Introduction
The treatment of bone defects arising from traumatic
events, congenital abnormalities and infection involves
autografting and allografting of bone tissues (1,2), or
replacement surgery (3–5). The satisfactory clinical outcomes of
autografting and allografting (6)
are tempered, however, by problems of insufficient supply,
significant morbidity and risk of disease transmission, promoting
the development of alternative technologies, such as bone tissue
engineering (7,8). The primary purpose of bone tissue
engineering is to generate synthetic bone grafting substitutes
through the combination of scaffolding material, viable cells and
growth factors. In particular, biocompatible scaffolds are
garnering increased attention due to their provision not only of
structural support for cells, but also of an environment promoting
cell adhesion, differentiation and proliferation (9,10).
Chitosan (CS) is a newly developed biomedical
material with biocompatibility, low toxicity and biodegradability
(11,12), and its polysaccharide side chains
contain numerous amino and hydroxyl groups that serve as convenient
links for surface modifications. In addition, hydroxyapatite (HA),
a common biomaterial with chemical components similar to bone
mineral, has been widely used in orthopedics to facilitate bone
ingrowth and osseointegration (13,14).
The recent appreciation that the requirements of bone tissue
engineering scaffolds cannot be fulfilled by any single material,
has given rise to the concept of composite bone scaffolds. In this
context, CS/HA composites generated by wet chemical methods, such
as sol-gel, co-precipitation, in situ compositing and
electrochemical deposition (15–17),
have been shown to accelerate osseointegration (18).
Since cell differentiation and proliferation can
only occur following adhesion of cells to a biocompatible surface
(19), facilitation of this
process by improving cell-scaffold interactions is a desirable
property of a bone scaffold (20,21).
To this end, cell-scaffold adhesion has been shown to be promoted
by modification of the scaffold using small signaling molecules,
such as arginine-glycine-aspartic (RGD) or cell growth factors
(22), although another study
suggested that RGD peptide may actually inhibit bone formation
(23). The present study
investigated the material-cell interaction characteristics and
osseointegrative properties of an RGD-modified CS/HA (RGD-CS/HA)
composite scaffold to evaluate its potential application in bone
tissue engineering.
Materials and methods
Isolation and cultivation of bone marrow
stromal cells (BMSCs)
The present study was approved by the ethics
committee of The First Hospital of Harbin City (Harbin, China).
Fisher rats (n=100; weight range, 100–150 g) were provided by
Centre of Experimental Animal of Harbin Medical University (Harbin,
China). The animals were maintained in standard lab conditions at
25±2°C in a 12 h light/dark cycle. The animals were provided with
food and water ad libitum. Care of the rats was in
compliance with the 'Guide for the Care and Use of Laboratory
Animals' published by the National Institute of Health (24). BMSCs were isolated from rat femurs
according to the method reported by Tamada and Ikada (25), and cultivated in the control media
consisting of complete Dulbecco's modified Eagle's medium (DMEM,
Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS, Gibco-BRL) and 1% penicillin/streptomycin antibiotics
(Mediatech, Inc., Mooresville, NC, USA) in a 5% CO2
atmosphere at 37°C. The culture medium was replaced every 2–3 days
and cells were passaged at 90% confluence.
Preparation of the RGD-CS/HA
scaffold
The CS/HA composite was prepared by in situ
compositing combined with lyophilization. Briefly, 1.25 g
Ca(NO3)2•4H2O and 0.43 g
KH2PO4 were added to 100 ml acetic acid, and
the solution was stirred until fully dissolved. Then, 5.0 g CS
(Haihui Bioengineering Co., Ltd., Qingdao, China) was added to the
solution, and the mixture was stirred for 4 h at room temperature
to obtain a homogeneous 5 wt% CS solution. Subsequently, the CS
solution was cast onto the internal surface of a custom-made mold,
and the mold was soaked in 5 wt% NaOH to form a CS/HA hydrogel. The
hydrogel was washed with deionized water until the pH value of the
water was ~7, then air-dried in an oven at 60°C until a diameter of
10 mm was reached. The semi-dried CS/HA composite was lyophilized,
and cut into CS/HA disks with a diameter of 10 mm and a height of
1.5 mm. The CS/HA disks were sterilized in an autoclave at 120°C
for 30 min.
The RGD-CS/HA scaffold was prepared by physical
adsorption of the RGD peptide onto the CS/HA disks. Briefly, the
sterilized CS/HA samples were immersed in RGD solution (100 mg/l
C12H22N6O6;
Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 24 h. The RGD-CS/HA
scaffold was obtained after washing in phosphate-buffered saline
(PBS) three times to remove free reagents, followed by air drying.
Spatial distribution of the RGD peptide on the CS/HA samples was
determined by labeling the RGD solution with fluorescein
isothiocyanate (FITC) followed by visualization using a Nikon E800
fluorescence microscope (Nikon Instruments Inc., Melville, NY,
USA).
RGD-CS/HA scaffold-BMSC interactions
Cell adhesion rate
A total of six RGD-CS/HA scaffolds were placed into
the well of a 24-well culture plate. In total, 1×104
cells diluted into 2 ml of control media supplemented with
10−9 mol/l Na-β-glycerophosphate (Trust and
Pharmaceutical Co., Ltd., Shanghai, China) and 10−8
mol/l dexamethasone were then added to each well. The scaffold-cell
construct was maintained in cell culture for 4 h at 37°C in a 5%
CO2 atmosphere, after which the RGD-CS/HA scaffolds were
washed three times with PBS to remove unattached cells. Adherent
cells were removed from the RGD-CS/HA scaffolds using trypsin-EDTA,
and counted using a Cell Counting kit-8 (Dojindo Molecular
Technologies, Inc., Rockville, MA, USA). The absorbance values were
measured at 450 nm using a Multiskan Mk3 (Thermo Fisher Scientific,
Waltham, MA, USA) for the initial cell solution (Ai) and
the unattached cell solution (Afree), and the cell
adhesion rate was estimated using the following equation: Cell
adhesion rate=(1-Afree/Ai) × 100. The average
cell adhesion rate of the six RGD-CS/HA scaffolds was
calculated.
