Introduction
Myelodysplastic syndrome (MDS) comprises a
heterogenous group of acquired bone marrow disorders, which is
characterized by the dysplastic growth of hematopoietic stem cells.
MDS typically exerts its effects in multiple cell lines and has a
propensity to progress to acute myelogenous leukemia. Although
hematopoietic stem cell transplantation represents the only mode of
treatment with curative potential, the relatively high morbidity
and mortality of this approach limits its usefulness (1,2).
Therefore, effective agents with more benign toxicity profiles are
urgently required for the treatment of this disease.
At present, the search for novel antitumor
substances obtained from plants is intensifying. Wogonin
(5,7-dihydroxy-8-methoxy-flavone), a flavonoid from the root of the
medicinal herb Scutellaria baicalensis Georgi, has been
recognized as a potent anticancer agent due to its broad toxicity
to various types of tumor cell lines (3–7).
Notably, at doses which are lethal to tumor cells, wogonin revealed
no, or little, toxicity for normal cells and exhibited no evident
toxicity in animals (4,6,8). The
molecular mechanisms underlying these growth-suppressive effects
are considered to include changes in the oxidation/reduction
status, cell cycle inhibition (9),
induction of apoptosis (8) and
phosphorylation of the vascular endothelial growth factor receptor
2 (VEGFR-2) (10). However, the
underlying molecular mechanism of its anticancer activity remains
to be fully elucidated.
Apoptosis, or programmed cell death, is critical for
the normal development, function and homeostasis of multi-cellular
organisms. Apoptosis is considered as a process which may be
exploited for the removal of precancerous and cancerous cells
(11), and reduced levels of
apoptosis are considered to be a fundamental component in the
pathogenesis of cancer. Therefore, further development of agents
which are able to induce, or enhance, the extent of apoptosis may
provide a promising strategy for enhancing the anticancer potential
of several anticancer drugs (12).
An accumulating body of evidence supports that cell cycle arrest
and apoptosis are closely associated with cell proliferation in
mammalian cells (9,13). It is well known that the transition
from one cell cycle phase to another occurs in an orderly fashion,
and cell cycle control is the major regulatory mechanism underlying
cell growth, which is regulated by cyclin-dependent kinases (CDKs)
and their cyclin partners (14,15).
Although the effects of wogonin on antiproliferative and apoptotic
activity have been documented in various human cancer cells, its
therapeutic efficacy and the underlying mechanisms in MDS remain to
be elucidated. The present study aimed to investigate the potency
of wogonin and to assess the signaling pathways involved in
modulating apoptosis in MDS cells.
Materials and methods
Reagents
Wogonin (purity >98%; Jiangsu Key Lab of
Carcinogenesis and Intervention, China Pharmaceutical University,
Nanjing, China) was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA), and the final concentration of
DMSO was >0.15%, which exhibited no toxicity to the SKM-1 cells.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) reagent was supplied by Sigma-Aldrich, the annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection and cell
cycle detection kits were obtained from BD Biosciences (Franklin
Lakes, NJ, USA), 4′,6-diamidino-2-phenylindole (DAPI) was purchased
from Beyotime Institute of Biotechnology (Haimen, China), the
bicinchoninic acid (BCA) protein assay kit was from Biosynthesis
Biotechnology Co., Ltd. (Beijing, China) and monoclonal antibodies,
including rabbit antihuman CDK4 (cat. no. 12790), rabbit antihuman
cyclin D1 (cat. no. 2978), mouse antihuman p21Cip1 (cat.
no. 2946), rabbit antihuman p27Kip1 (cat. no. 3688),
rabbit antihuman Bax (cat. no. 5023), rabbit antihuman Bcl-2 (cat.
no. 4223), rabbit antihuman poly(ADP-ribose) polymerase (PARP)
(cat. no. 9532), rabbit antihuman cleaved (c)-PARP (cat. no. 5625),
rabbit antihuman cytochrome c (cat. no. 11940), rabbit
antihuman caspase-3 (cat. no. 14220), mouse antihuman caspase-9
(cat. no. 9508) and mouse antihuman β-actin (cat. no. 3700), were
supplied by Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell line and cell culture
SKM-1, an MDS cell line, was obtained from the
Japanese Collection of Research Bioresources Cell Bank (Osaka,
Japan) (16). The cells were
cultured in RPMI-1640 medium (Gibco Life Technologies, Carlsbad,
CA, USA) containing 10% fetal bovine serum (Gibco Life
Technologies), 100 U/ml penicillin (Sigma-Aldrich, St. Louis, MO,
USA) and 100 µg/ml streptomycin (Sigma-Aldrich) in an
environment of saturated humidity, 5% CO2 at 37°C. Cells
in the logarithmic growth phase were used in all experiments.
