Introduction
Isoflurane is widely used in the clinic as a
volatile anaesthetic. Previous studies revealed that
preconditioning with isoflurane mimics the protective effects of
early and delayed ischemic preconditioning in the brain
(1–6). Isoflurane produced early preconditioning against
spinal cord ischemic injury (6).
However, whether isoflurane produces delayed preconditioning in
neural stem cells (NSCs) remains to be fully elucidated. The
serine/threonine kinase, LKB1 (also known as STK11), has assumed a
prominent position among tumour suppressors (7–9). LKB1 is
inactivated in individuals with Peutz-Jeghers syndrome, which is a
familial cancer-prone disease (10). Best characterized as an upstream
activator of the adenine monophosphate-activated protein kinase and
the mammalian target of rapamycin pathways (10), LKB1 regulates cellular and
organismal metabolism, cell polarity and a variety of other
functions, ranging from proliferation and migration to senescence,
apoptosis, the DNA damage response and differentiation (7,11).
Zeng et al (12) reported
that LKB1 physically associates with p53 in the nucleus and
phosphorylates p53 at the amino acid residues Ser15 and Ser392.
LKB1 is also recruited directly to the p21/WAF1 promoter and other
p53-activated promoters, and thereby regulates the cell cycle.
Furthermore, Liang et al (13) identified that knockdown of the
endogenous LKB1 facilitated cell cycle progression through the G1
and S phases, which was, at least in part, due to a reduced
activity of the p53 and p16 pathways (13). In addition, Liu et al
(8) reported that the inactivation
of LKB1 in the presence of active Ras facilitated metastasis, which
was proposed to be mediated via the Src family kinase-dependent
expansion of a CD24+ melanoma prometastatic tumour
subpopulation (8). On the basis of
these studies, the present study aimed to investigate whether the
Lkb1-p53-p21/WAF1 axis has a regulatory role in the modulation of
normal mouse NSC proliferation during isoflurane treatment.
Materials and methods
Isolation of the mouse NSCs
Mouse NSCs were isolated from the corpus striatum of
the embryonic brain in a 13-day-old pregnant mouse. Isolation of
the NSCs was performed as previously described (14). Briefly, the embryonic brain tissue
was sectioned into small segments with a razor. The segments were
digested with 0.125% trypsin-EDTA (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C for 15 min. The resulting cell suspension was seeded
in a six-well plate (Falcon, BD Biosciences, Franklin Lakes, NJ,
USA) in Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1) (Gibco,
Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 1%
(v/v) B27® Supplement, 20 ng/ml epidermal growth factor,
20 ng/ml basic fibroblast growth factor, 5 µg/ml heparin, 1
µg/ml laminin, 100 U/ml penicillin and 0.3 mg/ml glutamine
(all from Gibco), and incubated in a humidified tissue culture
incubator containing 5% CO2 at 37°C. The mouse
NSC (p53−/−) cell line, NE-4C, was kindly provided by
the Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China).
The NE-4C cells were cultured in DMEM:F12 (1:1) medium,
supplemented with 15% FBS, 1 mM sodium pyruvate, 2 mM L-glutamine,
0.1 mM non-essential amino acids and penicillin (25
U/ml)-streptomycin (925 mg/ml) (all from Gibco), prior to mixing.
The cells were subsequently incubated in a humidified tissue
culture incubator containing 5% CO2 at 37°C. The
cells were cultured on an identical feeder until passage 2, prior
to the subsequent experiments.
RNA extraction and analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA from each cell was isolated using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), according to the manufacturer's instructions. The RNA
samples were treated with DNase I (Sigma-Aldrich), quantified, and
reverse-transcribed into cDNA using the ReverTra Ace-α First Strand
cDNA Synthesis kit (Toyobo Co., Ltd., Osaka, Japan). RT-qPCR was
performed using a RealPlex4 real-time PCR detection system from
Eppendorf Co., Ltd. (Hamburg, Germany), with SYBR-Green RealTime
PCR Master mix (Toyobo Co., Ltd.) used as the detection dye.