Cell viability
BMSC viability on the RGD-CS/HA scaffold was
evaluated after 3 days of culture in DMEM supplemented with 10% FBS
using the Live/Dead Viability kit for mammalian cells (Life
Technologies, Saint Aubin, France). In this assay, calcein-AM
penetrates into the cytosol of viable cells and stains the cells
green, while ethidium homodimer-1 stains dead cell nuclei red.
Following 30 min of incubation at room temperature and rinsing with
PBS, BMSCs were observed using a FluoVie FV300 confocal laser
microscope (Olympus, Tokyo, Japan) and a Nikon E800 fluorescence
microscope.
Cell morphology
BMSC morphology on the RGD-CS/HA scaffold was
evaluated after 4, 24 and 48 h of culture in DMEM supplemented with
10% FBS. The RGD-CS/HA scaffolds were rinsed thoroughly with PBS to
remove the unattached cells, and then fixed using a 2.5%
glutaraldehyde solution (Sigma-Aldrich). Fixed cells were
dehydrated progressively in a graded ethanol series, after which
RGD-CS/HA scaffolds were sputter-coated with a gold-palladium layer
and examined using a scanning electron microscope (SEM, S-3400 N;
Hitachi, Toyko, Japan).
Alkaline phosphatase (ALP)
activity
Following 14 days of culture under osteogenic
conditions (DMEM supplemented with 10% FBS, 50 µg/ml vitamin
C(Trust and Pharmaceutical Co., Ltd.,), 10−9 mol/l
Na-β-glycerophosphate and 10−8 mol/l dexamethasone; Hao
Yuan Biological Pharmaceutical Co., Ltd., Shanghai, China), the
RGD-CS/HA scaffold was transferred to a 1.5-ml centrifuge tube. The
cell membrane was ruptured by ultrasonic cleaning to release the
proteins and DNA into the supernatant. Supernatant ALP activity was
measured using a rat alkaline phosphatase ELISA kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
instructions. DNA was quantified using a PicoGreen kit (Genmed
Scientifics Inc., Wilmington, DE, USA) following standard protocol.
The absorbance was read at an excitation/emission of 480–530 nm on
a microplate reader. Three measurements were taken and the results
are reported as the mean ± standard deviation.
In vivo heterotopic ossification
The biocompatibility of the RGD-CS/HA scaffold was
evaluated through in vivo heterotopic ossification. Male
Fisher rats (n=60) were randomly divided into two groups. Following
anesthesia with 10% chloral hydrate (Trust and Pharmaceutical Co.,
Ltd.), a 2-cm incision was made in the back, and the subcutaneous
tissue and fascia were separated. Subsequently, RGD-CS/HA
(RGD-CS/HA group), or CS/HA (CS/HA control group) scaffolds were
implanted. The incision was then sutured using Yiqiao absorbable
sutures (Johnson & Johnson, New Brunswick, NJ, USA) and the rat
was injected with gentamicin (5 mg/kg; Shanxi Kang Pharmaceutical
Co., Ltd., Lihua Yuan, China). After implantation for 1 day, 1
week, 2 weeks, 4 weeks or 6 weeks, respectively (six rats at each
time point), scaffolds were collected and examined by X-ray
radiography (YSX0101; Yueshen Medical Equipment Co., Ltd.,
Guangzhou, China). The radiographs were imported using Image Pro
Plus software (Media Cybernetics, Inc., Rockville, MD, USA), and
the optical density value was calculated. Scaffolds were then fixed
using a 10% glutaraldehyde solution, decalcified, stained with
hematoxylin and eosin (H&E), mounted in resin and sectioned
into 5-µm samples prior to examination using an optical
microscope (S8; Olympus Corporation, Toyko, Japan).
In vivo bone defect repair
The osseointegrative properties of the RGD-CS/HA
scaffold were investigated using an in vivo bone defect
repair experiment. Briefly, 36 female New Zealand rabbits (weight
range, 2.0–2.5 kg) provided by the Centre of Experimental Animal of
Harbin Medical University were randomly divided into three groups.
Rabbits were intravenously anesthetized with 2% pentobarbital
(Wegene Co., Ltd., Shanghai, China), after which a 3-cm incision
was made in the radius of the front leg. After separation of
subcutaneous tissue and muscle, a bone defect with a length of 1.5
cm was established, with the osteotomy line ~3 cm away from the
distal end of the radius. For the RGD-CS/HA group, the RGD-CS/HA
scaffold was implanted into the bone defect area, and for the CS/HA
group, the CS/HA scaffold was used. The incision was then washed
with gentamicin and sutured. For the control group, no scaffold was
implanted and the incision was sutured directly. The rabbits were
injected with 10 mg/kg penicillin (Shanxi Kang Pharmaceutical Co.,
Ltd.) for 3 days, then examined by X-ray radiography after
implantation for 2, 4 and 8 weeks (four rabbits at each time
point). Additionally, for each group, three-dimensional (3D)
reconstruction of the bone defect area was performed 8 weeks after
implantation of the scaffolds using a electronic computer X-ray
tomography technique (Aquilion ONE, Toshiba, Tokyo, Japan;
thickness: 0.5 mm). Furthermore, the radius of the rabbit in the
RGD-CS/HA and CS/HA scaffold groups was obtained 8 weeks after
implantation, and the attached ligaments and muscles were carefully
dissected. The ends of the radius were mounted with denture powder
(Pigeon Dental Mfg. Co., Ltd., Shanghai, China), then placed in an
SMD-10 universal material tester (Mechanical Science Co., Ltd.,
Changchun, China) for three-point bending test with a loading speed
of 0.5 mm/min. The maximum applied load that the radius could bear,
and the bending rigidity, were recorded during the experiment, and
compared with values obtained for a normal radius. The average
value for four rabbits The average value of four rabbits from each
group were calculated, and the results were reported as the mean ±
standard deviation. The statistical significance was determined by
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. The animals were sacrificed
by injecting 4% pentobarbital sodium (4 ml/kg; Xitang Biological
Technology Co., Ltd., Shanghai, China).
