Introduction
Extracellular matrix metalloproteinase inducer
(EMMPRIN) is a markedly glycosylated transmembrane glycoprotein
(1–4). EMMPRIN is widely expressed in human
tissues and exerts important roles in numerous tissue types,
including the lung, thymus, retina, skin, cornea and nervous
system, which involves various cellular processes (5,6). In
oncology research, EMMPRIN has been the subject of numerous
previous studies in the field of oncology, due to its consistently
high expression levels on the surface of various tumor types
(7–9). EMMPRIN is associated with cancer
progression and exerts important functions in tumor migration,
invasion, proliferation, angiogenesis, glycolysis and therapy
resistance (7–9). EMMPRIN stimulates surrounding
fibroblasts and endothelial cells to produce matrix
metalloproteinases and urokinase in a paracrine fashion, leading to
tumor cell invasion (10,11). Numerous previous reports
demonstrated that the expression levels of EMMPRIN were
significantly increased in esophageal cancer, as compared with
adjacent tissues (12–20). This indicated that EMMPRIN may be a
prognostic indicator for malignant tumors.
Hypoxia is a common characteristic of solid tumors,
and promotes cancer cell proliferation, angiogenesis, apoptosis
resistance, drug resistance and metastasis (21). Under hypoxic conditions,
hypoxia-inducible factors (HIFs) are upregulated and affect various
cellular biological processes, which allow the cancerous cells to
adapt to their environments (22).
HIF-1α is the most important subunit, and combines with a β subunit
to form a heterodimer, which in turn has important roles in tumor
hypoxia (22,23). Previous studies indicated that the
expression of EMMPRIN is upregulated under ischemic conditions in
neuronal and cardiac cells (24,25).
A previous study demonstrated that the expression of EMMPRIN may be
induced under hypoxic conditions in a colon carcinoma cell line,
LS174 (26), and suggested the
existence of an HIF-1 binding site, determined by chromatin
immunoprecipitation-on-chip assay (27–29).
However, the mechanism underlying the hypoxia-induced increase in
the expression of EMMPRIN remains to be elucidated in esophageal
cancer.
Based on these previous reports, the present study
hypothesized that EMMPRIN has an important role in HIF-1α-regulated
metastasis and the epithelial-mesenchymal transition (EMT) in
esophageal cells. The present study, therefore, investigated the
expression of EMMPRIN in hypoxic esophageal cancer cells, as well
as the role of EMMPRIN in metastasis and the EMT under hypoxic
conditions.
Materials and methods
Cell culture and hypoxia treatment
Human esophageal cancer cell lines, including
EC9709, EC-1 and EC-109, and the SHEE and HEEC normal esophageal
cell lines were purchased from the Shanghai Institute for
Biological Sciences (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (Gibco Life Technologies,
Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (PAA
Laboratories, Pasching, Australia) in a humidified incubator at
37°C, containing 5% CO2. For hypoxic exposure, the cells
were placed in a HERAcell 240 hypoxia incubator (Thermo Fisher
Scientific, Waltham, MA, USA) flushed with 1% O2, 5%
CO2, and 94% N2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer's instructions. The total RNA (2
µg) was reverse transcribed using a SYBR® PCR kit
(Takara Biotechnology Co., Ltd., Dalian, China). To quantify the
mRNA expression levels, qPCR was performed on a ABI Prism SDS 7000
sequence detector (Applied Biosystems Life Technologies, Foster
City, CA, USA). The program of qPCR was as follows: 50°C 2 min, one
cycle; 95°C 10 min, one cycle; 95°C 15 sec, 60°C 30 sec, 72°C 30
sec, 40 cycles; 72°C 10 min, one cycle. The relative expression
levels were normalized to the levels of GAPDH and were analyzed
using the comparative cycle threshold method (2−ΔΔCT).
