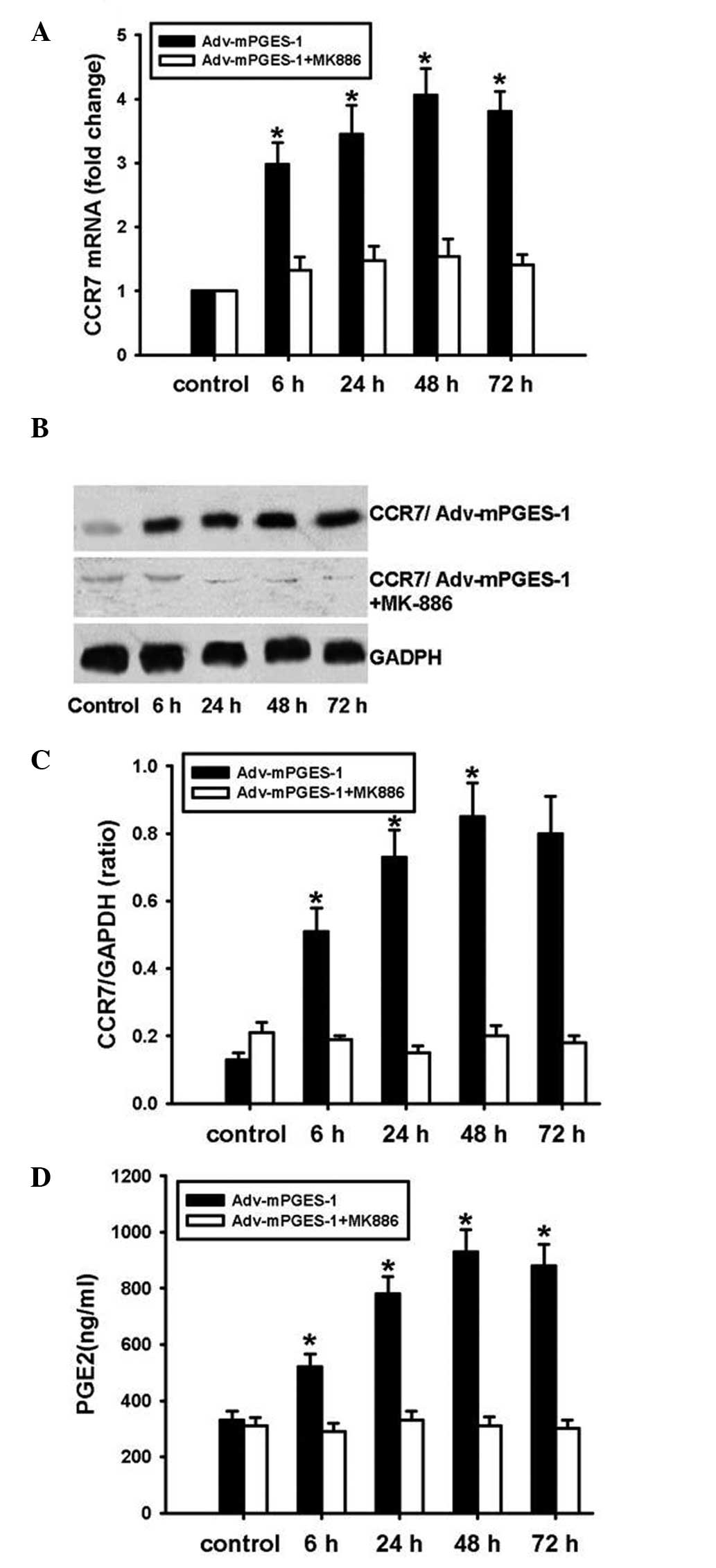
mPGES-1/PGE2 promotes CCR7 expression in
SW620 cells
SW620 cells were transfected with Adv-mPGES-1 vector
for 6, 24, 48 or 72 h and mRNA expression of mPGES-1 and CCR7 was
assessed by RT-qPCR. PGE2 levels in the cell culture supernatant
were determined by ELISA. The results showed that after
transfection with Adv-mPGES-1 vector, expression of mPGES-1 and
CCR7 mRNA was markedly enhanced (Fig.
