Introduction
The incidence of non-small cell lung cancer (NSCLC)
has increased in recent years with the deterioration of air quality
in China (1). In addition,
mutations in oncogenes and tumor-suppressor genes trigger the
progressive accumulation of NSCLC cases (2–5).
Chinese patients with NSCLC have a high mortality rate and to date,
limited therapeutic options are available for surgery-resistant
NSCLC, leading to poor prognosis (1). Molecular targeting of
cancer-associated signaling pathways represents a promising
treatment option for cancer and has improved the 5-year survival
rates of patients with metastatic cancer (6,7).
Although several targeted therapies, including the inhibition of
BRAF (8), have been adopted for
NSCLC treatment, challenges remain, similarly to those for other
cancer types: i) Activation of collateral pathways often
circumvents therapeutic blockage and induces resistance during
targeted therapies; ii) Generic tumor-suppressive drugs are
challenging to develop into clinical drugs.
MicroRNAs (miRNAs/miRs), small RNAs of ~22
nucleotides in length, are non-coding single-stranded RNAs which
are able to regulate gene expression via binding to the
3′-untranslated regions (UTR) of target mRNAs (9). Altered miRNA expression profiles have
been reported in NSCLC, which result in differential expression of
oncogenes and tumor suppressors, and affects downstream signaling
pathways (10,11). For instance, miRNA-21 (12), miRNA-205 (13), miR-1254 (14) and miR-574-5p (14) were found to be overexpressed in
NSCLC. Furthermore, single-nucleotide polymorphisms in the
complementary site of the 3′-UTR of target genes such as KRAS
(rs712) are correlated with the risk of NSCLC, suggesting that
let-7 miRs participate in the progression of NSCLC (15).
In the human genome, miR-361 is encoded by Xq21.2
between exons 9 and 10 of the choroideremia gene enconding for Rab
escort protein 1 and produces two mature miRNAs, including
miR-361-3p and miR-361-5p (16).
miR-361-5p was downregulated in the cells of a transplantable
metastatic xenograft compared to that in a non-metastatic xenograft
(17). An in vitro study
demonstrated that miR-361-5p was expressed in matched primary and
metastatic carcinoma, which suggested that miR-361-5p inhibits the
growth and proliferation of cancer cells (18). Kanitz et al (19) demonstrated that miR-361-5p is
inversely correlated to vascular endothelial growth factor A in
human cutaneous squamous cell carcinoma. In prostate cancer,
miR-361-5p was shown to act as a tumor suppressor by targeting
signal transducer and activator of transcription 6 (STAT6)
(20). As it was hypothesized that
miR-361-5p may also be a potential suppressor of other cancer
types, the present study examined the effects of miR-361-5p in
NSCLC.
The present study used miR-chips in order to
determine the expression of miR-361-5p in NSCLC tissues and their
adjacent normal tissues. Furthermore, the present study assessed
the suppressive effects of miR-361-5p on the NSCLC cell line H23
using Cell Counting Kit 8 (CCK-8), colony formation and flow
cytometric assays as well as a xenograft experiment. Transfection
with miR-361-5p overexpression vector was used to upregulate
miR-361-5p, and a luciferase reporter assay was used to identify a
direct target as well as downstream signaling effects.
Small-interfering (si)RNA was further used to knockdown a target
gene of miR-361-5p in order to explore the downstream effects. The
present study suggested that miR-361-5p inhibited NSCLC progression
and may therefore be used as a novel therapeutic tool in NSCLC.
Materials and methods
Patients and samples
The NSCLC and adjacent tissues were obtained from 25
early-stage patients that underwent surgical resection and who had
received no previous treatment. All patients were in T2 stage,
including 7-patients with a Gleason score <7, 10 patients with a
Gleason score of 7 and 8 patients with a Gleason score >7
(21). All of these specimens were
examined using haematoxylin and eosin (HE) staining (Beyotime
Institute of Biotechnology, Inc., Wuhan, China). Only samples with
>70% tumor content were used as NSCLC tissues in the present
study. The samples were frozen in liquid nitrogen for microarray
and reverse-transcription polymerase chain reaction (RT-qPCR)
analyses. All of the specimens were used according to institutional
guidelines and approved protocols. The ethics committees of The
Second Affiliated Hospital of the Medical School of Xi'an Jiaotong
University (Xi'an, China) approved the protocol of the present
study. Written informed consent was obtained from all patients.