Statistical Analysis
The results of the biomechanical assessments are
reported as the mean ± standard deviation and were analyzed using
Statistical Product and Service Solutions software (SPSS version
19.0; SPSS, Inc., Chicago, IL, USA). The statistical difference
among different groups was evaluated using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Scaffold implantation
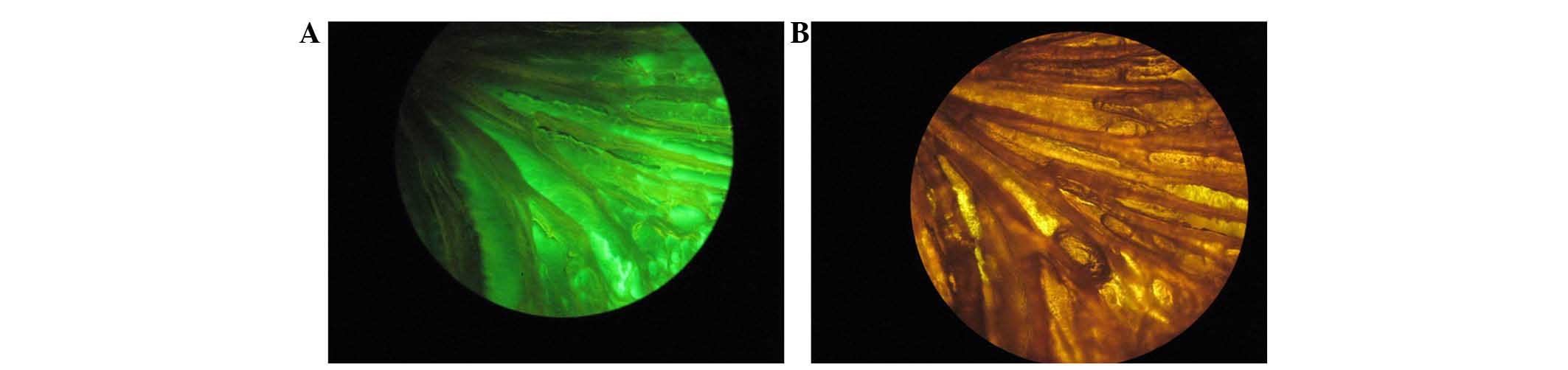
Fig. 1 shows
micrographs of the FITC-labeled RGD-CS/HA scaffold with and without
fluorescence. Under the non-fluorescent mode, the channel
morphology of the CS/HA composite was clearly visible, whilst under
the fluorescent mode, the CS/HA composite exhibited a homogeneous
bright green color, indicating uniform distribution of the RGD
peptide.
RGD-CS/HA scaffold-BMSC interactions
Cell adhesion rate
The adhesion rate of BMSCs to the RGD-CS/HA scaffold
in the initial incubation period (4 h) was 80.7% when the scaffold
was prepared using 100 mg/l CS solution, compared with 71.6% for
scaffold prepared using 50 mg/l CS solution. These results indicate
that RGD modification enhances the cell affinity of the CS/HA
composite.
Cell viability
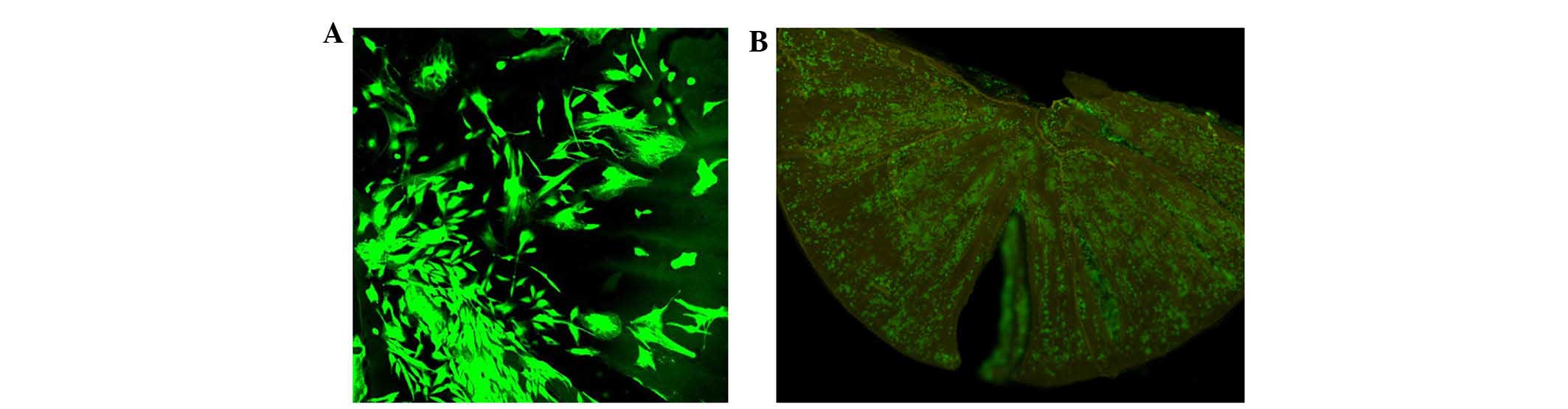
Confocal (Fig. 2A)
and fluorescence (Fig. 2B)
microscopy images of BMSCs in the Live/Dead cell assay show that
BMSCs were almost entirely viable, fusiform in shape, and evenly
distributed on the RGD-CS/HA scaffold, including the channel-shaped
pores. These results indicated the high cytocompatibility between
the RGD-CS/HA scaffold and BMSCs.