Measurement of cell growth and
viability
The SKM-1 cells were seeded at a density of
5×103 cells/well into 6-well plates and were allowed to
grow overnight. Subsequently, the cells were treated with 20, 40,
80, 120 or 160 µmol/l wogonin, whereas only RPMI-1640 medium
was added for the control group. Following incubation for 12, 24,
48 or 72 h, an MTT assay was performed to measure the cell
viability, as described previously (17), and the absorbance at 570 nm was
recorded using a Model 550 microplate reader (Bio-Rad Laboratories,
Tokyo, Japan).
Cell morphological assessment
Following culture as described above, the SKM-1
cells were collected and spread onto the slides. Subsequently, the
cells were fixed with ice-cold 4% paraformaldehyde for 20 min,
permeabilized with 0.1% Triton X-100 for 25 min and washed with
ice-cold phosphate-buffered saline (PBS), followed by staining with
DAPI (1 µg/ml) for 25 min at 4°C in the dark. Morphological
changes of the nuclei of cells were undergoing apoptosis (100
cells) were recorded at the reference points using a phase-contrast
microscope (Olympus CKx41; Olympus, London, UK).
Apoptosis assay by flow cytometric
analysis (FCM)
Cell apoptosis was identified by FCM using annexin
V/propidium iodide (PI) staining, as described previously (17). Briefly, the SKM-1 cells
(1×105 cells) were treated and incubated, as described
in the previous section. The cells were subsequently collected by
centrifugation at 920 x g for 5 min, and washed once in ice-cold
PBS, prior to the assessment of apoptotic cell death using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA USA),
according to the manufacturer's instructions.
Cell cycle distribution
The SKM-1 cells (1×105 cells/well) were
seeded into a 6-well plate for 24 h at 37°C. The cells were washed
once with PBS, the medium was replaced with fresh medium, and the
cells were subsequently treated with various concentrations of
wogonin. Following treatments for the various durations, the cells
were collected, fixed with 70% ethanol, incubated with 25
µg/ml ribonuclease A and stained with 50 µg/ml PI for
30 min at room temperature in the dark. Subsequently, the cell
cycle data were calculated by FCM (BD Biosciences), according to
the manufacturer's instructions.
Western blot analysis
Western blotting was performed, as described
previously (17,18). Briefly, the total protein was
isolated from the harvested cells and the quantity of protein was
measured using a BCA protein assay. Samples containing 25 µg
protein were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc. Hercules, CA,
USA). Non-specific binding sites were blocked for 1 h with 5%
non-fat milk, prior to an incubation with the following primary
antibodies diluted in PBS at 4°C overnight: Anti-CDK4 (1:1,000),
anti-cyclin D1 (1:1,000), anti-p21Cip1 (1:1,000),
anti-p27Kip1 (1:1,000), anti-cytochrome c
(1:1,000), anti-caspase-9 (1:1,000), anti-caspase-3 (1:1,000),
anti-PARP (1:1,000), anti-c-PARP (1:1,000), anti-Bcl-2 (1:1,000),
anti-Bax (1:1,000), and anti-β-actin (1:1,000) Subsequently, a
secondary horseradish peroxidase-conjugated goat anti-mouse
antibody (1:1,000; Santa Cruz Biotechnology, Inc.) was added for 1
h at room temperature. The blots were visualized using an enhanced
chemiluminescence system (Amersham, Buckinghamshire, UK), and the
density of β-actin served as an internal loading control.
Statistical analysis
All data are expressed as the mean ± standard
deviation for experiments performed in triplicate, and the data
were analyzed using the Statistical Package for Social Science
(SPPS version 13.0; SPSS, Inc., Chicago, IL, USA). Statistically
significant differences between the experimental groups were
determined using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of cell proliferation
Following incubation with different concentrations
of wogonin for the various durations, the proliferation of the
SKM-1 cells was markedly inhibited (Fig. 1). Significant differences were
observed between the different groups (P<0.05), and the
concentration of wogonin required to yield a half maximal
inhibitory concentration (IC50) of the proliferation, as
measured at the 24, 48 and 72 h time points, was 212.1, 58.0 and
43.4 µmol/l, respectively. Therefore, these results
demonstrated that wogonin markedly inhibited the proliferation of
SKM-1 cells in a time- and dose-dependent manner.
Cell morphological assessment following
treatment with wogonin
The morphological changes due to apoptosis occurring
in the nuclei of the cells were observed under a fluorescence
microscope. The untreated SKM-1 cells exhibited a pale blue
fluorescence, demonstrating an even pattern of distribution of the
chromatin in the nucleolus (Fig.
2A), whereas those treated with wogonin exhibited a
dose-associated increase in the fraction of apoptotic cells with
condensed and fragmented DNA, as revealed by the darker blue
fluorescence compared with that in the non-apoptotic cells
(Fig. 2B and C), suggesting that
wogonin induced cell apoptosis in a dose-dependent manner.