Microarray analysis
The expression of miRNAs in NSCLC and adjacent
tissues was assessed by miRNA-microarray. Briefly, total RNA was
isolated using the mir-Vana™ miRNA isolation kit (Applied
Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). After
isolation of total RNA, individual RNA samples from the NSCLC and
adjacent tissues were joined at equal amounts, respectively. One
µg mixed RNA of NSCLC or adjacent tissues was
reverse-transcribed into cDNA using a miRNA complete labeling and
Hyb kit (Agilent Technologies, Inc., Santa Clara, CA, USA),
respectively. Subsequently, the labeled probes were hybridized onto
three miRNA microarrays (Agilent Technologies, Inc.) for each
group. The Agilent SurePrint G3 Human GE 8x60K Microarrays were
scanned and analyzed using Agilent Feature Extraction (v10.7)
software (Agilent Technologies, Inc.). Agilent GeneSpring software
11.0 (Agilent Technologies, Inc.) was used for normalization of the
expression levels using the ΔΔCT method.
Cell culture
The H23 human NSCLC cell line was obtained from
Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) (Gibco-BRL, Invitrogen Life Technologies, Inc., Carlsbad,
CA, USA) with 10% fetal bovine serum (Gibco-BRL) at 37° C in an
incubator with an atmosphere of 5% CO2.
Cell transfection
The miRNA mimics and siRNA oligonucleotide duplexes
were synthesised by GenePharma (Shanghai, China). The sequences of
these miRNA mimics and siRNA oligonucleotide duplexes were those
designed by a previous study (20): miR-361-5p,
5′-ACCCCUGGAGAUUCUGAUAAUU-3′; negative control (NC),
5′-UUCUCCGAACGUGUCACGUTT-3′; STAT6-specific siRNA,
5′-AGACCUGUCCAUUCGCUCATT-3′ (sense) and 5′-UGAGCGAAUGGACAGGUCUTT-3′
(anti-sense). NC, 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAATT-3′ (anti-sense). Transfection was
performed using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer's instructions. The transfection
complexes were added to the cells and incubated for 6 h prior to
replacing the medium.
RT-qPCR
Total RNA was isolated from tissues or cells using
RNAiso Plus (Takara, Dalian, China). The RNA concentration was
measured using a BioPhotometer Plus (Eppendorf AG, Hamburg,
Germany). RNA was reverse-transcribed into cDNA using a miScript
SYBR Green PCR kit according to the manufacturer's instructions
(Qiagen, Hilden, Germany). The primer for miR-361-5p was purchased
from Sigma-Aldrich (St Louis, MO, USA; cat no. MIRAP00338). Reverse
transcription was performed using a PrimeScript RT reagent kit with
gDNA Eraser (Takara) according to the manufacturer's instructions.
Applied Biosystems 7500 Real-Time PCR System was used to conduct
the PCR. The PCR conditions were 95° C for 3 min followed by 40
cycles of 95° C for 12 sec and 62° C for 40 sec. GAPDH was used as
an internal control. The primers used were as follows: STAT6
forward, 5′-ACCCTCGAGTCCGCCACCATGGCTCTGTGGGGTCTG-3′ and reverse,
5′-CAGCTGGGATCGAATTCTGGGGTTGGCCCT-3′; B-cell lymphoma extra large
(Bcl-xL) forward, 5′-GAT CCCCATGGCAGCAGTAAAGCAAG-3′ and reverse,
5′-CCCCATCCCGGAAGAGTTCATTCACT-3′. GAPDH forward,
5′-TGCACCACCAACTGCTTAGC and reverse, 5′-GGCATGGACTGTGGTCATGAG-3′.
All reactions were performed in triplicate. Negative control
without template was used to eliminate contamination. The relative
expression was calculated using the 2−ΔΔCt method.
Western blot analysis
The protein expression levels of STAT6, Bcl-xL and
GAPDH were analyzed using western blot analysis. The cells were
homogenized using NP-40 lysis buffer [1% NP-40, 150 mM NaCl, 50 mM
Tris (pH 8.0)]. The protein concentration was determined using the
bicinchoninic acid protein kit (Beyotime Institute of
Biotechnology, Inc.). For each sample, 20 µg protein was
separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (Millipore, Billerica, MA, USA). After blocking
with 4% skimmed milk at room temperature for 1 h, the membranes
were incubated with rabbit STAT6 monoclonal antibody (cat. no.
#5397; 1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit
Bcl-xL monoclonal antibody (cat. no. sc-7195) or rabbit GAPDH
monoclonal antibody (cat. no. sc-25778) (both 1:1000; Santa-Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4° C overnight,
respectively. The membranes were then washed three times with TBST
(pH 7.6; 20 mM Tris-HCl, 137 mM NaCl and 0.01% Tween-20). The
membranes were subsequently stained with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
sc-2301; 1:1000; Santa-Cruz Biotechnology, Inc.) at 37° C for 1 h.
The blots were visualized using enhanced chemiluminescence reagent
(Millipore). Images were captured using a Kodak 440 digital imager
(San Diego, CA, USA). Image-Pro Plus≈6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) was used for analysis.