3A). Similarly to the trends in mRNA expression, the protein
expression of mPGES-1 and CCR7 was markedly increased following
forced overexpression of mPGES-1 (Fig.
3B and C). At the mRNA as well as the protein level,
transfection time-dependent changes in CCR7 expression were in
parallel with those of mPGES-1. To further examine the association
of mPGES-1 and CCR7 expression in SW620 cells, mPGES-1 inhibitor
MK886 was employed. Pre-treatment of SW620 cells with MK886 prior
to transfection with Adv-mPGES-1 blocked the transfection-mediated
increases in PGE2 release and CCR7 expression at the mRNA as well
as the protein level (Fig. 4A–D).
These results indicated that for the mRNA and the subsequent
protein expression of CCR7, PGE2 derived from mPGES-1 is required.
It can be concluded that in SW620 cells, expression of mPGES-1
leads to increases in the levels of PGE2, which then stimulates the
expression of CCR7.
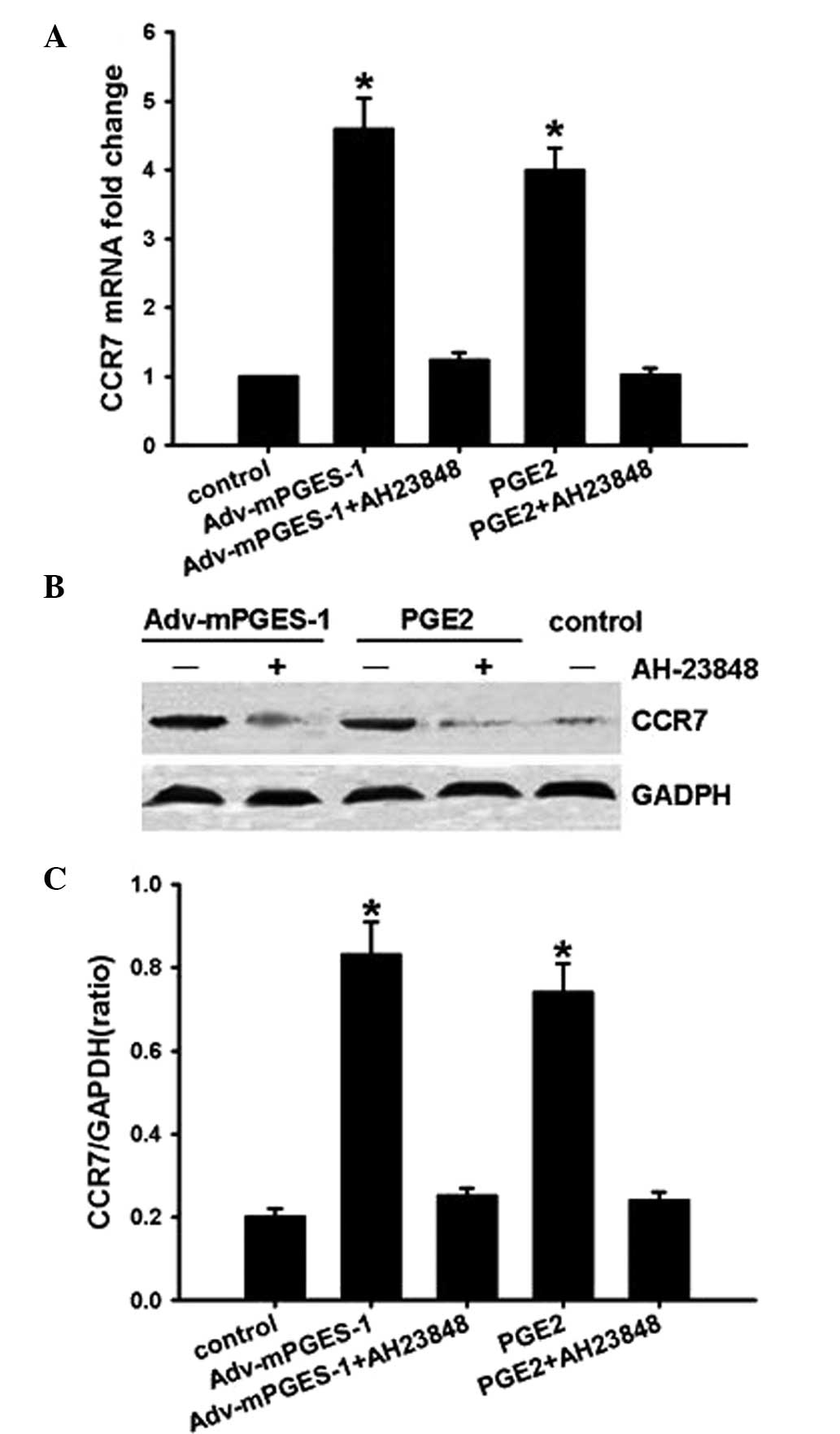
mPGES-1/PGE2 mediates CCR7 expression via
receptor EP4 and subsequent activation of the Akt-GSK3β
pathway
A previous study by our group reported that
COX2/PGE2 increased CCR7 expression via the EP4 receptor in cancer
cells (8). Therefore, the present