RT-qPCR amplification was performed over 40 cycles, with
denaturation at 95°C for 15 sec and annealing at 58°C
for 45 sec. The target cDNA was quantified using the relative
quantification method. A comparative threshold cycle (Ct) was used
to determine gene expression relative to a control (calibrator),
and the steady-state mRNA levels were reported as an n-fold
difference relative to the calibrator. For each sample, the Ct
values of the marker genes were normalized using the equation: ΔCt
= Ct_genes - Ct_18S RNA. The relative expression levels were
determined using the equation: ΔΔCt = ΔCt_sample_groups -
ΔCt_control_group. The values used to plot the relative expression
of markers were calculated using the expression 2−ΔΔCt.
The mRNA levels were calibrated, based on the levels of 18S rRNA.
The cDNA of each gene was amplified using the primers listed in
Table I.
 | Table IReverse-transcription quantitative
polymerase chain reaction primers used in the present study. |
Table I
Reverse-transcription quantitative
polymerase chain reaction primers used in the present study.
| Gene product | Forward and reverse
primers (5′→3′) | Size (bp) |
|---|
| Lkb1 | F:
GGGCAACCTGCTACTCACC | 103 |
| R:
CCAGATGTCCACCTTGAAAC | |
| p53 | F:
ATGAACCGCCGACCTATC | 98 |
| R:
AGGGCAGGCACAAACACG | |
| p21 | F:
GCCTTGTCGCTGTCTTGC | 95 |
| R:
GCTGGTCTGCCTCCGTTTT | |
| 18S rRNA | F:
AGGGGAGAGCGGGTAAGAGA | 241 |
| R:
GGACAGGACTAGGCGGAACA | |
Immunofluorescence staining
The cultured cells were washed three times with
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde
(Sigma-Aldrich) for 30 min. For blocking of non-specific binding
sites, the samples were incubated with phosphate-buffered saline
(Sigma-Aldrich) containing 0.5% Triton X-100 (Sigma-Aldrich) and 5%
calf serum (Gibco) for 40 min at 37°C. Subsequently, the cells were
first incubated with a primary antibody (Table II) overnight at 4°C and
then with fluorescein isothiocyanate-conjugated goat anti-rabbit
IgG antibody (1:200; Abcam, Cambridge, UK) and 5 µg/ml
4′,6-diamidino-2-phenylindole (Sigma-Aldrich) at 26°C for 30 min.
Subsequently, the cells were thoroughly washed with TBS-T buffer
and viewed under a fluorescence microscope (DMI3000; Leica,
Allendale, NJ, USA).
 | Table IIList of primary antibodies used in the
present study. |
Table II
List of primary antibodies used in the
present study.
| Antibody | Cat. no. | Company | Application |
|---|
| Rabbit anti-mouse
LKB1 | (sc-5638) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | WB (1:1,000) |
| | | IHC (1:200) |
| Rabbit anti-mouse
p53, Ser15-pho | (sc-101762) | Santa Cruz
Biotechnology, Inc. | IHC (1:200) |
| Rabbit anti-mouse
p21 | (2947S) | Cell Signaling
Technology, Inc. (Danvers, MA, USA) | WB (1:1,000) |
| | | IHC (1:200) |
| Rabbit
anti-GAPDH | (5174S) | Cell Signaling
Technology | WB (1:1,000) |
Co-immunoprecipitation assay
For each experiment, the cells were seeded at
3×105 cells/well in six-well plates, cultured until 85%
confluence was reached, and subsequently lysed (500 µl per
plate) in a modified cell lysis buffer [20 mM Tris/HCl, (pH 7.5),
150 mM NaCl, 1% Triton X-100, 1 mM EDTA, sodium pyrophosphate,
β-glycerophosphate, Na3VO4 and leupeptin;
Beyotime Institute of Biotechnology, Shanghai, China), prior to
western blot analysis and immunoprecipitation assay. Following
lysis, each sample was collected to clear the lysate of the
insoluble debris by centrifugation (Allegra 64R; Beckman Coulter,
Miami, FL, USA) at 10,000 × g for 10 min, prior to an incubation
with 20 µg protein A-agarose beads (Beyotime Institute of
Biotechnology) by agitation for 30 min at 4°C. Subsequently,
the samples were centrifuged again at 10,000 × g for 1 min and
transferred to a fresh 1.5 ml tube. The antibodies (Table II) were incubated with the samples
for 90 min prior to the re-addition of 20 µg protein
A-agarose beads to capture the immune complexes. The pelleted beads
were subsequently washed three times with 500 µl cell lysis
buffer, dissolved in 4X SDS-PAGE sample loading buffer, and heated
for 10 min at 95°C.