Immunohistochemistry (IHC)
The samples were fixed with 10% buffered formalin
and embedded in paraffin. Tissue blocks were cut into 6-µm
sections. The sections were blocked with normal rabbit serum (Santa
Cruz Biotechnology, Inc.)and then incubated with the same
monoclonal antibodies as those used for western blotting (1:100
dilution for STAT6 and Bcl-xL) at 4° C overnight. After washing
with TBST, the sections were stained with the horseradish
peroxidase streptavidin complex (Beyotime Institute of
Biotechnology, Inc.). The sections were color-developed with
diaminobenzidine (Beyotime Institute of Biotechnology, Inc.). The
sections were stained with hematoxylin and observed under a Leica
400 light microscope (Leica Microsystems, Inc., Buffalo Grove, IL,
USA).
Cell proliferation assay
H23 cells were seeded in six-well plates and
cultured for 24 h followed by transfection with miR-361-5p for 24
h. Subsequently, the cells were re-suspended and seeded into a
96-well plate at 2,000 cells/well. Cell proliferation was
determined using a CCK-8 assay kit (Beyotime Institute of
Biotechnology, Inc). The absorbance was detected using a
Bioluminescence 96-well microplate reader (GloMax 96-well
Luminometer; Promega Corporation, Madison, WI, USA). at a
wavelength of 450 nm according to the manufacturer's instructions.
Cell viability was determined by calculating the mean values.
Experiments were repeated three times and three wells were used for
each group.
Colony formation assay
After transfection with miR-361-5p or NC vector, 300
cells for each group were plated into each well of a six-well plate
and incubated for two weeks at 37° C in an incubator with an
atmosphere of 5% CO2. Subsequently, the cells were
washed three times using phosphate-buffered saline (PBS) and fixed
with methanol for 15 min. The cells were then stained with 0.2%
crystal violet (Beyotime Institute of Biotechnology, Inc.) for 20
min at room temperature. The colonies were counted and the results
were obtained by determining the mean values from three independent
experiments.
Flow cytometric analysis
To determine the apoptotic rate of the cells,
Annexin V-fluorescein isothiocyanate and propidium iodide
(BioVision, Milpitas, CA, USA) double staining was performed
according to manufacturer's instructions, followed by flow
cytometric analysis (BD FACSCanto II Flow; Beckman-Coulter, Brea,
CA, USA). At least 30,000 cells for each sample were processed. The
experiments were performed in triplicate.
Plasmid construction and luciferase
reporter assay
The 3′-UTR of STAT6 mRNA with affinity for
miR-361-5p was obtained by RT-PCR. The PCR products were then
cloned into the psiCHECK-2™ luciferase Vector (Promega Corporation)
using XhoI and NotI restriction sites (Promega
Corporation according to the protocol of a previous study (22). A vector containing a deletion at
the binding site was also generated as a mutant reporter. The cells
were then transfected with STAT6 3′-UTR (Sangon Biotech. Shanghai
Co., Ltd., Shanghai, China) and mutant reporter as well as
miR-361-5p mimics and NC. The Bcl-xL promoter region containing a
STAT6-binding sequence was cloned into the luciferase reporter
plasmid pGL3-basic (Promega Corporation) to generate a reporter of
Bcl-xL. The cells were then transfected with these vectors and
dual-luciferase assays were performed to detect luciferase activity
at 48 h after co-transfection.
Xenograft experiment
Five-week-old female nude mice (age, 5 weeks;
weight, 14.52±0.42 g; n=5/group) were obtained from the Animal
Center of The Second Affiliated Hospital (Medical School of Xi'an
Jiaotong University, Xi'an, China) and were used for the tumor
formation experiment. The mice were fed with a 0.012% rottlerin
diet and kept at 22° C and 30% humidity. The experiment was
approved by the ethics committee of the Second Affiliated Hospital
of Medical School, Xi'an Jiaotong University (Xi'an, China). The
mice were injected with H23 cells into the dorsal side. The tumor
volume was measured every two days. The formula V=LxWxDx3.14/6 was
used to calculate the tumor volume. Once the tumor volume was
>30 mm3, the mice were divided into two groups. One
group was treated with 200 pmol NC vector and the other group was
treated with 200 pmol miR-361-5p mimics with 10 µl
Lipofectamine 2000 (Invitrogen Life Technologies, Inc.). The
xenograft tumors were injected with the vectors at multiple sites
and injection was performed every 2 days over 19 days. The tumors
were harvested following 19 days and subjected to western blot
analysis, IHC and RT-qPCR. After 19 days post-experiment, the mice
were anesthetized by inhalation of 2.5% isoflurane (Invitrogen Life
Technologies, Inc.) and executed.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard deviation of at least three independent experiments.