study used EP4 inhibitor AH-23848 to investigate whether the
activation of EP4 is involved in the underlying molecular
mechanisms of the mediation of CCR7 expression by mPGES-1/PGE2.
SW620 cells were pre-treated with the inhibitor for 1 h prior to
transfection with Adv-mPGES-1 vector or treatment with PGE2 (10
µM) for 48 h. RT-qPCR and western blot analysis of CCR7
showed that the EP4 inhibitor completely blocked
mPGES-1/PGE2-mediated CCR7 expression at the mRNA as well as at the
protein level (Fig. 5), implying
that mPGES-1/PGE2 enhanced CCR7 expression through binding with
receptor EP4.
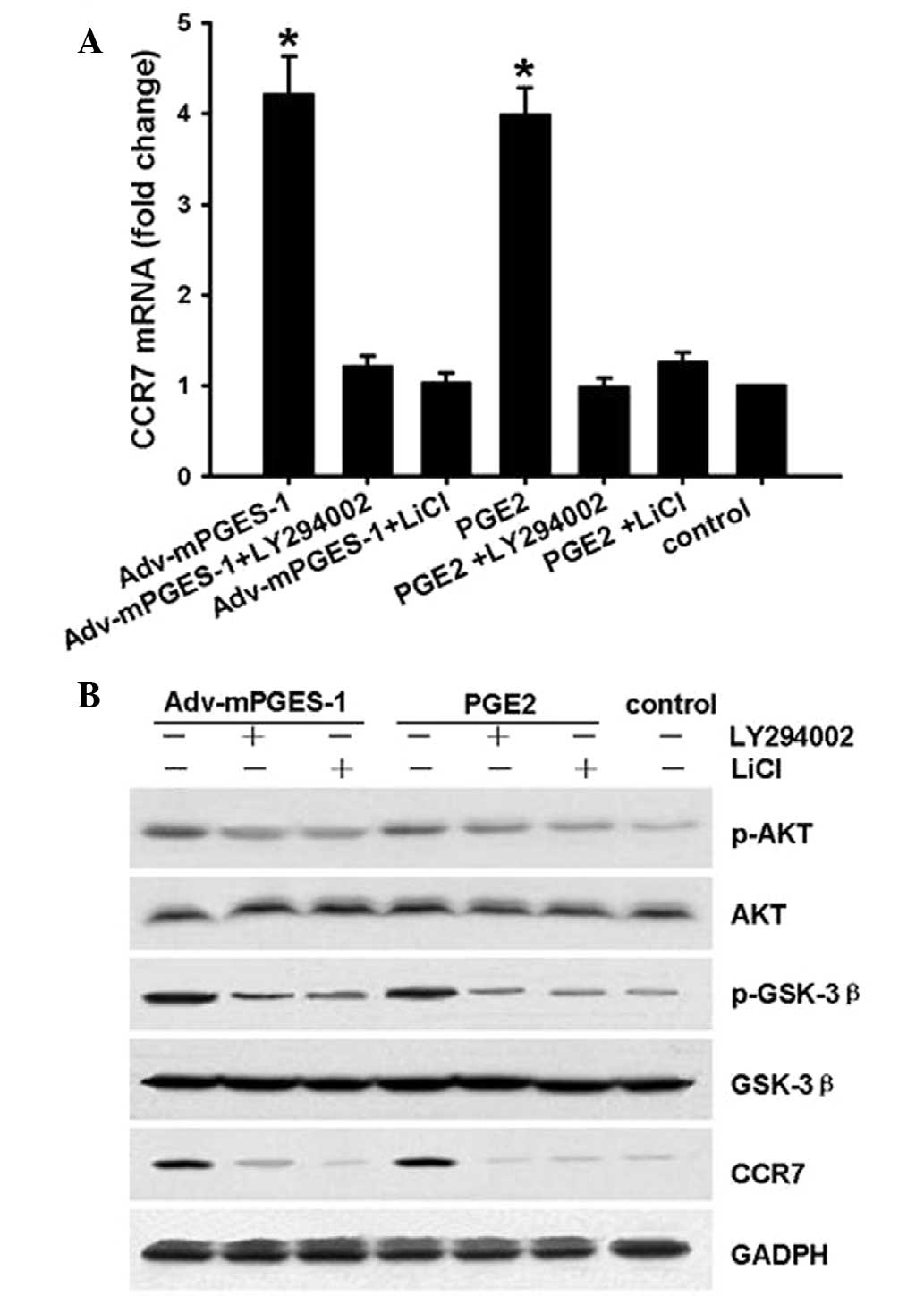
GSK3β is a kinase located downstream of the PI3K/AKT
pathway. GSK3β maintains an active state in its de-phosphorylated
form. The present study determined whether the mediation of CCR7
expression by mPGES-1/PGE2 involves the Akt-GSK3β pathway in SW620
cells. For this, SW620 cells were treated with PI3K/AKT inhibitor
LY294002 (20 µM) or GSK-3β inhibitor LiCl (20 µM) for
1 h prior to mPGES-1/PGE2 treatment, following which CCR7, p-AKT
and p-GSK3β levels were assessed by RT-qPCR or western blotting.
The results showed that after transfection with Adv-mPGES-1 vector
or treatment with PGE2 (1 µM) for 8 h, in parallel with
changes in CCR7 expression (Fig.
6A), the levels of p-AKT and p-GSK3β increased, while the
levels of AKT and GSK-3β were not affected (Fig. 6B). These results indicated that the
EP4-PI3K/AKT-GSK3β pathway is involved in the enhancement of CCR7
expression by mPGES-1/PGE2 in SW620 cells.
Discussion
CCR7 is overexpressed in numerous cancer types and
is involved in the occurrence, progression, metastasis and invasion
of cancer (1–5). Thus, identifying targetable
components of signaling pathways that regulate CCR7 expression may
have broad therapeutic implications. Previous studies by our group
have demonstrated that in colon cancer cells and tissues,
COX-2/PGE2 enhanced the expression of chemokine CCR7 through the
EP4 receptor, which contributed to cancer proliferation and
metastasis (6–8). mPGES-1 signals downstream of COX-2
and, as the last key enzyme of the PGE2 synthesis pathway,
contributes to the synthesis and secretion of PGE2 in cooperation
with COX-2. Previous studies, including those using
mPGES-1-knockout mice, indicated key roles of mPGES-1-generated
PGE2 in female reproduction and in pathological conditions,
including inflammation, pain, fever, anorexia, atherosclerosis,
stroke and tumorigenesis (13,14).