EMMPRIN and GAPDH primers were synthesized and obtained from
Invitrogen Life Technologies. The primer sequences were as follows:
EMMPRIN, forward 5′-CGGGGCTGCCGGCACAGTCTTC-3′ and reverse
5′-AGCAGCCTCAGGTGGAACT-3′; and GAPDH, forward
5′-CATGACAACTTTGGTATCGTGG-3′ and reverse
5′-CCTGCTTCACCACCTTCTTG-3′. The mRNA expression levels of EMMPRIN
were normalized against those of GAPDH.
Small interfering (si)RNA
transfection
HIF-1α-specific siRNA (Shanghai GenePharma Co.,
Ltd., Shanghai, China) was used to downregulate the expression of
HIF-1α. A total of 2×105 cancer cells were seeded into
6-well plates 24 h prior to transfection. The cells were
transfected with HIF-1α siRNA or control siRNAs (100 nM) using
Lipofectamine® 2000 (Invitrogen Life Technologies),
according to the manufacturer's instructions, and the medium was
changed 6 h post-transfection. After 48 h, the total RNA or protein
was extracted for RT-qPCR or western blotting, respectively.
Western blot analysis
Esophageal cancer cells were treated with human
recombinant (hr)EMMPRIN (ACROBiosystems, Newark, DE, USA) or
transfected with HIF-1α siRNA, and the total protein was isolated
for western blotting. Briefly, the cells were lysed in
radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) to extract the proteins and the protein
concentration was determined using a Bradford assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The protein was separated
by 12.5% SDS-PAGE and transferred onto nitrocellulose (NC)
membranes (EMD Millipore, Billerica, MA, USA) at 55 V for 4 h at
4°C. The NC membranes were blocked with 5% non-fat milk in
Tris-buffered saline (TBS) and incubated with primary anti-bodies
at 1:1,000 dilution in TBS overnight at 4°C. The primary antibodies
included mouse anti human EMMPRIN antibody (F-5; cat. no.
sc-374101; 1:500), rabbit anti human E-cadherin antibody (H-108;
cat. no. sc-7870; 1:500), mouse anti human fibronectin antibody
(A-11; cat. no. sc-271098; 1:500), rabbit anti human SNAI 1
antibody (H-130; cat. no. sc-28199; 1:500), mouse anti human GAPDH
antibody (G-9; cat. no. sc-365062; 1:500) and mouse anti human
HIF-1α antibody (28b; cat. no. sc-13515; 1:500), which were
purchased from Santa Cruz Biotechnology, Inc. Anti-αSMA (1A4;
1:2,000) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rabbit anti-human FSP-1 antibody (cat. no. 13018; 1:1,000) was
purchased from Cell Signalling Technology, Inc. (Danvers, MA, USA).
The membranes were then washed three times (10 min each) in TBS,
containing Tween 20 (TBST), and were subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies, including
goat-anti-mouse-horseradish-peroxidase (HRP)-conjugated IgG (cat.
no. sc-2005; 1:5,000) and goat anti-rabbit HRP-conjugated IgG (cat.
no. sc-2004; 1:5,000) from Santa Cruz Biotechnology, Inc., in TBST
for 1 h at room temperature. The membranes were washed as before.
The protein bands were visualized on X-ray film using an ECL
Western Blotting kit (Pierce Biotechnology, Appleton, WI, USA).
Protein band density was quantified using the gel-pro analyzer
(Media Cybernetics, Inc., Rockville, MD, USA).
Migration and invasion assay
For the migration assay, 1×105 EC109
cells were plated into the top chamber on the non-coated membranes
of 24-well plates (Corning Life Sciences, Lowell, MA, USA) and
allowed to migrate toward the hrEMMPRIN-containing medium in the
lower chamber. For the invasion assay, 1×105 cells were
plated into the top chamber of 24-well plates onto Matrigel coated
membranes (BD Biosciences, Franklin Lakes, NJ, USA) and cultured
for 24 h at 37°C. Each insert was coated with 60 µg Matrigel
prior to the invasion assay. The cells were added to medium with or
without hrEMMPRIN. The cells that failed to invade through the
pores were removed using a cotton swab. The invading cells were
fixed with 10% methanol and subsequently stained with 0.1% crystal
violet (Sigma-Aldrich, St. Louis, MO, USA). The stained cells were
counted under a microscope (BX51; Olympus Corporation, Toyko,
Japan) at magnification, ×40 in three random fields per well.