Significant differences were confirmed using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-361-5p is downregulated in NSCLC
tissues
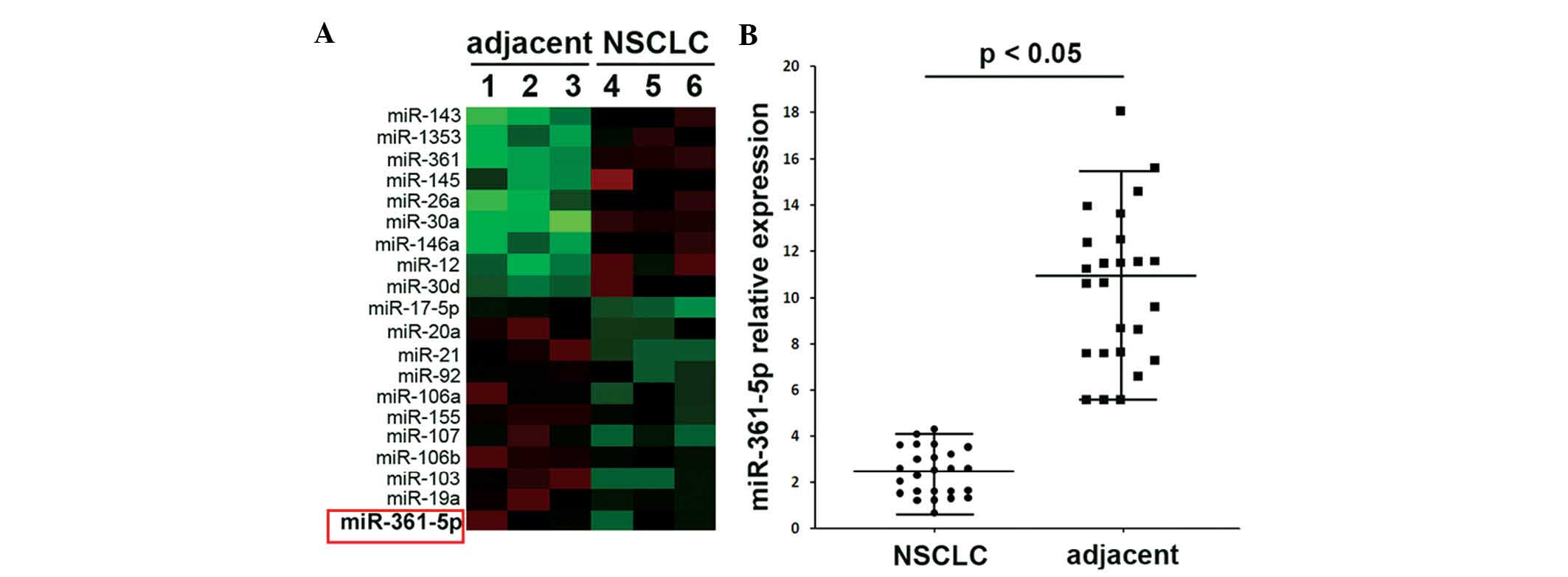
The miRNA array of NSCLC samples and adjacent
tissues indicated lower expression of miR-361-5p in NSCLC tissues
compared to that in adjacent tissues (Fig. 1A). Expressional changes of
miR-361-5p in 25 NSCLC and 25 adjacent tissues were also confirmed
by RT-qPCR (Fig. 1B) with the
results showing similar results to those of the miRNA array. It is
therefore concluded that the reduced expression of miR-361-5p may
participate in the progression of NSCLC.