Cell morphology
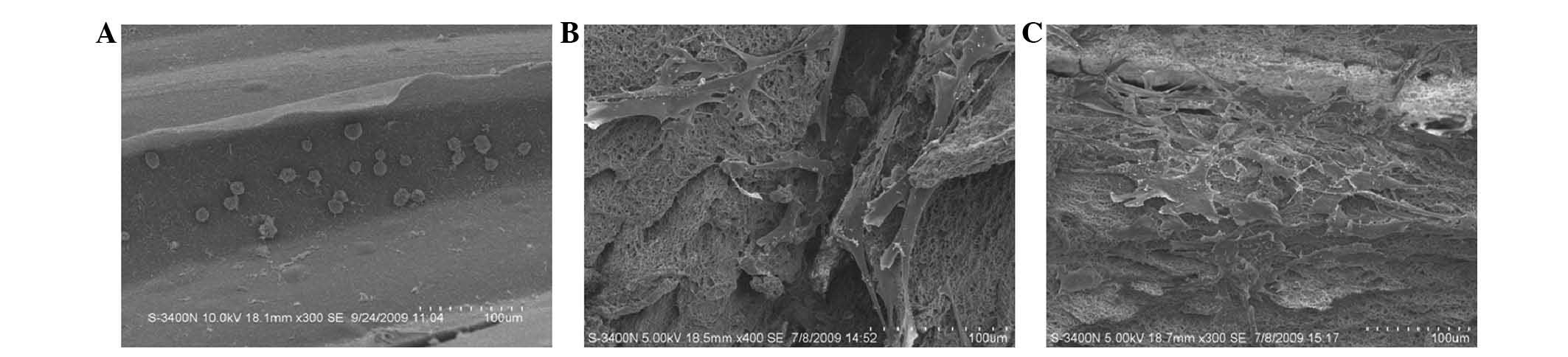
SEM images of BMSCs seeded on the RGD-CS/HA scaffold
indicate that after 4 h the BMSCs exhibited a round or elliptical
shape with almost no spread on the scaffold (Fig. 3A), whereas after 24 h of culture,
they had a flattened polygonal shape and exhibited pseudopodia
(Fig. 3B). After 48 h of culture
(Fig. 3C), BMSCs were well
distributed on the scaffold, had formed extracellular matrix, and
had made numerous cell-cell interactions.
Alkaline phosphatase (ALP)
activity
ALP is an essential enzyme for ossification and is a
well-defined marker for cell differentiation to an osteogenic
lineage. After 14 days of culture under osteogenic conditions, the
ALP content of the BMSCs on the RGD-CS/HA scaffold was elevated
(0.006±0.0008 U/l/ng), indicating that the BMSCs on the RGD-CS/HA
scaffold differentiated toward an osteogenic phenotype.
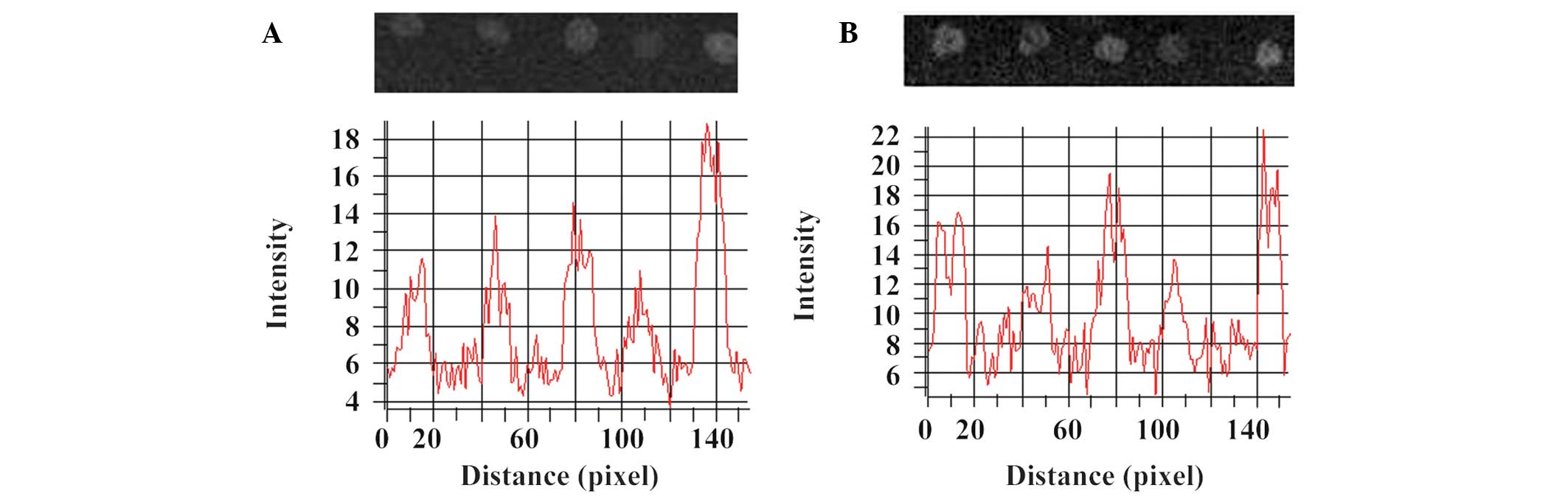
In vivo heterotopic ossification
X-ray radiographs and optical density values of the
implanted scaffolds in the RGD-CS/HA (Fig. 4A) and CS/HA (Fig. 4B) rat groups after 1 day, and 1, 2,
4 and 6 weeks indicate that, in the two groups, the lowest and
highest optical density values were observed 4 and 6 weeks after
implantation, respectively.