Immunofluorescence
The cells grown on sterile glass coverslips were
briefly washed with PBS, fixed with cold 100% methanol for 5 min,
and then air dried. The slides were first incubated with 10%
species-specific serum for blocking for 30 min, followed by
incubation with primary antibodies or nonspecific isotype
antibodies (E-cadherin mouse monoclonal antibody; cat. no.
sc-21791; Santa Cruz Biotechnology, Inc.) on ice overnight.
Following incubation with the primary anti-bodies, the slides were
washed twice with PBS, and were then incubated with FITC-labeled
goat-anti-mouse IgG (1:300; cat. no. A11001; Invitrogen Life
Technologies) in a dark chamber for 1 h at room temperature. The
cell nuclei were stained with DAPI (1:20,000 in PBS). The slides
were viewed under a microscope (BX51) and images were captured.
Statistical analysis
Each experiment was repeated in triplicate. The data
are presented as the mean ± standard deviation. The statistical
differences between the groups were analyzed by one or two-way
analysis of variance, followed by Bonferoni's multiple comparison
tests using PRISM statistical analysis software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
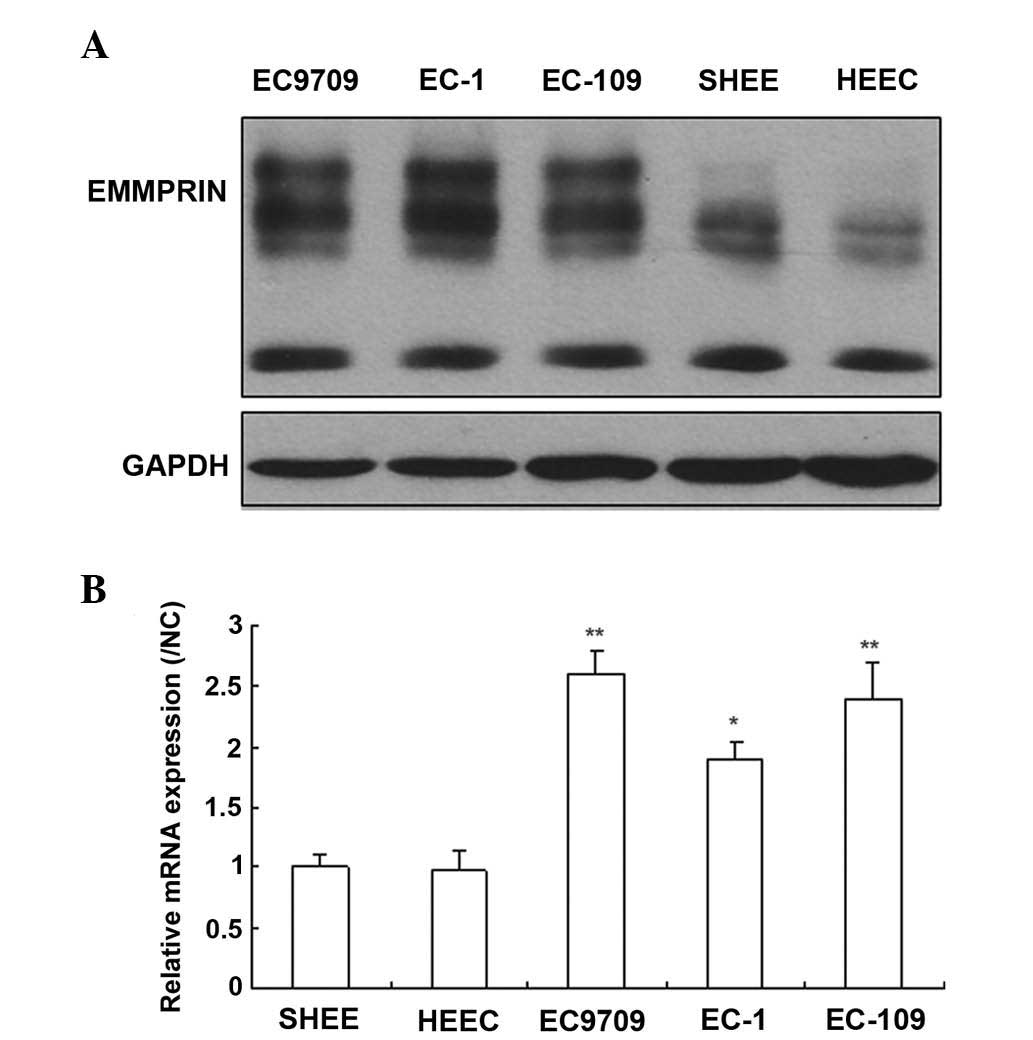
EMMPRIN is expressed in esophageal
carcinoma cells
To investigate the role of EMMPRIN in esophageal
carcinoma cells, the expression levels of EMMPRIN was quantified in
both normal and cancerous esophageal cells by western blotting and
RT-qPCR. The results demonstrated that the EMMPRIN protein was
expressed in the majority of esophageal cancer cells, however, only
marginally detectable in normal esophageal cells (Fig. 1A). The results of the RT-qPCR
revealed that the mRNA expression levels of EMMPRIN were similar to
the protein expression levels of EMMPRIN (Fig. 1B). These results suggested that
EMMPRIN may exert an important function in the progression of
esophageal carcinoma.
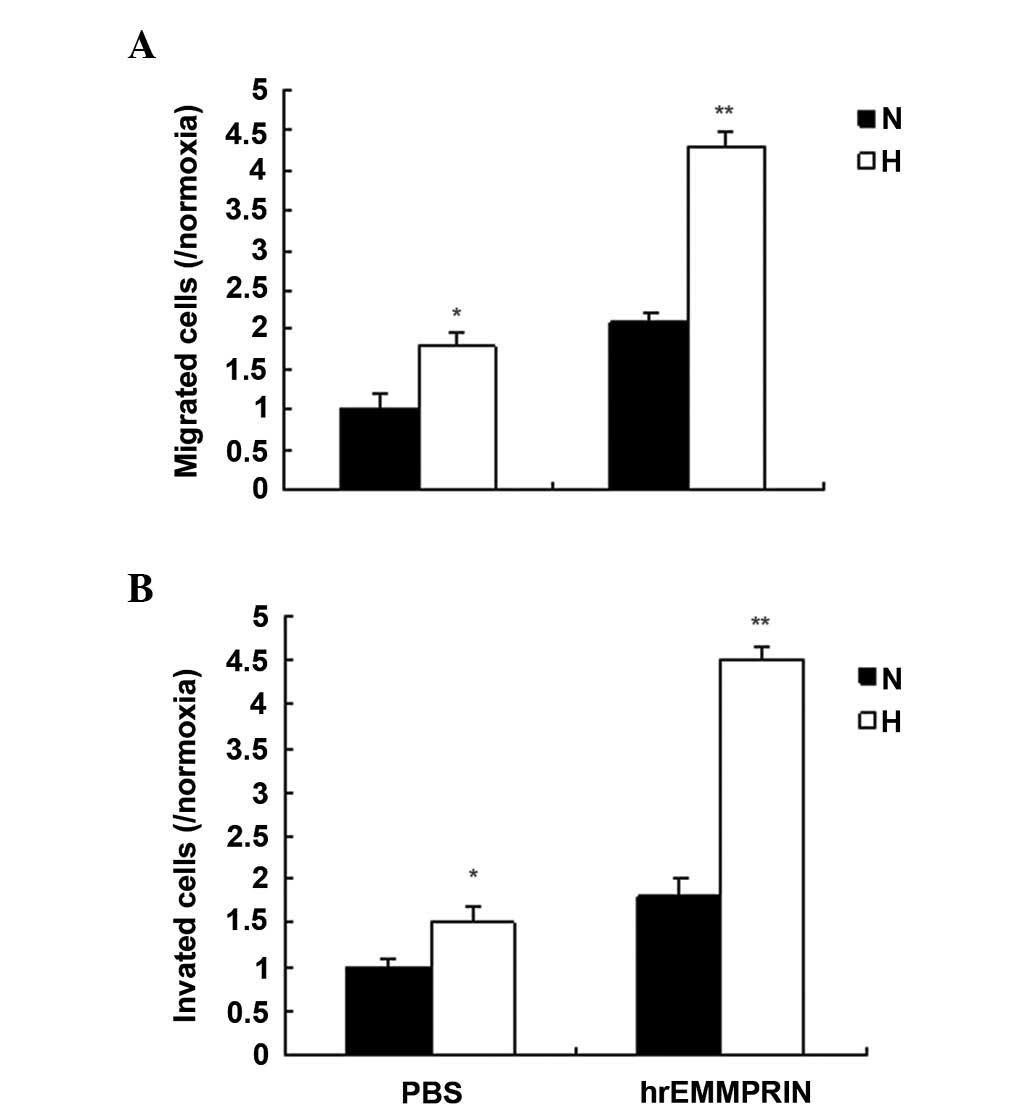
EMMPRIN promotes metastasis in a hypoxic
environment
EMMPRIN has important roles in cancer metastasis,
and a hypoxic environment is a common characteristic of solid
tumors. To investigate the role of EMMPRIN in esophageal cancer,
the migration and invasion ability of EC109 cells treated with
hrEMMPRIN was analyzed using a Transwell assay. The results
demonstrated that cell migration increased in the EC109 cells
treated with hrEMMPRIN, and more cell migration and invasion were