Chromatin immunoprecipitation (ChIP)
assays
ChIP experiments were performed using the primary
antibodies provided in Table II
and normal rabbit immunoglobulin G (IgG; Upstate Biotechnology,
Inc., Lake Placid, NY, USA) as a negative control. All the steps
were performed as previously described (9,12).
Briefly, the cells were fixed with 1% formaldehyde for 30 min at
37°C, and the reaction was subsequently quenched by the
addition of 125 mM glycine for 10 min at room temperature to form
DNA-protein cross-links. The products were sheared by sonication
using 4–5 sets of 10-sec pulses on wet ice with a Cole-Parmer High
Intensity Ultrasonic Sonicator (50-watt model; Cole-Parmer
Instrument Co., San Diego, CA, USA), equipped with a 2-mm tip and
set to 30% of the maximum power, which produced the appropriate
200–800 bp DNA fragments. Subsequently, the samples were incubated
with antibodies at 4°C overnight. The PCR amplification
experiment was performed under the following conditions: 33 cycles
run by denaturation at 95°C for 30 sec, annealing at
55°C for 30 sec, and extension at 72°C for 30 sec.
Changes in the binding were expressed as the relative fold
enrichment, following the subtraction of the matched IgG negative
control.
Western blot analysis
The total protein extracts from each treatment group
were resolved by 12% SDS-PAGE at 20°C over 120 min and transferred
onto polyvinylidene fluoride (PVDF) membranes (Millipore,
Billerica, MA, USA). Following blocking, the PVDF membranes were
washed four times for 15 min with TBS-T buffer at room temperature,
and subsequently incubated with the primary antibodies listed in
Table II. Following extensive
washing, the membranes were incubated with secondary
peroxidase-linked goat anti-rabbit IgG (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h. Following a
further four washing steps (15 min each) with TBS-T buffer at room
temperature, the immunoreactivity was visualized by enhanced
chemiluminescence (ECL) using the ECL kit of Pierce Biotechnology,
Inc. (Rockford, IL, USA). The membranes were subsequently exposed
to Kodak XAR-5 films (Sigma-Aldrich). GAPDH was used as a loading
control.
Flow cytometric (FCM) analysis of the
cell cycle by propidium iodide (PI) staining
Each group of cells was seeded at 3×105
cells/well in six-well plates and cultured until 85% confluence was
reached. The cells were washed three times with PBS, prior to
collection by centrifugation (Allegra X-22R, Beckman Coulter,
Miami, FL, USA) at 1,000 × g for 5 min. The cell pellets were
subsequently resuspended in 1 ml PBS, fixed in 70% ice-cold ethanol
and kept in a freezer for >48 h. Prior to flow cytometric
analysis, the fixed cells were centrifuged at 1,000 × g for 5 min,
washed twice with PBS, and resuspended in PI staining solution
(Sigma-Aldrich), containing 50 µl/ml PI and 250 µg/ml
RNase A (Sigma-Aldrich). The cell suspension, which was protected
from the light, was incubated for 30 min at 4°C and analysed
by fluorescence-activated cell sorting (FACS) using a BD FACSAria
cell sorter (BD Biosciences, Frankin Lakes, NJ, USA). A total of
20,000 events were acquired for analysis using CellQuest software
version 2.0 (BD Biosciences).