In the present study, the link between CCR7 and
mPGES-1 was investigated. The results suggested that PGE2 secretion
was markedly enhanced by overexpression mPGES-1, and that CCR7
expression in colon cancer cells was significantly upregulated
following overexpression mPGES-1 or treatment with PGE2. These
increases, however, were blocked by mPGES-1 inhibitor MK886. It is
implied that CCR7 expression is upregulated by mPGES-1-derived PGE2
in colon cancer cells, which is consistent with previous studies by
our group reporting that COX-2 and mPGES-1 promoted cancer
progression (6,7). These studies indicated that in colon
cells, CCR7 expression is upregulated by COX-2 and mPGES-1;
however, their influence on cancer progression requires further
investigation. Another study by our group and a previous study
indicated that mPGES-1-derived PGE2 enhanced CCR7 expression
through binding to the EP4 receptor (8). Similarly to the findings by our
group, the COX-2/mPGES-1/EP-4 axis has been indicated to be
involved in the proliferation, migration and angiogenesis of human
tenocytes and cancer cells as well as to be implicated in human
abdominal aortic aneurysm (15,16).
By contrast, other studies showed that COX-2 and mPGES-1-derived
PGE2 promoted cancer progression through the EP2 receptor in canine
and feline malignant mammary tumors (17,18).
These studies indicated that there cell type- and species-dependent
differences may exist, possibly due to different biological
functions of mPGES-1-derived PGE2 through binding to different
receptors.
GSK-3β is a multi-functional serine/threonine
protein kinase located downstream of the PI3K/AKT pathway, which
has a variety of regulatory activities and participates in numerous
signaling pathways. It has a regulatory role in the genesis and
progression of a variety of malignant tumor types through
regulating cancer-associated processes, including proliferation and
apoptosis (19,20). GSK3β maintains an active state in
its de-phosphorylated form. The present study determined that
Akt-GSK3β signal transduction was involved in the enhancement of
CC7 expression by mPGES-1 through PGE2 binding to the EP4 receptor.
Thus, in addition to inhibiting mPGES-1 expression in cancer cells,
EP4 antagonists and AKT/GSK-3β inhibitors may emerge as potential
therapeutics to reduce CCR7 expression in cancer. It is likely that
CCR7 has a crucial role in mPGES-1/PGE2-mediated cancer-cell
migration. The underlying mechanisms of the interaction of mPGES-1
and CCR7 in cancer-cell migration and the resulting formation of
metastases deserves further investigation.
Acknowledgments
The present study was supported by the Nation
Natural Science Foundation of China (grant nos. 81101476 and
81460613) and the Guangxi Nature Science Foundation of China (grant
no. 2013GXNSFBA019126 to J.L.).
References
|
1
|
Coffelt SB and de Visser KE: Cancer:
Inflammation lights the way to metastasis. Nature. 507:48–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lipp M, Burgstahler R, Müller G, Pevzner
V, Kremmer E, Wolf E and Förster R: Functional organization of
secondary lymphoid organs by the chemokine system. Curr Top
Microbiol Immunol. 251:173–179. 2000.PubMed/NCBI
|
|
3
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowan JE, McCarthy NI, Parnell SM, White
AJ, Bacon A, Serge A, Irla M, Lane PJ, Jenkinson EJ, Jenkinson WE
and Anderson G: Differential requirement for CCR4 and CCR7 during
the development of innate and adaptive αβT cells in the adult
thymus. J Immunol. 193:1204–1212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinhardt A, Ravens S, Fleige H, Haas JD,
Oberdörfer L, Łyszkiewicz M, Förster R and Prinz I: CCR7-mediated
migration in the thymus controls γδ T-cell development. Eur J
Immunol. 44:1320–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu S, Li J, Ji W and Chen Y: CCR7
expression is regulated by COX-2 in colon cancer cells. Chin J Mod
Med. 21:5–9. 2011.In Chinese.