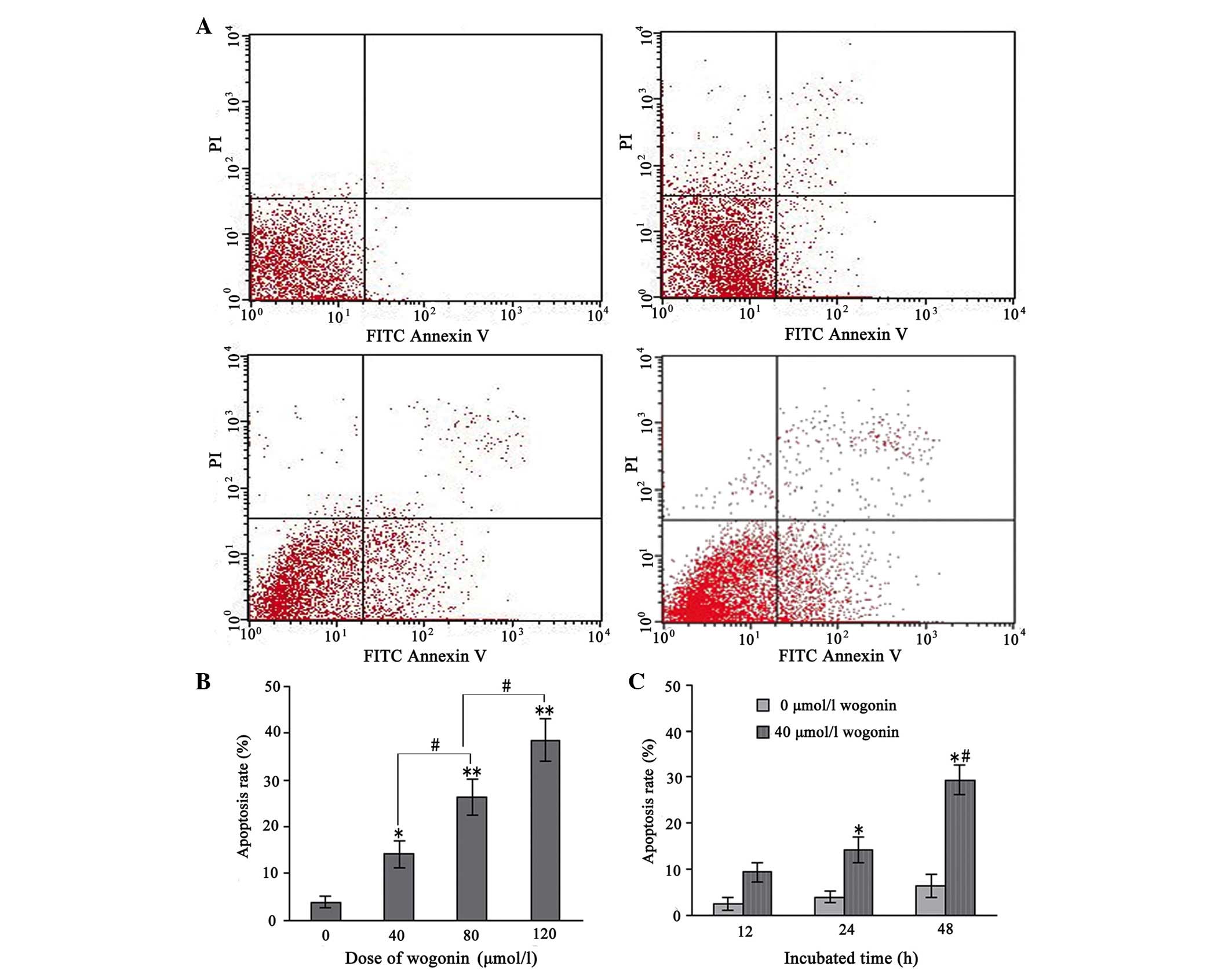
Wogonin treatment induces cell
apoptosis
The apoptotic cells were further quantified using
the annexin V/PI double-staining assay. Following treatment with
various concentrations of wogonin for 24 h, the total percentages
of cells in an apoptotic state, as calculated by the sum of those
cells undergoing early and late apoptosis, are shown in Fig. 3A and B. These results indicated
that wogonin induced apoptosis in the SKM-1 cells in a
dose-dependent manner. Furthermore, when the SKM-1 cells were
incubated with 40 µmol/l wogonin for 12, 24 and 48 h, the
apoptotic rate increased significantly with the duration of the
experiment (P<0.05; Fig. 3C).
These data provided evidence that apoptosis was induced in the
wogonin-treated SKM-1 cells.