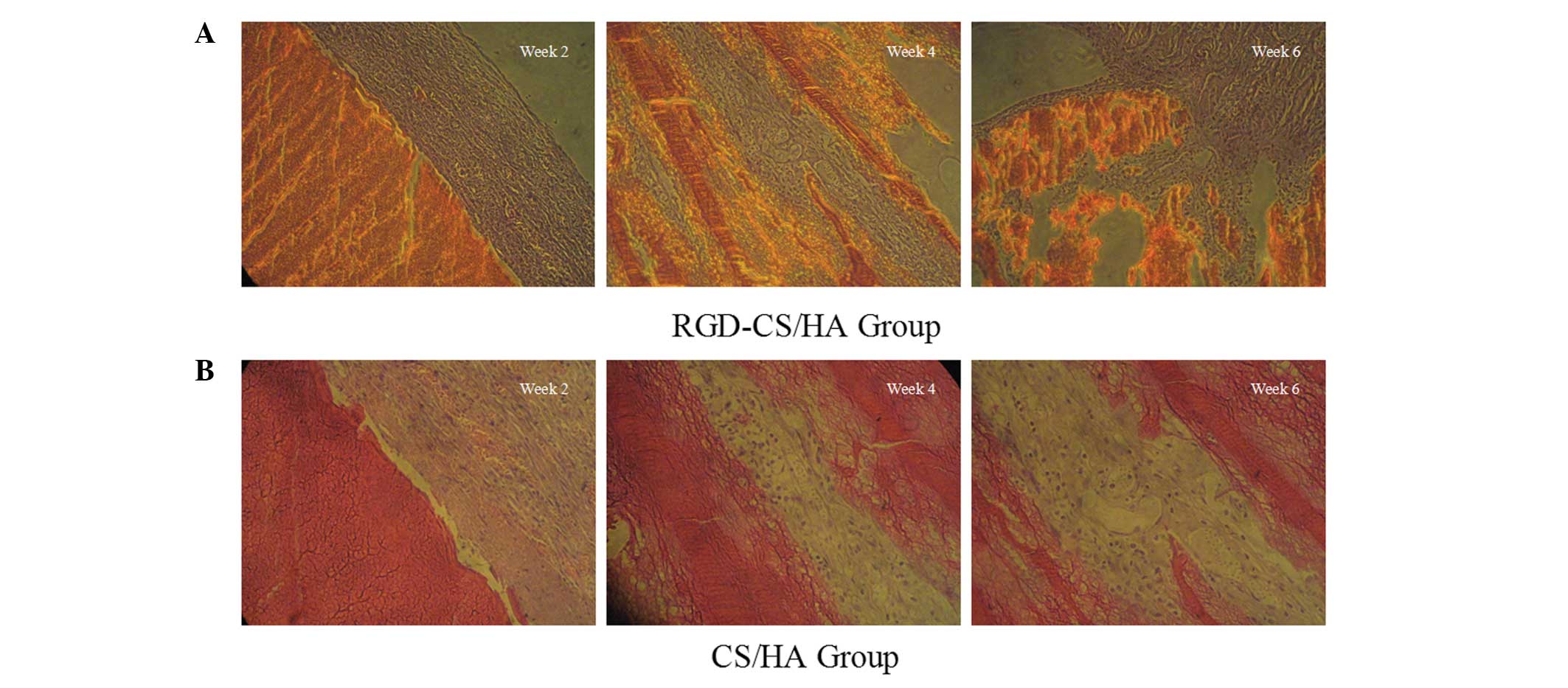
Optical micrographs
Optical micrographs of the implanted scaffolds in
the RGD-CS/HA rat group at 2, 4 and 6 weeks (Fig. 5A), indicate the presence of a small
number of chondrocytes and abundant blood vessels in the newly
formed tissues 2 weeks after implantation. Osteoclasts and
osteoblasts were evident after 4 weeks, whereas 6 weeks after
implantation, mature trabecular bone and medullary cavity were
observed, with blood vessels in between. By contrast, CS/HA
scaffolds were characterized by fibroblast hyperplasia giving rise
to predominantly fibrillar connective tissues 4 weeks after
implantation on the CS/HA scaffold, with only a small amount of
trabecular bone and number of collagen fibers visible after 6
weeks. These results indicated the superior biocompatibility of the
RGD-CS/HA compared with the CS/HA scaffold.
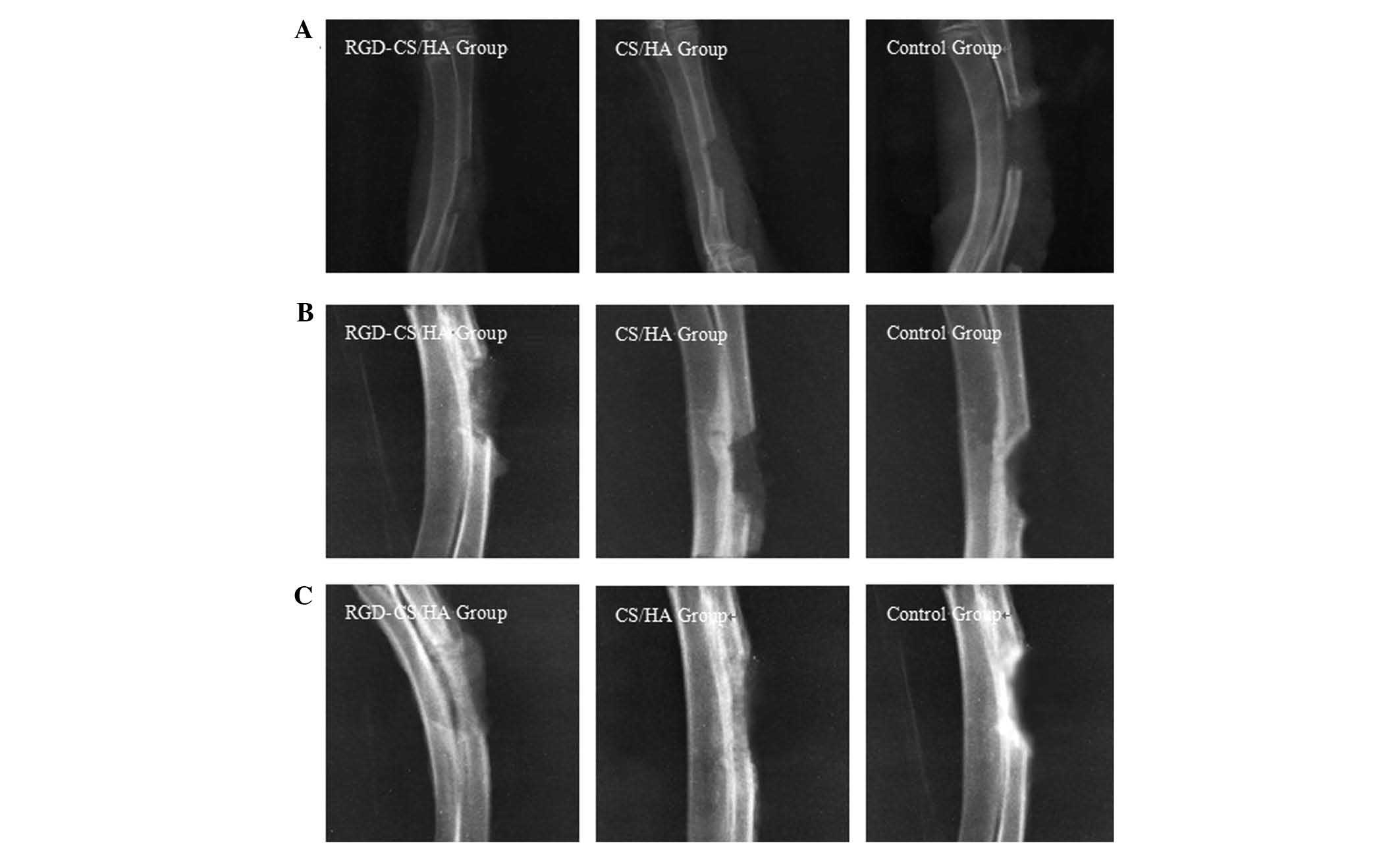
In vivo bone defect repair
X-ray radiographs comparing rabbit radial bone
defects in the RGD-CS/HA, CS/HA and control groups indicate a low
density shadow in the RGD-CS/HA group only 2 weeks after
implantation (Fig. 6A), whereas 4
weeks after implantation (Fig.