observed in hypoxic conditions, compared with the normoxic
conditions (Fig. 2A and B).
EMMPRIN induces the EMT in hypoxic
cells
The EMT is closely associated with metastasis. To
investigate whether EMMPRIN is associated with the EMT, EMT
markers, including E-cadherin, fibronectin, vimentin and α-smooth
muscle actin (SMA) were detected by immunofluoresence and western
blot analysis. The protein expression levels of the epithelial
marker, E-cadherin, were markedly decreased, whereas those of
fibronectin, vimentin, FSP1, Snail1 and α-SMA were markedly
increased in the EC109 cells (Fig.
3A and 3B). In addition, the
mRNA expression levels of E-cadherin were markedly decreased,
whereas those of fibronectin, α-SMA, snail family zinc finger 1,
and fibroblast secretory protein 1 were markedly increased in the
EC109 cells, as determined by RT-qPCR (Fig. 3C).
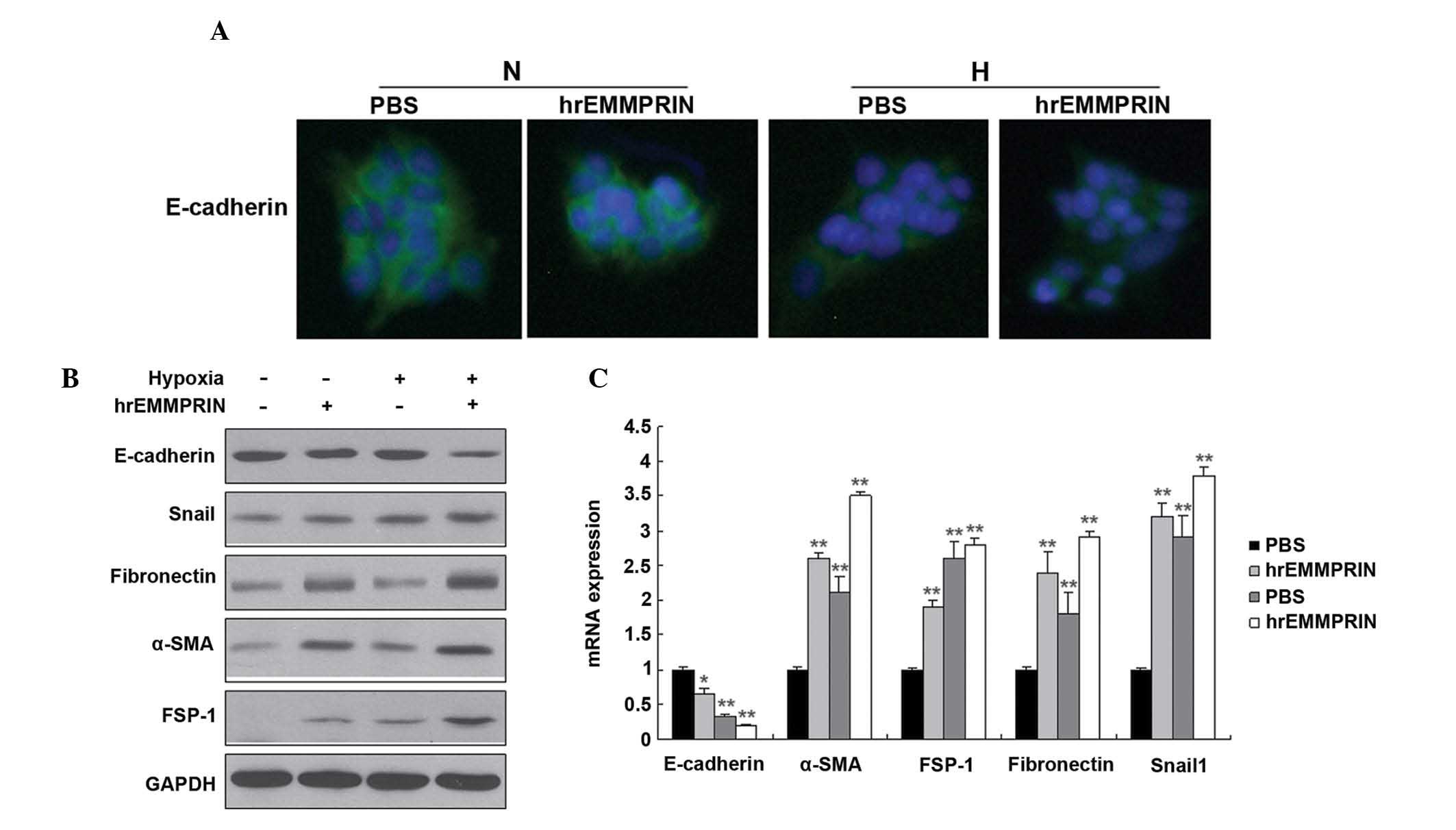 | Figure 3EMMPRIN induces the EMT when the cells
are under hypoxic, however, not normoxic conditions. (A) The
expression of E-cadherin was quantified by immunofluorescence. The
EC109 cells were seeded into 12-well plates, treated with hypoxia
(1% O2) and hrEMMPRIN (10 µg/ml), fixed and