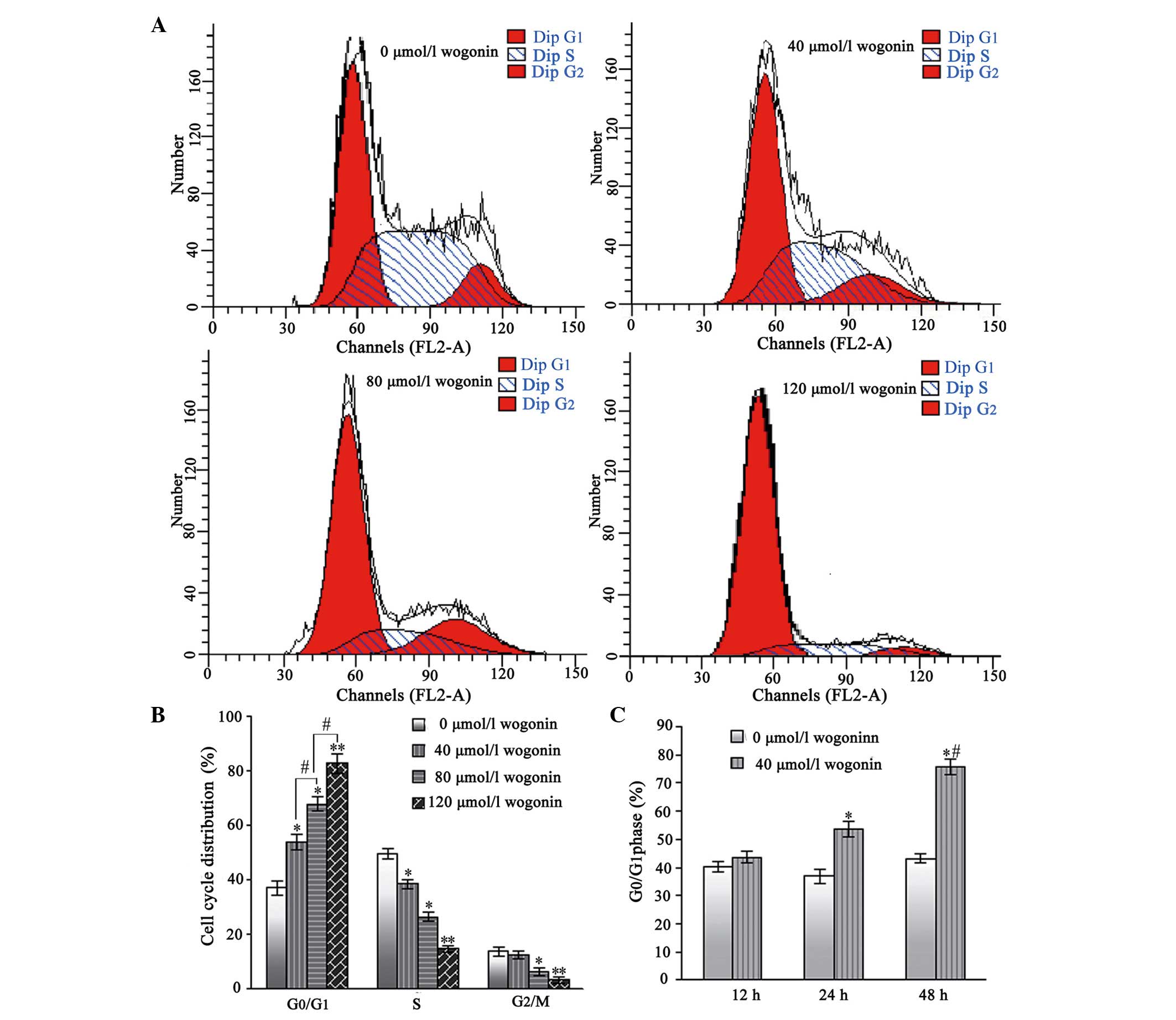
Wogonin treatment causes
G0/G1 phase arrest in the SKM-1 cells
Following treatment with wogonin for 24 h, the
percentages of SKM-1 cells in the G0/G1 phase
increased from 38.96±2.62% in the untreated cells, to 51.53±2.76,
63.68±2.82 and 76.52±3.46% in the cells treated with increasing
concentrations of wogonin (40, 80 and 120 µmol/l,
respectively; Fig. 4A and B).
Furthermore, the number of SKM-1 cells in the S and
G2/mitosis phases correspondingly decreased. Therefore,
these results indicated that wogonin induced a
G0/G1 cell-cycle arrest in a dose-and
time-dependent manner.