6B), high and low density shadows were observed for the
RGD-CS/HA and CS/HA groups, respectively. Eight weeks after
implantation (Fig. 6C), de
novo bone tissue in the RGD-CS/HA group had a density
comparable to that of normal bone, whereas the relatively small
diameter of the de novo bone tissue in the CS/HA group
indicated that the scaffold had collapsed in vivo. In the
control group, a periosteal reaction was observed after 2 and 4
weeks, whereas after 8 weeks an increase in the density of the ends
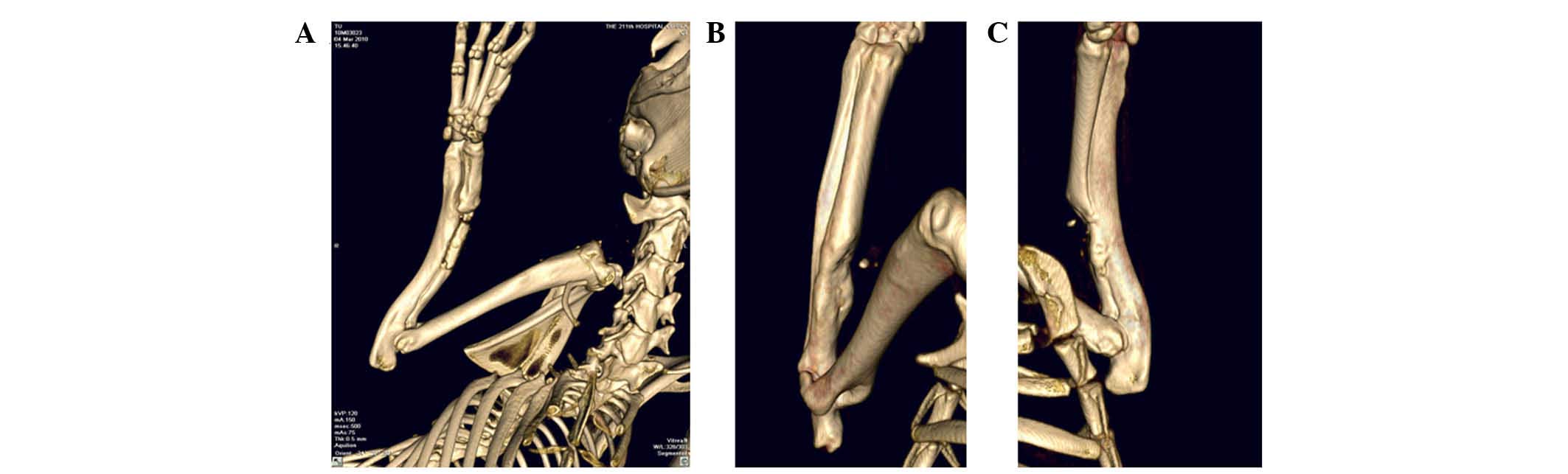
of the radius was observed. 3D images obtained by X-ray tomography
(Fig. 7) confirmed that formation
of bone tissue after 8 weeks implantation was optimal on the
RGD-CS/HA scaffold. Consistent with these data, the maximum applied
load and bending rigidity of the radius in the RGD-CS/HA group were
comparable with those of the normal group (P>0.05) and
significantly higher than those of the CS/HA group (P<0.05;
Table I).
 | Table IMaximum applied load and bending
rigidity of the radius in the different groups. |
Table I
Maximum applied load and bending
rigidity of the radius in the different groups.
| Group | Maximum applied
load (N) | Bending rigidity
(N/mm) |
|---|
| Normal radius |
166.22±18.83a | 62.90±4.88a |
| RGD-CS/HA |
152.57±14.63a | 57.80±5.33a |
| CS/HA | 79.25±18.35 | 36.36±6.35 |
Discussion
Bone tissue engineering has emerged as an important
method in the repair of bone defects, as well as replacement
surgery (26–28) and, in this context, there is
increasing interest in the development of composite scaffolds with
osseointegrative properties. Highly porous scaffolds with open
structures are considered to be the optimal candidates for bone
substitutes due to their facilitation of bone oxygenation and
angiogenesis (29). We have
previously shown that a CS/HA composite prepared by in situ
compositing in combination with lyophilization contained
channel-shaped and spherical pores with average sizes of 400 and
7.8 µm, respectively, and high porosity (30). The channel-shaped pores provide a
framework for bone ingrowth into the pores, and the spherical pores
are suitable for capillary ingrowth and extracellular matrix
interactions. In the present study, it was demonstrated that an RGD
peptide-modified CS/HA composite with increased cellular affinity
was superior to the CS/HA composite with respect to cell adhesion
rate, cell viability, cell morphology and ALP activity.
Furthermore, the scaffold had favorable osseointegrative
properties, as measured by in vivo heterotopic ossification
and bone defect repair, and possessed biomechanical properties
comparable to those of a normal radius. The present results
indicate that the RGD-CS/HA scaffold has promising potential for
bone tissue engineering applications.