Methyl thiazolyl tetrazolium (MTT) assay
for cell proliferation
Each group of cells was seeded at 2×103
cells/well in six-well plates, and cultured in DMEM supplemented
with 10% FBS at 37°C with 5% CO2 until 85%
confluence was reached. The MTT reagent (5 mg/ml; Sigma-Aldrich)
was added to the maintenance cell medium containing different
concentrations of isoflurane (0, 0.125, 0.25 and 0.5 mM) for
different time periods (0, 12, 24 and 48 h), and incubated at
37°C for an additional 4 h. The reaction was terminated by
the addition of 150 µl dimethylsulfoxide (DMSO;
Sigma-Aldrich) per well, and the cells were lysed for 15 min. The
plates were subsequently gently agitated for 5 min. The absorbance
was measured at 490 nm using an enzyme-linked immunosorbent assay
reader (Model 680; Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The data in each experiment are shown as the mean ±
standard error of the mean where applicable; differences were
evaluated using the Student's t-test. GraphPad Prism version 5.00
(GraphPad Software Inc, La Jolla, CA, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isoflurane suppresses proliferation of WT
NSCs by inducing cell cycle arrest
The results of the MTT assay indicated that
isoflurane significantly inhibited the proliferation of WT NSCs
(P<0.01, n=3) in a dose-dependent manner (Fig. 1). Furthermore, following treatment
of the WT NSCs with the concentration of isoflurane which caused
50% inhibition (IC50=0.246 mM), cell proliferation was
significantly inhibited at all time points studied (P<0.01,
n=3). By contrast, no difference was observed in the proliferation
rate of NE-4C (p53−/−) cells in the presence of
isoflurane compared with the controls at any time point (P>0.05,
n=3; Fig. 1). The complete
inhibition of proliferation induced by isoflurane in WT NSCs
appears to occur during the G0/G1 phase, as determined from the
FACS profile (Fig. 1).
Isoflurane activates p53-dependent
Lkb1-p21 signalling in NSCs
RT-qPCR revealed that the mRNA expression levels of
Lkb1, p53 and p21 were increased in WT NSCs
treated with the IC50 concentration of isoflurane
(P>0.05, n=3 compared with the control) (Fig. 2). However, the expression levels of
the NSC marker, Nestin, and a cell proliferation marker,
survivin, were significantly lower under these conditions
(P>0.05, n=3) (Fig. 2). The
situation was more variable in NE-4C (p53−/−) cells. The
expression levels of Lkb1, nestin and survivin
were significantly different following isoflurane treatment
(P<0.01, n=3), whereas the expression levels of p53 and
p21 remained unchanged (Fig.
2). Western blotting revealed that the expression levels of
Lkb1 and p21 in isoflurane-treated WT NSCs were significantly