Effects of miR-361-5p on H23 in
vitro
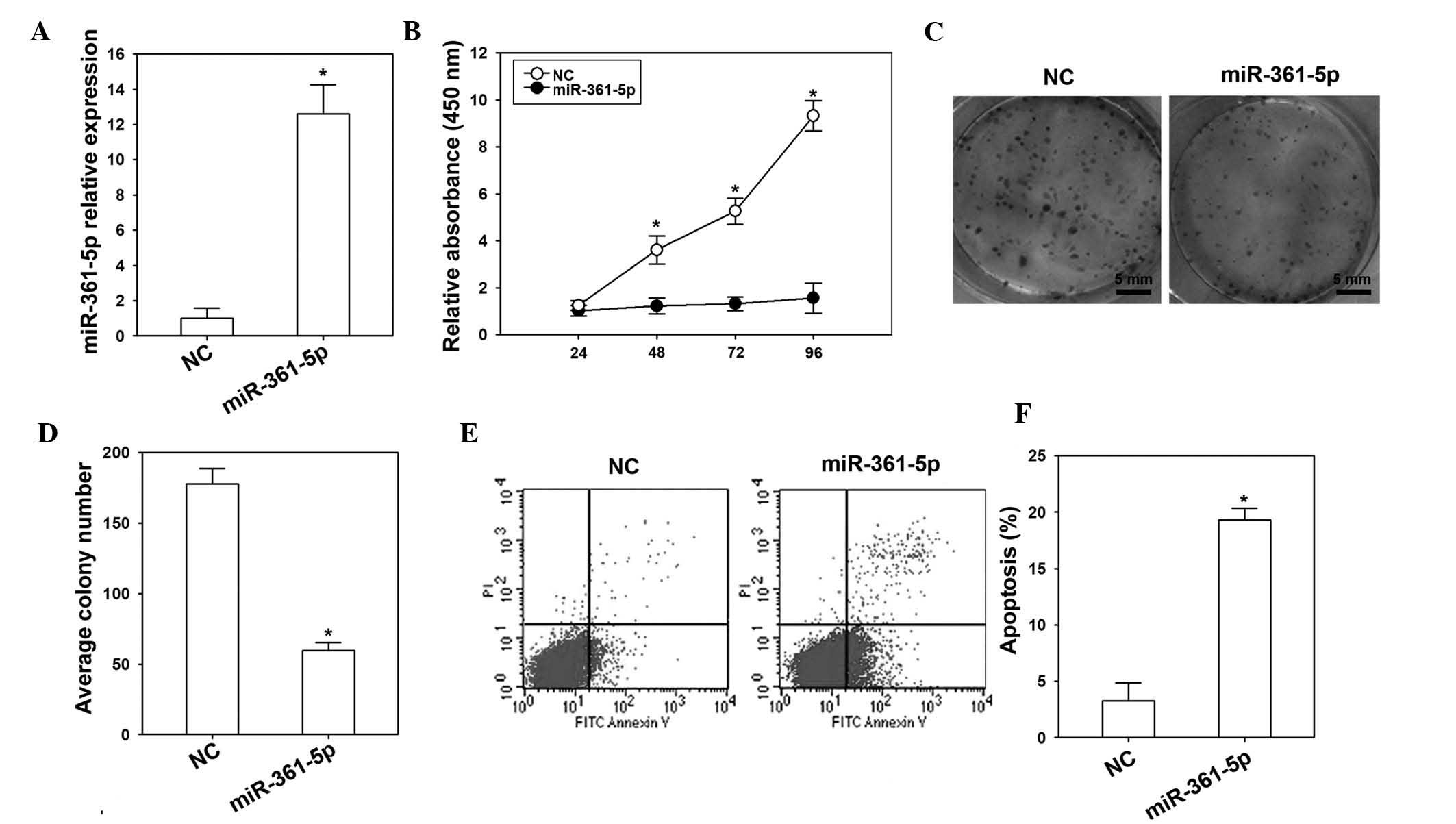
The effects of miR-361-5p in H23 cells were detected
by transfection with miR-361-5p mimics or NC vector. RT-qPCR
confirmed that miR-361-5p was overexpressed in the miR-361-5p
mimics group compared to that in the NC group (Fig. 2A). Of note, overexpression of
miR-361-5p inhibited the proliferation and colony formation ability
of H23 cells (Fig. 2B–D). Flow
cytometric analysis showed that the apoptotic rate of H23 cells
transfected with miR-361-5p mimics was higher than that in the NC
group (Fig. 2E and F).
STAT6 is a direct target of miR-361-5p
and promotes Bcl-xL expression
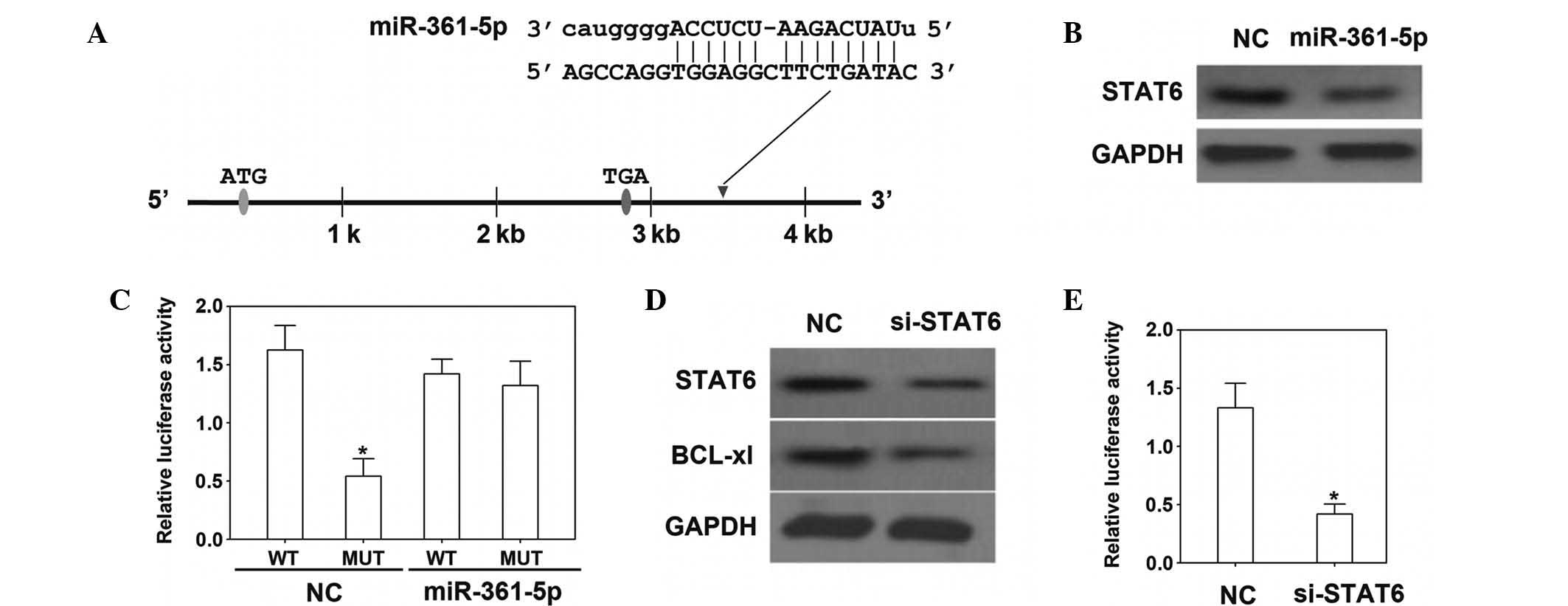
The 3′-UTR of STAT6 mRNA was identified as a target
of miR-361-5p (Fig. 3A). In the
present study, the mRNA and protein levels of STAT6 were depressed
by transfection with miR-361-5p mimics (Fig. 3B and C). To confirm the direct
interaction of miR-361-5p with the STAT6 3′-UTR, a dual-luciferase
reporter vector containing the STAT6 3′-UTR was constructed and
transfected into H23 cells, which were co-transfected with
miR-361-5p mimics or control vector. The results showed that
luciferase activity was significantly decreased when miR-361-5p was
overexpressed. Moreover, following transfection with STAT6-specific
siRNA, the expression of Bcl-xL was downregulated, indicating that
STAT6 is required for Bcl-xL expression in H23 cells (Fig. 3D). A further luciferase reporter
assay demonstrated that STAT6 was able to bind to the Bcl-xL
promoter and that STAT6 siRNA was able to decrease Bcl-xL
expression and luciferase activity (Fig. 3E).
miR-361-5p suppresses tumor growth in
vivo
Following transfection with miR-361-5p every other
day, the volume of tumors derived from H23 cells was significantly
suppressed from days 5–18 of the xenograft experiment (Fig. 4A). Vector-mediated overexpression
of miR-361-5p in the tumors led to the down-regulation of STAT6 and
Bcl-xL at the mRNA as well as the protein level (Fig. 4B–D), which indicated that