Expression of cell cycle proteins
following treatment with wogonin
Following treatment with increasing concentrations
of wogonin (40, 80 and 120 µmol/l) for 48 h, the levels of
cyclin D1 and its upstream kinase, CDK4, in the SKM-1 cells were
reduced in a dose-dependent manner (Fig. 5). By contrast, a marked increase in
the protein expression levels of p21Cip1 and
p27Kip1 were observed in the SKM-1 cells following
treatment with the identical doses of wogonin for 48 h (Fig. 5). Taken together, these data
indicated that wogonin also exerts a growth-inhibitory effect on
the cells by inducing cell cycle arrest at the G1/S
phase transition, followed by the upregulation of the expression of
p21Cip1 and p27Kip1, and the downregulation
of the expression of cyclin D1 and CDK4 in the SKM-1 cells.
Expression of apoptosis-associated
proteins following treatment with wogonin
Following treatment for 48 h, wogonin increased the
expression levels of the proapoptotic proteins Bax, activated
caspases 3 and 9, and activated c-PARP in the SKM-1 cells, whereas
it caused a decrease in the expression of the antiapoptotic
protein, Bcl-2, and of the inactive form of PARP. A significant
difference was observed between them (P<0.05; data not shown).
Notably, the dose at which wogonin affected the expression of the
apoptosis-associated proteins was similar to that at which the cell
proliferation was suppressed and apoptosis was induced. Therefore,
these results clearly suggested that the growth-inhibitory effects
of wogonin on the SKM-1 cells were mediated by cell apoptosis.
Discussion
Chinese herbal medicines have been used for
thousands of years in China and are increasingly being recognized
as novel remedies for enhancing the efficacy, or alleviating the
adverse effects, of tumor therapies (19). An accumulating body of evidence has
demonstrated that wogonin induces cell death via cell cycle arrest
and the induction of apoptosis in numerous types of human cancer
cell lines (9), however,
information on wogonin-induced apoptosis in human MDS cells is
lacking. To provide an improved understanding of the mechanism
underlying its anticancer activity, the effect of wogonin on human
MDS cells was investigated in the present study.
The induction of apoptosis in cancer cells is one of
the most important and direct ways to control the development of
cancer and to eliminate tumors. An imbalance between proliferation
and apoptosis may lead to unlimited cell proliferation, which may
result in tumor development (20).
Therefore, the effects of wogonin in antagonizing the proliferation
of SKM-1 cells and cell apoptosis were initially investigated in
the present study using MTT and annexin V-PI double-staining
assays. The results demonstrated that wogonin significantly
inhibited the proliferation of SKM-1 cells, in a time- and
dose-dependent manner (Fig. 1).
Furthermore, the present study also confirmed that wogonin
suppressed cell proliferation, predominantly as a result of the
induction of apoptosis, as demonstrated by the percentage of
annexin V-positive cells that were present and the characteristic
morphological changes associated with apoptosis in the nucleus. As
shown in Fig. 2, the nuclear
morphological analysis revealed a dose-associated increase in the
fraction of apoptotic cells with condensed and fragmented nuclei,
as indicated by the darker blue fluorescence exhibited in
wogonin-treated SKM-1 cells (Fig. 2A
and B) compared with those cells with pale blue fluorescence in
the control group (Fig. 2C), as
observed with DAPI staining. Previously, it was reported that
treatment with wogonin induces apoptosis and inhibits cell
proliferation in other diseases of the hematopoietic system,
including myeloma and B-cell non-Hodgkin's lymphoma cell lines
(21,22). This is consistent with the results
of the present study and these findings clearly demonstrated that
wogonin exerts anticancer effects through the induction of cell
apoptosis.
Apoptosis results as a consequence of a series of
precisely regulated events, which are frequently altered in tumor
cells, and the apoptotic process is tightly regulated by a
fine-tuned balance between proapoptotic and antiapoptotic factors.
Various dysfunctions of the apoptotic pathway and uncontrolled
cellular proliferation ultimately lead to carcinogenesis and tumor
progression (23). In general,
apoptotic cell death may be executed by the key molecular
mechanism, involving mitochondria (the intrinsic pathway) and the
death receptor pathways (the extrinsic pathway) (24). In each pathway, an apoptotic
stimulus results in the activation of caspases either directly, or
via the activation of the mitochondrial death program (25). Therefore, the expression levels of
certain key apoptotic proteins, including caspases-3 and -9, Bax,
Bcl-2, PARP and c-PARP, were assessed to determine the possible
mechanism of wogonin-induced apoptosis. The Bcl-2 family of
proteins (antiapoptotic proteins, including Bcl-2 and Bcl-xl, and
proapoptotic proteins, including Bax and Bad), exert an important
role in the apoptosis of cancer cells, since the interactions among
these proteins set the threshold for cell survival (26,27).