increased compared with the control (P<0.01, n=3). By contrast,
only Lkb1 was induced at the protein level in isoflurane-treated
NE-4C cells (P<0.01, n=3). GAPDH was used as a loading control.
The immunofluorescence analysis yielded results which were
consistent with those of western blotting (Figs. 3 and 4).
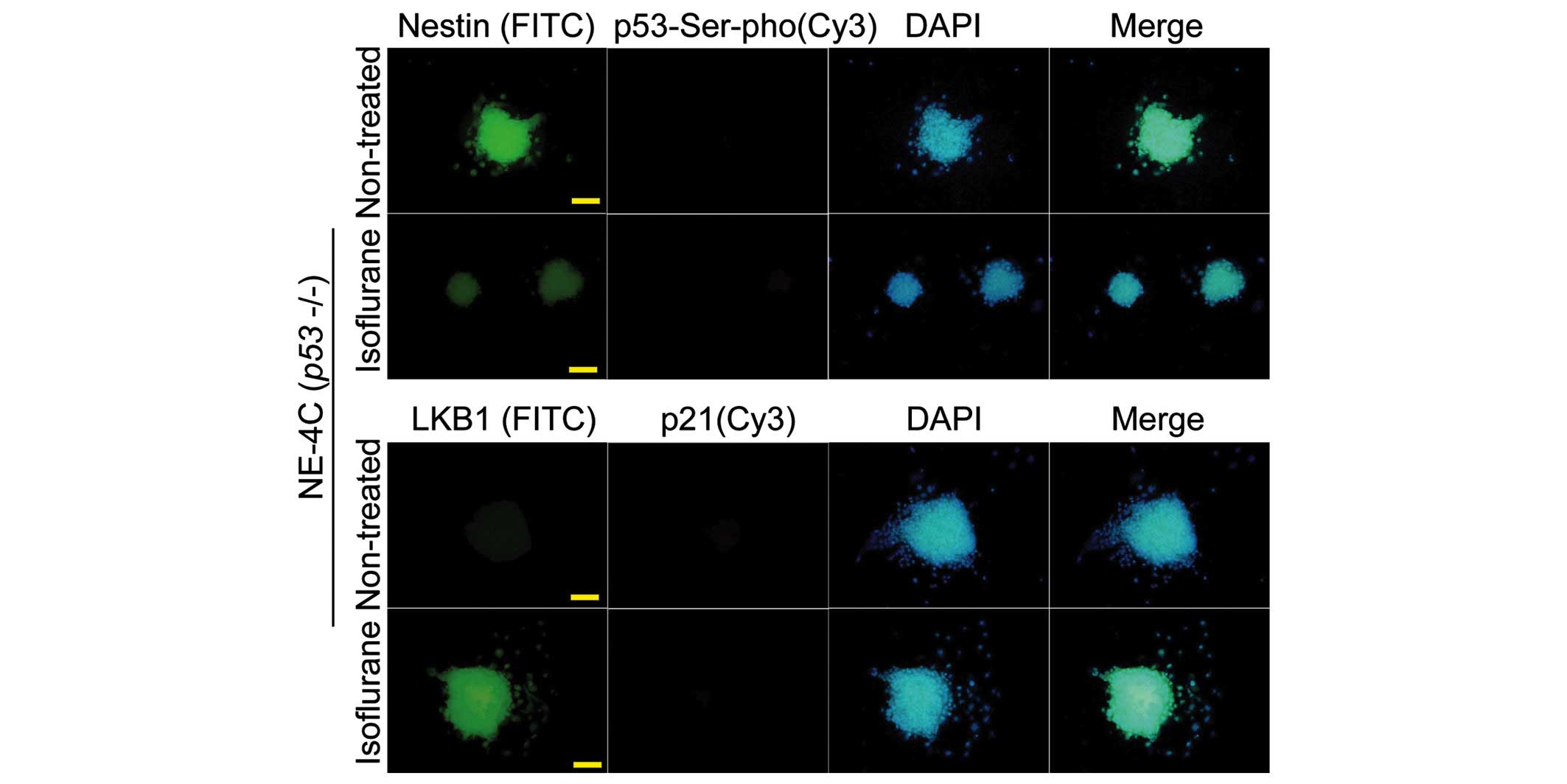
Isoflurane induces p53 Ser15
phosphorylation and increased levels of promoter binding in WT
NSCs
An increase in p53 Ser15 phosphorylation was
observed following treatment of the WT NSCs with the
IC50 concentration of isoflurane (P<0.01, n=3
compared with the control) (Fig.
5A). However, in the NE-4C (p53−/−) cells, the
protein signal of p53 and Ser15-phosphorylated p53 in the
isoflurane-treated group was not significantly different compared
with that in the untreated cells (P>0.05, n=3) (Fig. 5A). Subsequently, a ChIP assay was
used to determine whether isoflurane increased the quantity of p53
protein binding a target promoter. The binding efficiency of p53 to
two independent p53 protein-binding sites located in the p21
promoter was much higher following treatment (P<0.01, n=3
compared with the control) (Fig.
5B). However, no significant differences were identified in the
isoflurane-treated NE-4C (p53−/−) group or the untreated
group (P>0.05, n=3) (Fig. 5B).
These results indicated that the gene promoter activity of p21 is
increased in WT NSCs in the presence of isoflurane.
Discussion
Following mammalian nerve cell injury, there is a
spontaneous induction of NSC migration to sites of nerve injury,
followed by appropriate repair of the damaged tissue to restore the
original neuronal function (14).
Therefore, NSCs have an important role in the repair of nerve
injury and in nerve regeneration (14). As a well-established inhalational
anesthetic, isoflurane is generally considered to induce ischemic
tolerance in the myocardium, cerebrum and spinal cord (1–6),
however, the effects of isoflurane on NSCs have not been
extensively studied. In the present study, a dose-dependent
inhibition of mouse neural cell proliferation was observed. These
results suggested that this may contribute to certain of the side
effects of isoflurane. The additional experiments gave further
credence to the hypothesis that the Lkb1-p53-p21 signalling pathway
exerts a role in this response. A number of reports have indicated
that this pathway is important in the regulation of cell
proliferation (7–13). In response to specific stimuli, LKB1
phosphorylates the p53 transcription factor at specific sites
(9,12), which increases the affinity of p53
for its target promoters, including that of the p21 gene,
thereby increasing target gene expression (9,12).