miR-361-5p reduced STAT6/Bcl-xL signaling and tumorigenicity in
vivo.
Discussion
NSCLC is one of the most prevalent cancer types in
China and has a high mortality rate; however, the underlying
mechanisms for the progression of NSCLC have largely remained
elusive. Accumulating evidence has indicated a deregulated
expression of miRNAs in various types of cancer, including NSCLC
(11,23,24).
These miRNAs are likely to have crucial roles in tumorigenesis and
may be employed as suitable targets or tools in cancer treatment.
For instance, miR-21 represses tumor suppressor phosphatase and
tensin homolog and promotes growth (25) and invasion of NSCLC, while miR-494
is able to modulate apoptosis induced by tumor necrosis
factor-related apoptosis-inducing ligand in NSCLC (26). In addition, miR-101, miR-1254,
miR-574-5p, miR-143 and miR-181a were demonstrated to be involved
in NSCLC (27,28). The present study demonstrated that
miR-361-5p was down-regulated in NSCLC tissues compared with that
in adjacent tissues, which suggested that miR-361-5p may inhibit
the progression of NSCLC. Thus, the present study further examined
the role of miR-361-5p in the proliferation of proliferation NSCLC
cells in vitro as well as in vivo.
The present study demonstrated that transfection of
H23 cells with miR-361-5p suppressed their proliferation and colony
formation ability. Furthermore, overexpression of miR-361-5p
induced apoptosis in H23 cells. These results indicated that
miR-361-5p acted as a tumor suppressor in NSCLC. Similar results
were previously obtained for the castration-resistant prostate
cancer cell line DU145 (20). By
contrast, miR-361-5p functioned as an oncogene in cervical
carcinoma cells by enhancing their proliferation; it was indicated
that miR-361-5p is an important factor for the progression of
cervical cancer (29). Due to
these contradictory results, further studies should scan different
types of cancer in order to elucidate the function of
miR-361-5p.