In the present study, the results of the western blot analyses
revealed that wogonin increased the expression of the proapoptotic
protein, Bax, in the SKM-1 cells following treatment for 48 h,
whereas it led to a decrease in the expression of the antiapoptotic
protein, Bcl-2, thereby resulting in an increase in the ratio of
proapoptotic to antiapoptotic Bcl-2 family proteins, in favor of
apoptosis in the MDS cells. It was reported that the induction of
mitochondrial apoptosis requires the involvement of the Bcl-2
protein family (21), in which Bax
and Bcl-2 are key proteins involved in the regulation of the
mitochondrial pathway. The predominant mechanism by which Bcl-2
family members initiate or prevent apoptosis is by controlling the
release of cytochrome c and other proapoptotic factors from
the mitochondria (28–30). During this process, cytosolic
cytochrome c activates procaspase-9 by binding to apoptotic
protease-activating factor 1 in the presence of dATP, leading to
the activation of caspase-9 (31).
Subsequently, downstream effector caspases, including caspase-3,
are activated, which eventually trigger apoptosis (32,33).
The present study provided evidence to suggest that wogonin
increased the expression of cytochrome c and activated
(cleaved) caspases-3 and -9 in SKM-1 cells following treatment for
48 h. The caspase signaling pathway, which is activated
extrinsically and intrinsically, is the major mechanism of
apoptosis in most cellular systems (34). Notably, the present study
identified that the increase in apoptosis mediated by wogonin was
accompanied by a decreased level of PARP and the emergence of
c-PARP, and markedly increased levels of Bax protein with
concomitantly decreased levels of Bcl-2. An accumulating body of
evidence has identified that PARP is one component of the
caspase-mediated pathway of apoptosis, and c-PARP is considered to
be a marker of apoptosis, being critically involved in the
intrinsic apoptosis pathway (35–38).
It has long been known that wogonin exerts apoptosis-inducing
effects in malignant cells by changing the mitochondrial membrane
potential (36,38). The crucial step in the
mitochondrial apoptotic pathway is the loss of the mitochondrial
membrane potential, triggering the activation of the apoptotic
cascade and the execution of cell death (39). The wogonin-induced decreased ratio
of Bcl-2 to Bax, and increased expression of cleaved caspases-3 and
-9, was demonstrated in human breast cancer cells and in vascular
smooth muscle cells (4,40). Therefore, it is reasonable to
hypothesize that wogonin-induced apoptosis of the MDS cells is
mediated by the mitochondrial pathway through modulating the ratio
of Bcl-2 to Bax.
Cell growth is tightly regulated by cell-cycle
checkpoints and apoptosis is often associated with G1
phase arrest of the cell cycle (41). In the present study, the cell cycle
analysis by FCM revealed that wogonin induced a
G0/G1 cell-cycle arrest of the SKM-1 cells in
a dose-and a time-dependent manner. Evidence in the literature
suggests that wogonin may induce the G1 phase arrest of
cells associated with several human malignancies, including
non-Hodgkin's lymphoma Raji (22),
leukemia U-937 (42), human
colorectal cancer carcinoma (43)
and human cervical carcinoma HeLa (9) cells. All these reports suggested that
one of the mechanisms by which wogonin may act to inhibit the
proliferation of cancer cells is through an inhibition of cell
cycle progression. It is well documented that cell cycle
progression is partly controlled by a family of protein kinase
complexes in eukaryotic cells, including CDKs and their activating
partners, the cyclins (44–47).
During the G0/G1 phase progression, cyclin D1
binds to CDK4/CDK6, resulting in the formation of the cyclin E/CDK4
complex, eventually driving the cell from G1 to S phase
(48). In the present study, it
was revealed that treatment of the SKM-1 cells with wogonin
markedly decreased the expression of cyclin D1 and its upstream
kinase, CDK4, in a dose-dependent manner. It is noteworthy that the
exposure of different cancer cell lines to wogonin was previously
shown to reduce the protein levels of cyclin D, resulting in growth
inhibition at the G0/G1 phase (5). Therefore, it is possible that the
antiproliferative effects of wogonin are due to the inhibition of
cyclin D1 expression in MDS cells. The cell cycle progression in
eukaryotic cells is also regulated by the relative balance between
the cellular concentrations of CDK inhibitors, including the
p21Cip1 and p27Kip1 proteins of the Cip/Kip
family (49). Furthermore, the
overexpression of p27Kip1 was revealed to prevent the
activation of CDKs and entry into the S-transition phase (50), and p21Cip1 was
demonstrated to exert an important role in regulating the
G1-S and G2 checkpoints (51). The present study revealed that a
marked increase in the protein expression levels of
p27Kip1 and p21Cip1 in the SKM-1 cells was
observed following treatment with wogonin for 48 h. Taken together,
these data suggested that wogonin induced
G0/G1 phase arrest in the MDS cells by
modulating several key G1 regulatory proteins, including
CDK4 and cyclin D1, in addition to upregulating the expression of
p27Kip1 and p21Cip1.