The expression of p21 elicits cell cycle arrest, which, in certain
contexts, may be followed by apoptosis (9,12).
The present study revealed that the expression of Lkb1, p53 and p21
increased in WT NSCs, although not in the NE-4C (p53−/−)
cells, following isoflurane treatment. Furthermore, isoflurane
promoted p53 Ser15 phosphorylation and increased the binding of p53
to the p21 promoter.
Taken together, the results suggest that the
Lkb1-p53-p21 signalling pathway is important in the response of
NSCs to isoflurane. Further studies are required to determine
whether this is of clinical relevance, and whether the manipulation
of p53 activation may help to attenuate the side effects of
isoflurane.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81202811) and a project
funded by the China Postdoctoral Science Foundation (nos.
2014M550250 and 2015T80455) and the Shanghai Municipal Health
Bureau Fund (no. 20124320).
References
|
1
|
Sang H, Cao L, Qiu P, Xiong L, Wang R and
Yan G: Isoflurane produces delayed preconditioning against spinal
cord ischemic injury via release of free radicals in rabbits.
Anesthesiology. 105:953–960. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng S and Zuo Z: Isoflurane
preconditioning reduces purkinje cell death in an in vitro model of
rat cerebellar ischemia. Neuroscience. 118:99–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong L, Zheng Y, Wu M, Hou L, Zhu Z,
Zhang X and Lu Z: Preconditioning with isoflurane produces dose-
dependent neuroprotection via activation of adenosine triphosphate-
regulated potassium channels after focal cerebral ischemia in rats.
Anesth Analg. 6:233–237, table of contents. 2003.
|
|
4
|
Zheng S and Zuo Z: Isoflurane
preconditioning induces neuroprotection against ischemia via
activation of P38 mitogen- activated protein kinases. Mol
Pharmacol. 65:1172–1180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapinya KJ, Löwl D, Fütterer C, Maurer M,
Waschke KF, Isaev NK and Dirnagl U: Tolerance against ischemic
neuronal injury can be induced by volatile anesthetics and is
inducible NO synthase dependent. Stroke. 33:1889–1898. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park HP, Jeon YT, Hwang JW, Kang H, Lim
SW, Kim CS and Oh YS: Isoflurane preconditioning protects motor
neurons from spinal cord ischemia: Its dose- response effects and
activation of mitochondrial adenosine triphosphate- dependent
potassium channel. Neurosci Lett. 387:90–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ollila S and Mäkelä TP: The tumor
suppressor kinase LKB1: Lessons from mouse models. J Mol Cell Biol.
3:330–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Monahan KB, Pfefferle AD, Shimamura
T, Sorrentino J, Chan KT, Roadcap DW, Ollila DW, Thomas NE and
Castrillon DH: LKB1/STK11 inactivation leads to expansion of a
prometastatic tumor subpopulation in melanoma. Cancer Cell.
21:751–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Qin W, Hou L and Huang Y: MicroRNA-
17 promotes normal ovarian cancer cells to cancer stem cells
development via suppression of the LKB1- p53- p21/WAF1 pathway.
Tumour Biol. 36:1881–1893. 2015. View Article : Google Scholar
|
|
10
|
Krock B, Skuli N and Simon MC: The tumor
suppressor LKB1 emerges as a critical factor in hematopoietic stem
cell biology. Cell Metab. 13:8–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veeranki S, Hwang SH, Sun T, Kim B and Kim
L: LKB1 regulates development and the stress response.
Dictyostelium Dev Biol. 360:351–357. 2011. View Article : Google Scholar
|
|
12
|
Zeng PY and Berger SL: LKB1 is recruited
to the p21/WAF1 promoter by p53 to mediate transcriptional
activation. Cancer Res. 66:10701–10708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang X, Wang P, Gao Q, Xiang T and Tao X:
Endogenous LKB1 knockdown accelerates G(1)/S transition through p53
and p16 pathways. Cancer Biol Ther. 9:156–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren W, Guo Q, Yang Y and Chen F: bFGF and
heparin but not laminin are necessary factors in the mediums that
affect NSCs differentiation into cholinergic neurons. Neurol Res.
28:87–90. 2006. View Article : Google Scholar : PubMed/NCBI
|