STAT6, a member of the STAT family, is able to
activate cytokines and growth factors (30). In cancers, the expression of STAT6
was shown to be upregulated (31,32).
Cui et al (33) found that
unphosphorylated STAT6 triggers the upregulation of
cyclooxygenase-2 in NSCL. In addition, they demonstrated that
knockout of STAT6 resulted in a decrease of cyclo-oxygenase-2
expression. Cyclooxygenase-2 is a well-known marker of malignant
cancer phenotypes (34). Thus,
STAT6 also participates in the proliferation and invasion of cancer
cells. In mammary carcinoma, STAT6-deficient mice showed a markedly
enhanced immunity against tumors and metastasis (35). In prostate cancer, miR-361-5p
inhibited STAT6 as its target gene, and overexpression of
miR-361-5p in prostate neoplasms depressed STAT6 in vivo as
well as in vitro (20). In
analogy with this, the results of the present study also showed
that in NSCLC, miR-361-5p inhibited the expression of STAT6 in
vivo as well as in vitro. In order to further elucidate
the roles of the STAT6/Bcl-xL signaling pathway, the present study
examined the expression of Bcl-xL following STAT6 inhibition and
used a luciferase reporter assay to demonstrate that STAT6 directly
regulates Bcl-xL expression in NSCLC.
The crosstalk between the STAT6/Bcl-xL signaling
pathway and miR-361-5p may be a key incidence for the development
of metastatic of NSCLC. STAT6 knockout was previously associated
with the depression of proliferation and induction of apoptosis of
cancer cells (33). Furthermore,
STAT6 signaling was shown to contribute to cancer cell growth. The
present study demonstrated that Bcl-xL levels were reduced
following downregulation of STAT6 by miR-361-5p, providing a novel
approach for treating NSCLC by inhibiting the STAT6/Bcl-xL
signaling pathway. Several miRNAs have been previously shown to
inhibit the proliferation and metastasis of tumor cells. However,
to date, no suitable therapeutic targets for NSCLC have been
identified. In order to uncover therapeutic targets, a
comprehensive screening analysis of miRNAs in NSCLC is required.
The present study used a miRNA array to scan NSCLC tissues for
potential targets, revealing a regulatory mechanism of miR-361-5p
in NSCLC. It was speculated that the downregulation of miR-361-5p
may be responsible for the dysregulation of STAT6/Bcl-xL signaling,
resulting in tumor progression.
In conclusion, the present study demonstrated that
miR-361-5p was decreased in NSCLC tissues. Furthermore,
upregulation of miR-361-5p depressed proliferation and colony
formation, and promoted apoptosis of H23 cells via the STAT6/Bcl-xL
pathway in vitro and in vivo. These results indicated
that miR-361-5p has a tumor-suppressive function in NSCLC, which
may be harnessed as a strategy for treating NSCLC; the feasibility
of this approach requires further evaluation.
Acknowledgments
The present study was supported in part by a grant
from the Science and Technology R&D Program of Shaanxi Province
[Grant no. 2010K14-02 (22)].
References
|
1
|
Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang
Y, Zhao H, Wu J, Zhang Y, Zhao L, et al: National survey of the
medical treatment status for non-small cell lung cancer (NSCLC) in
China. Lung Cancer. 77:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: Biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T, Viallet J, Mulshine JL,
Linnoila RI, Minna JD and Gazdar AF: Mutations of ras genes
distinguish a subset of non-small-cell lung cancer cell lines from
small-cell lung cancer cell lines. Oncogene. 6:1353–1362.
1991.PubMed/NCBI
|
|
5
|
Stephens P, Hunter C, Bignell G, Edkins S,
Davies H, Teague J, Stevens C, O'Meara S, Smith R, Parker A, et al:
Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature.