In conclusion, the present study provided evidence
that wogonin acted on several regulatory factors (cyclin D1, CDK4,
p21Cip1 and p27Kip1), causing a
G0/G1 phase arrest, halting cell cycle
progression, and inducing apoptosis in the MDS cells, which may be
mediated by the mitochondrial pathway through a modulation of the
ratio of Bcl-2 to Bax. Therefore, the findings in the present study
revealed that wogonin may be a putative target for therapeutic
action in the treatment of MDS. However, further studies,including
exploring the effects of long-term in vivo exposure to
wogonin, are required.
Acknowledgments
This study was supported by the National Nature
Science Foundation of People's Republic China (no. 81372985) and
the National Nature Science Foundation of People's Republic China
(no. 81200877).
References
|
1
|
Chen Y, Liu K, Xu L, Chen H, Liu D, Zhang
X, Shi H, Han W, Wang Y, Zhao T, et al: HLA-mismatched
hematopoietic SCT without in vitro T-cell depletion for
myelodysplastic syndrome. Bone Marrow Transplant. 45:1333–1339.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kindwall-Keller T and Isola LM: The
evolution of hematopoietic SCT in myelodysplastic syndrome. Bone
Marrow Transplant. 43:597–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polier G, Ding J, Konkimalla BV, Eick D,
Ribeiro N, Köhler R, Giaisi M, Efferth T, Desaubry L, Krammer PH
and Li-Weber M: Wogonin and related natural flavones are inhibitors
of CDK9 that induce apoptosis in cancer cells by transcriptional
suppression of Mcl-1. Cell Death Dis. 2:e1822011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung HY, Jung YM, Shin DH, Lee JY, Oh MY,
Kim HJ, Jang KS, Jeon SJ, Son KH and Kong G: Anticancer effects of
wogonin in both estrogen receptor-positive and -negative human
breast cancer cell lines in vitro and in nude mice xenografts. Int
J Cancer. 122:816–822. 2008. View Article : Google Scholar
|
|
5
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
6
|
Baumann S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCgamma1-and Ca2+-dependent
apoptosis. Blood. 111:2354–2363. 2008. View Article : Google Scholar
|
|
7
|
Chow SE, Chang YL, Chuang SF and Wang JS:
Wogonin induced apoptosis in human nasopharyngeal carcinoma cells
by targeting GSK-3β and ΔNp63. Cancer Chemother Pharmacol.
68:835–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CC, Kuo CL, Lee MH, Lai KC, Lin JP,
Yang JS, Yu CS, Lu CC, Chiang JH, Chueh FS and Chung JG: Wogonin
triggers apoptosis in human osteosarcoma U-2 OS cells through the
endoplasmic reticulum stress, mitochondrial dysfunction and
caspase-3-dependent signaling pathways. Int J Oncol. 39:217–224.
2011.PubMed/NCBI
|
|
9
|
Yang L, Zhang HW, Hu R, Yang Y, Qi Q, Lu
N, Liu W, Chu YY, You QD and Guo QL: Wogonin induces G(1) phase
arrest through inhibiting Cdk4 and cyclin D1 concomitant with an
elevation in p21Cip1 in human cervical carcinoma HeLa cells.
Biochem Cell Biol. 87:933–942. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu N, Gao Y, Ling Y, Chen Y, Yang Y, Gu
HY, Qi Q, Liu W, Wang XT, You QD and Guo QL: Wogonin suppresses
tumor growth in vivo and VEGF-induced angiogenesis through
inhibiting tyrosine phosphorylation of VEGFR2. Life Sci.
82:956–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelloff GJ, Crowell JA, Steele VE, Lubet
RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R,
Lawrence JA, et al: Progress in cancer chemoprevention: Development
of diet-derived chemopreventive agents. J Nutr. 130(2 Suppl):
S467–S471. 2000.
|
|
12
|
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen
YL, Tsai NM, Liu YC, Tzao C, Yu DS and Harn HJ: Anti-proliferative
A549 human activity of Bupleurum scrozonerifolium in lung cancer
cells in vitro and in vivo. Cancer Lett. 222:183–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trivedi PP, Roberts PC, Wolf NA and
Swanborg RH: NK cells inhibit T cell proliferation via p21-mediated
cell cycle arrest. J Immunol. 174:4590–4597. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: Potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leukaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia G, Chen B, Ding J, Gao C, Lu H, Shao
Z, Gao F and Wang X: Effect of magnetic Fe3O4
nanoparticles with 2-methoxyestradiol on the cell-cycle progression
and apoptosis of myelodysplastic syndrome cells. Int J
Nanomedicine. 6:1921–1927. 2011.
|
|
18
|
Jiang Z, Chen BA, Xia GH, Wu Q, Zhang Y,
Hong TY, Zhang W, Cheng J, Gao F, Liu LJ, et al: The reversal
effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on
SKOV3/DDP ovarian carcinoma cells. Int J Nanomedicine. 4:107–114.