The initial cell adhesion rate on a bone tissue
engineering scaffold is crucial for subsequent cell proliferation
and differentiation, and exerts a significant influence on the
subsequent morphogenesis of artificial tissues. The adhesion rate
of BMSCs in the current study is markedly higher than that of rat
osteosarcoma cells on RGD-modified CS scaffolds after 1 week of
culture (31). Although the
difference in the identity of the seed cells may contribute to this
discrepancy, it is hypothesized that it is also attributable to the
increased access of the BMSCs to the interior of the RGD-CS/HA
scaffold afforded by the presence of the channel-shaped pores. Such
a notion is supported by our visualization of BMSCs in the
channel-shaped pores of the scaffold, as well as by numerous
cell-cell interactions and the elevated ALP activity of BMSCs on
the RGD-CS/HA scaffold, which compares favorably to that of a
previous study (32).
The cell affinity of a scaffold is a key
consideration in bone tissue engineering. Scaffolds with a 3D
porous structure, such as that described, support nutrient supply
and promote cell adhesion and proliferation (33,34).
The cell adhesion, spreading and cytoskeletal properties of bone
scaffolds can be augmented by RGD modification of the scaffold
(35), a phenomenon attributed to
the formation of focal adhesion between RGD peptide and cell
membrane integrins (36). In the
present study, the RGD peptide was uniformly immobilized on the
CS/HA composite through physical adsorption, a method considered
superior to chemical grafting methods employed in other studies
(37,38).
The results of the in vivo heterotopic
ossification experiments showed that the post-implantation X-ray
optical densities of the RGD-CS/HA and CS/HA scaffolds were lowest
after 4 weeks and highest after 6 weeks. This may be attributed to
the fact that, relative to the rates of osseointegrative indices,
rates of CS degradation and HA adsorption were higher at 4 weeks,
and lower at 6 weeks post-implantation. In addition, the superior
performance of the RGD-CS/HA scaffold relative to the CS/HA
scaffold in the in vivo bone defect repair experiment is
attributable to presence of channel-shaped and spherical pores,
which stimulate the differentiation of BMSCs into osteoblasts and
chondrocytes, and support formation of new bone tissues (39). Furthermore, the elevated Ca and P
levels that have been reported to accompany HA adsorption may
enhance the osseointegrative properties of the differentiated
osteoblasts (21).
In conclusion, the present study demonstrated that
an RGD-CS/HA scaffold prepared by in situ compositing
combined with lyophilization had high biocompatibility,
cytocompatibility, histocompatibility and osseointegrative
properties, and represents a promising bone substitute for use in
bone tissue engineering applications.
Acknowledgments
The present study was financially supported by
Harbin Introduction of Talent Special Fund 2013, Harbin Special
Funds for Scientific and Technological Innovation Talent Research
(grant no. 2014RFQGJ038). The authors would like to thank Medjaden
Bioscience Limited for assisting in the preparation of this
manuscript.
References
|
1
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Temple HT and Malinin TI: Microparticulate
cortical allograft: An alternative to autograft in the treatment of
osseous defects. Open Orthop J. 2:91–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang HY, Zhang SH, Luo JB, Liu YH, Qian
SH, Liang FH and Huang YL: Investigation of protein adsorption
mechanism and biotribological properties at simulated stem-cement
interface. J Tribology Transactions of ASME. 135:0323012013.
View Article : Google Scholar
|
|
4
|
Zanasi S: Innovations in total knee
replacement: New trends in operative treatment and changes in
peri-operative management. Eur Orthop Traumatol. 2:21–31. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HY, Luo JB, Zhou M, Zhang Y and
Huang YL: Biotribological properties at the stem-cement interface
lubricated with different media. J Mech Behav Biomed Mater.
20:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller MA, Ivkovic A, Porter R, Harris MB,
Estok DM II, Smith RM, Evans CH and Vrahas MS: Autologous bone
grafting on steroids: Preliminary clinical results. A novel
treatment for nonunions and segmental bone defects. Int Orthop.
35:599–605. 2011. View Article : Google Scholar :
|
|
7
|
Carne GM, Ishaug SL and Mikos AG: Bone
tissue engineering. Nat Med. 1:1322–1324. 1995. View Article : Google Scholar
|
|
8
|
Baroli B: From natural bone grafts to
tissue engineering therapeutics: Brainstorming on pharmaceutical
formulative requirements and challenges. J Pharm Sci. 98:1317–1375.
2009. View Article : Google Scholar
|
|
9
|
Kamath MS, Ahmed SS, Dhanasekaran M and
Santosh SW: Polycaprolactone scaffold engineered for sustained
release of resveratrol: Therapeutic enhancement in bone tissue
engineering. Int J Nanomedicine. 9:183–195. 2014.PubMed/NCBI
|
|
10
|
Shahini A, Yazdimamaghani M, Walker KJ,
Eastman MA, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV,
Vashaee D and Tayebi L: 3D conductive nanocomposite scaffold for
bone tissue engineering. Int J Nanomedicine. 9:167–181.
2014.PubMed/NCBI
|
|
11
|
Saravanan S, Sameera DK, Moorthi A and
Selvamurugan N: Chitosan scaffolds containing chicken feather
keratin nanoparticles for bone tissue engineering. Int J Biol
Macromol. 62:481–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dessì M, Borzacchiello A, Mohamed TH,
Abdel-Fattah WI and Ambrosio L: Novel biomimetic thermosensitive
β-tricalcium phosphate/chitosan-based hydrogels for bone tissue
engineering. J Biomed Mater Res A. 101:2984–2993. 2013. View Article : Google Scholar
|
|
13
|
Shuai C, Gao C, Feng P, Peng S and Wen X:
Grain growth associates mechanical properties in
nano-hydroxyapatite bone scaffolds. J Nanosci Nanotechnol.
13:5340–5345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia Y, Zhou P, Cheng X, Xie Y, Liang C, Li
C and Xu S: Selective laser sintering fabrication of
nano-hydroxy-apatite/poly-ε-caprolactone scaffolds for bone tissue
engineering applications. Int J Nanomedicine. 8:4197–4213.
2013.
|
|
15
|
Yamaguchi I, Tokuchi K, Fukuzaki H, Koyama
Y, Takakuda K, Monma H and Tanaka J: Preparation and microstructure
analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater
Res. 55:20–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Venugopal JR, El-Turki A,
Ramakrishna S, Su B and Lim CT: Electrospun biomimetic
nanocomposite nano-fibers of hydroxyapatite/chitosan for bone
tissue engineering. Biomaterials. 29:4314–4322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan K and Wang RZ: Surface modifications
of bone implants through wet chemistry. J Mater Chem. 16:2309–2321.