431:525–526. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Temel JS, Greer JA, Muzikansky A,
Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD,
Jacobsen J, Pirl WF, et al: Early palliative care for patients with
metastatic non-small-cell lung cancer. N Engl J Med. 363:733–742.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schilsky RL: Personalized medicine in
oncology: The future is now. Nat Rev Drug Discov. 9:363–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerber DE and Minna JD: ALK inhibition for
non-small cell lung cancer: From discovery to therapy in record
time. Cancer Cell. 18:548–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu SL, Chen HY, Chang GC, Chen CY, Chen
HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang
KM and De W: Prognostic significance of serum miRNA-21 expression
in human non-small cell lung cancer. J Surg Oncol. 104:847–851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larzabal L, de Aberasturi AL, Redrado M,
Rueda P, Rodriguez MJ, Bodegas ME, Montuenga LM and Calvo A:
TMPRSS4 regulates levels of integrin α5 in NSCLC through miR-205
activity to promote metastasis. Br J Cancer. 110:764–774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foss KM, Sima C, Ugolini D, Neri M, Allen
KE and Weiss GJ: MiR-1254 and miR-574-5p: Serum-based microRNA
biomarkers for early-stage non-small cell lung cancer. J Thorac
Oncol. 6:482–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chin LJ, Ratner E, Leng S, Zhai R, Nallur
S, Babar I, Muller RU, Straka E, Su L, Burki EA, et al: A SNP in a
let-7 microRNA complementary site in the KRAS 3′ untranslated
region increases non-small cell lung cancer risk. Cancer Res.
68:8535–8540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roth C, Stückrath I, Pantel K, Izbicki JR,
Tachezy M and Schwarzenbach H: Low levels of cell-free circulating
miR-361-3p and miR-625* as blood-based markers for
discriminating malignant from benign lung tumors. PLoS One.
7:e382482012. View Article : Google Scholar
|
|
17
|
Watahiki A and Wang Y, Morris J, Dennis K,
O'Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PLoS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khella HW, White NM, Faragalla H, Gabril
M, Boazak M, Dorian D, Khalil B, Antonios H, Bao TT, Pasic MD, et
al: Exploring the role of miRNAs in renal cell carcinoma
progression and metastasis through bioinformatic and experimental
analyses. Tumor Biol. 33:131–140. 2012. View Article : Google Scholar
|
|
19
|
Kanitz A, Imig J, Dziunycz PJ, Primorac A,
Galgano A, Hofbauer GF, Gerber AP and Detmar M: The expression
levels of microRNA-361-5p and its target VEGFA are inversely
correlated in human cutaneous squamous cell carcinoma. PLoS One.
7:e495682012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: MiR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Humphrey PA: Gleason grading and
prognostic factors in carcinoma of the prostate. Mod Pathol.
17:292–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nature Genetics. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romano G, Acunzo M, Garofalo M, Di Leva G,
Cascione L, Zanca C, Bolon B, Condorelli G and Croce CM: MiR-494 is
regulated by ERK1/2 and modulates TRAIL-induced apoptosis in
non-small-cell lung cancer through BIM down-regulation. Proc Natl
Acad Sci USA. 109:16570–16575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan P, Yin Z, Li X, Wu W and Zhou B:
Meta-analysis of human lung cancer microRNA expression profiling
studies comparing cancer tissues with normal tissues. J Exp Clin
Cancer Res. 31:542012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Wang S, Wang H, Li P and Ma Z:
MicroRNAs: Novel biomarkers for lung cancer diagnosis, prediction
and treatment. Exp Biol Med (Maywood). 237:227–235. 2012.
View Article : Google Scholar
|
|
29
|
Wu X, Xi X, Yan Q, Zhang Z, Cai B, Lu W
and Wan X: MicroRNA-361-5p facilitates cervical cancer progression
through mediation of epithelial-to-mesenchymal transition. Med
Oncol. 30:7512013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wurster AL, Tanaka T and Grusby MJ: The
biology of Stat4 and Stat6. Oncogene. 19:2577–2584. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Das S, Shetty P, Valapala M, Dasgupta S,
Gryczynski Z and Vishwanatha JK: Signal transducer and activator of
transcription 6 (STAT6) is a novel interactor of annexin A2 in
prostate cancer cells. Biochemistry. 49:2216–2226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li BH, Yang XZ, Li PD, Yuan Q, Liu XH,
Yuan J and Zhang WJ: IL-4/Stat6 activities correlate with apoptosis
and metastasis in colon cancer cells. Biochem Biophys Res Commun.
369:554–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui X, Zhang L, Luo J, Rajasekaran A,
Hazra S, Cacalano N and Dubinett SM: Unphosphorylated STAT6
contributes to constitutive cyclooxygenase-2 expression in human
non-small cell lung cancer. Oncogene. 26:4253–4260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hida T, Yatabe Y, Achiwa H, Muramatsu H,
Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T and Takahashi
T: Increased expression of cyclooxygenase 2 occurs frequently in
human lung cancers, specifically in adenocarcinomas. Cancer Res.
58:3761–3764. 1998.PubMed/NCBI
|
|
35
|
Ostrand-Rosenberg S, Grusby MJ and
Clements VK: Cutting edge: STAT6-deficient mice have enhanced tumor
immunity to primary and metastatic mammary carcinoma. J Immunol.
165:6015–6019. 2000. View Article : Google Scholar : PubMed/NCBI
|