2009.PubMed/NCBI
|
|
19
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karunagaran D, Joseph J and Kumar TR: Cell
growth regulation. Adv Exp Med Biol. 595:245–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Liu LP, Chen Y, Tian XY, Qin J,
Wang D, Li Z and Mo SL: Wogonin induces apoptosis in RPMI 8226, a
human myeloma cell line, by downregulating phospho-Akt and
overexpressing Bax. Life Sci. 92:55–62. 2013. View Article : Google Scholar
|
|
22
|
Wang L, Zhang H, Chen B, Xia G, Wang S,
Cheng J, Shao Z, Gao C, Bao W, Tian L, et al: Effect of magnetic
nanoparticles on apoptosis and cell cycle induced by wogonin in
Raji cells. Int J Nanomedicine. 7:789–798. 2012.PubMed/NCBI
|
|
23
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen B, Liang Y, Wu W, Cheng J, Xia G, Gao
F, Ding J, Gao C, Shao Z, Li G, et al: Synergistic effect of
magnetic nanoparticles of Fe3O4 with gambogic
acid on apoptosis of K562 leukemia cells. Int J Nanomedicine.
4:251–259. 2009. View Article : Google Scholar :
|
|
26
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leber B, Lin J and Andrews DW: Still
embedded together binding to membranes regulates Bcl-2 protein
interactions. Oncogene. 29:5221–5230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
29
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB,
Korsmeyer SJ, et al: Proapoptotic BAX and BAK: A requisite gateway
to mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanchez-Alczar JA, Khodjakov A and
Schneider E: Anticancer drugs induce increased mitochondrial
cytochrome c expression that precedes cell death. Cancer Res.
61:1038–1044. 2001.
|
|
32
|
Riedl SJ and Salvesen GS: The apoptosome:
Signaling platform of cell death. Nat Rev Mol Cell Biol. 8:405–413.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caserta TM, Smith AN, Gultice AD, Reedy MA
and Brown TL: Q-VD-OPh, a broad spectrum caspase inhibitor with
potent antiapoptotic properties. Apoptosis. 8:345–352. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agarwal A, Mahfouz RZ, Sharma RK, Sarkar
O, Mangrola D and Mathur PP: Potential biological role of poly
(ADP-ribose) polymerase (PARP) in male gametes. Reprod Biol
Endocrinol. 7:1432009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danial NN: BCL-2 family proteins: Critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahfouz RZ, Said TM, Mangrola D, et al:
Potential roles of poly (ADP-ribose) polymerase in male
reproduction. Arch Med Sci. 5:S92–S98. 2009.
|
|
38
|
Zhang PX, Li HM, Chen D, Ni J, Kang Y and
Wang S: Oleanolic acid induces apoptosis in human leukemia cells
through caspase activation and poly (ADP-ribose) polymerase
cleavage. Acta Biochim Biophys Sin (Shanghai). 39:803–809. 2007.
View Article : Google Scholar
|
|
39
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu YM, Wang X, Nawaz A, Kong ZH, Hong Y,
Wang CH and Zhang JJ: Wogonin ameliorates lipotoxicity-induced
apoptosis of cultured vascular smooth muscle cells via interfering
with DAG-PKC pathway. Acta Pharmacol Sin. 32:1475–1482. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Q, Hilsenbeck S and Gazitt Y: Arsenic
trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or
G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and
synergy with APO2/TRAIL. Blood. 101:4078–4087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang HW, Yang Y, Zhang K, Qiang L, Yang
L, Yang L, Hu Y, Wang XT, You QD and Guo QL: Wogonin induced
differentiation and G1 phase arrest of human U-937 leukemia cells
via PKC delta phosphorylation. Eur J Pharmacol. 591:7–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H,
Li Z, You Q and Guo Q: Wogonin induced G1 cell cycle arrest by
regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in
human colorectal cancer carcinoma cells. Toxicology. 312:36–47.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paulovich AG and Hartwell LH: A checkpoint
regulates the rate of progression through S phase in S. cerevisiae
in response to DNA damage. Cell. 82:841–847. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Walker JL and Assoian RK:
Integrin-dependent signal trans,duction regulating cyclin D1
expression and G1 phase cell cycle progression. Cancer Metastasis
Rev. 24:383–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lew DJ and Kornbluth S: Regulatory roles
of cyclin dependent kinase phosphorylation in cell cycle control.
Curr Opin Cell Biol. 8:795–804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Harbour JW and Dean DC: Rb function in
cell-cycle regulation and apoptosis. Nat Cell Biol. 2:E65–E67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|