2006. View
Article : Google Scholar
|
|
18
|
Wang F, Zhang YC, Zhou H, Guo YC and Su
XX: Evaluation of in vitro and in vivo osteogenic differentiation
of nano-hydroxy-apatite/chitosan/poly (lactide-co-glycolide)
scaffolds with human umbilical cord mesenchymal stem cells. J
Biomed Mater Res A. 102:760–768. 2014. View Article : Google Scholar
|
|
19
|
Zhang HY, Han JM, Sun YL, Huang YL and
Zhou M: MC3T3-E1 cell response to stainless steel 316L with
different surface treatments. Mater Sci Eng C Mater Biol Appl.
56:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Ghannam AR, Ducheyne P, Risbud M, Adams
CS, Shapiro IM, Castner D, Golledge S and Composto RJ: Model
surfaces engineered with nanoscale roughness and RGD tripeptides
promote osteoblast activity. J Biomed Mater Res A. 68:615–627.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Secchi AG, Grigoriou V, Shapiro IM,
Cavalcanti-Adam EA, Composto RJ, Ducheyne P and Adams CS: RGDS
peptides immobilized on titanium alloy stimulate bone cell
attachment, differentiation and confer resistance to apoptosis. J
Biomed Mater Res A. 83:577–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang F, Williams CG, Wang DA, Lee H,
Manson PN and Elisseeff J: The effect of incorporating RGD adhesive
peptide in polyethylene glycol diacrylate hydrogel on osteogenesis
of bone marrow stromal cells. Biomaterials. 26:5991–5998. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawyer AA, Hennessy KM and Bellis SL:
Regulation of mesenchymal stem cell attachment and spreading on
hydroxyapatite by RGD peptides and adsorbed serum proteins.
Biomaterials. 26:1467–1475. 2005. View Article : Google Scholar
|
|
24
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: The 1996 Guide for the Care and Use of Laboratory
Animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar
|
|
25
|
Tamada Y and Ikada Y: Fibroblast growth on
polymer surfaces and biosynthesis of collagen. J Biomed Mater Res.
28:783–789. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Blunt L, Jiang X, Brown L and
Barrans S: The significance of the micropores at the stem-cement
interface in total hip replacement. J Biomater Sci Polym Ed.
22:845–856. 2011. View Article : Google Scholar
|
|
27
|
Amini AR, Laurencin CT and Nukavarapu SP:
Bone tissue engineering: Recent advances and challenges. Crit Rev
Biomed Eng. 40:363–408. 2012. View Article : Google Scholar
|
|
28
|
Zhang HY, Blunt LA, Jiang XQ, Brown LT and
Barrans SM: The influence of bone cement type on production of
fretting wear on the femoral stem surface: A preliminary study.
Clin Biomech (Bristol Avon). 27:666–672. 2012. View Article : Google Scholar
|
|
29
|
Roohani SI, Tavangarian F and Emadi R:
Nanostructured bioactive glass coating on porous hydroxyapatite
scaffold for strength enhancement. Mater Lett. 62:3428–3430. 2008.
View Article : Google Scholar
|
|
30
|
Qu Z, Yan J, Li B, Zhuang J and Huang Y:
Improving bone marrow stromal cell attachment on
chitosan/hydroxyapatite scaffolds by an immobilized RGD peptide.
Biomed Mater. 5:0650012010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho MH, Wang DM, Hsieh HJ, Liu HC, Hsien
TY, Lai JY and Hou LT: Preparation and characterization of
RGD-immobilized chitosan scaffolds. Biomaterials. 26:3197–3206.
2005. View Article : Google Scholar
|
|
32
|
Hofmann S, Hagenmuller H, Koch AM, Müller
R, Vunjak-Novakovic G, Kaplan DL, Merkle HP and Meinel L: Control
of in vitro tissue-engineered bone-like structures using human
mesenchymal stem cells and porous silk scaffolds. Biomaterials.
28:1152–1162. 2007. View Article : Google Scholar
|
|
33
|
Hutmacher DW: Scaffolds in tissue
engineering bone and cartilage. Biomaterials. 21:2529–2543. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mistry AS, Pham QP, Schouten C, Yeh T,
Christenson EM, Mikos AG and Jansen JA: In vivo bone
biocompatibility and degradation of porous fumarate-based
polymer/alumoxane nanocomposites for bone tissue engineering. J
Biomed Mater Res A. 92:451–462. 2010.
|
|
35
|
El-Ghannam AR, Ducheyne P, Risbud M, Adams
CS, Shapiro IM, Castner D, Golledge S and Composto RJ: Model
surfaces engineered with nanoscale roughness and RGD tripeptides
promote osteoblast activity. J Biomed Mater Res A. 68:615–627.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
LeBaron RG and Athanasiou KA:
Extracellular matrix cell adhesion peptides: Functional
applications in orthopedic materials. Tissue Eng. 6:85–103. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kantlehner M, Finsinger D, Meyer J,
Schaffner P, Jonczyk A, Diefenbach B, Nies B and Kessler H:
Selective RGD-mediated adhesion of osteoblasts at surfaces of
implants. Angew Chem Int Ed. 38:560–562. 1999. View Article : Google Scholar
|
|
38
|
Haubner R, Bruchertseifer F, Bock M,
Kessler H, Schwaiger M and Wester HJ: Synthesis and biological
evaluation of a (99m) Tc-labelled cyclic RGD peptide for imaging
the alphavbeta3 expression. Nuklearmedizin. 43:26–32.
2004.PubMed/NCBI
|
|
39
|
Oliveira JM, Rodrigues MT, Silva SS,
Malafaya PB, Gomes ME, Viegas CA, Dias IR, Azevedo JT, Mano JF and
Reis RL: Novel hydroxyapatite/chitosan bilayered scaffold for
osteochondral tissue-engineering applications: Scaffold design and
its performance when seeded with goat bone marrow stromal cells.
Biomaterials. 27:6123–6137. 2006. View Article : Google Scholar : PubMed/NCBI
|