|
|
7
|
Yu S, Li J, Ji W and Chen Y: The
clinicopathological features of COX2 and CCR7 in human breast
carcinoma tissue. Chin J Mod Med. 21:990–992. 2011.In Chinese.
|
|
8
|
Yu S, Duan J, Zhou Z, Pang Q, Wuyang J,
Liu T, He X, Xinfa L and Chen Y: CCR7 is a critical role in
invasion and metastasis of colon cancer in vivo and in vitro.
Cancer Biol Ther. 7:1037–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruan D and So SP: Prostaglandin E2
produced by inducible COX-2 and mPGES-1 promoting cancer cell
proliferation in vitro and in vivo. Life Sci. 116:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casós K, Siguero L, Fernández-Figueras MT,
León X, Sardá MP, Vila L and Camacho M: Tumor cells induce COX-2
and mPGES-1 expression in microvascular endothelial cells mainly by
means of IL-1 receptor activation. Microvasc Res. 81:261–268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang HH and Meuillet EJ: Identification
and development of mPGES-1 inhibitors: Where we are at? Future Med
Chem. 3:1909–1934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bett AJ, Haddara W, Prevec L and Graham
FL: An efficient and flexible system for construction of adenovirus
vectors with insertions or deletions in early regions 1 and 3. Proc
Natl Acad Sci USA. 91:8802–8806. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elander N, Ungerbäck J, Olsson H, Uematsu
S, Akira S and Söderkvist P: Genetic deletion of mPGES-1
accelerates intestinal tumorigenesis in APC (Min/+) mice. Biochem
Biophys Res Commun. 372:249–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samuelsson B, Morgenstern R and Jakobsson
PJ: Membrane prostaglandin E synthase-1: A novel therapeutic
target. Pharmacol Rev. 59:207–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan D and So SP: Prostaglandin E2
produced by inducible COX-2 and mPGES-1 promoting cancer cell
proliferation in vitro and in vivo. Life Sci. 116:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dolkart O, Liron T, Chechik O, Somjen D,
Brosh T, Maman E and Gabet Y: Statins enhance rotator cuff healing
by stimulating the COX2/PGE2/EP4 pathway: An in vivo and in vitro
study. Am J Sports Med. 42:2869–2876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camacho M, Dilmé J, Solà-Villà D,
Rodríguez C, Bellmunt S, Siguero L, Alcolea S, Romero JM, Escudero
JR, Mar tínez-González J and Vila L: Microvascular
COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J Lipid
Res. 54:3506–3515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Millanta F, Asproni P, Canale A, Citi S
and Poli A: COX-2, mPGES-1 and EP2 receptor immunohistochemical
expression in canine and feline malignant mammary tumours. Vet Comp
Oncol. May 14–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mai W, Miyashita K, Shakoori A, Zhang B,
Yu ZW, Takahashi Y, Motoo Y, Kawakami K and Minamoto T: Detection
of active fraction of glycogen synthase kinase 3beta in cancer
cells by nonradioisotopic in vitro kinase assay. Oncology.
71:297–305. 2006. View Article : Google Scholar
|
|
20
|
Shakoori A, Ougolkov A, Yu ZW, Zhang B,
Modarressi MH, Billadeau DD, Mai M, Takahashi Y and Minamoto T:
Deregulated GSK3beta activity in colorectal cancer: Its association
with tumor cell survival and proliferation. Biochem Biophys Res
Commun. 334:1365–1373. 2005. View Article : Google Scholar : PubMed/NCBI
|