incubated with E-cadherin and α-SMA antibodies. The cells were then
analyzed using immunofluorescence microscopy. Green staining
indicates the E-cadherin protein. (B) The expression levels of the
EMT markers in pancreatic cancer cells were analyzed. The EC109
cells were seeded into 6-well plates, and treated with hypoxia (1%
O2) and hrEMMPRIN (10 µg/ml). The total protein
was isolated from the cells and the protein expression levels of
E-cadherin, fibronectin, α-SMA, FSP-1 and Snail1 were quantified by
western blot analysis. The protein expression levels of Snail1,
fibronectin, α-SMA and FSP-1 were increased, where as thh
expression of E-cadherin was decreased. (C) The mRNA expression
levels of E-cadherin, fibronectin, vimentin, α-SMA, FSP-1 and
Snail1 were quantified by reverse transcription-quantitative
polymerase chain reaction and revealed similar results to the
protein expression levels. *P<0.05 and
**P<0.01, vs. control. hrEMMPRIN, extracellular
matrix metalloproteinase inducer; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; α-SMA, α smooth muscle actin; FSP-1, fibroblast
secretory protein 1; Snail1, snail family zinc finger 1; PBS,
phosphate-buffered saline. |
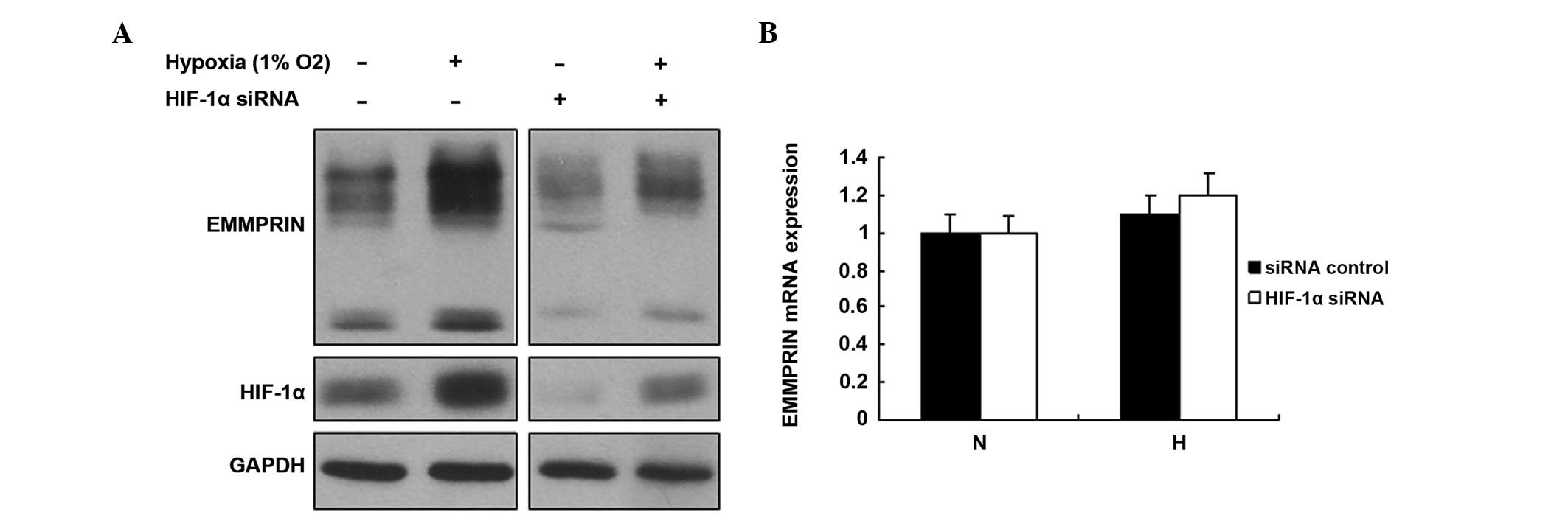
HIF-1α increases the expression of
EMMPRIN in esophageal cancer cells
HIF-1α is an important transcription factor, which
regulates numerous target genes involved in various biological
processes. Hypoxia increases esophageal cancer cell metastasis. In
order to determine whether EMMPRIN was regulated by HIF-1α, the
EC109 cells were treated with HIF-1α siRNA. The expression levels
of EMMPRIN increased following hypoxic treatment, and conversely
decreased following treatment with HIF-1α siRNA (Fig. 4A). No changes in the mRNA
expression levels of EMMPRIN were observed in the cells with
downregulated HIF-1α (Fig. 4B).
These data suggested that HIF-1α regulates the protein expression
of EMMPRIN in esophageal cancer cells in a hypoxic
microenvironment.
Discussion
Previous studies demonstrated that the expression of
EMMPRIN was higher in esophageal cancer tissue samples, as compared
with their normal counterparts (16,17)
and that the expression of EMMPRIN was associated with lymph node
metastasis, the severity of tumor invasion and differentiation
(18–20). The present study demonstrated that
the expression of EMMPRIN was higher in esophageal cancer cells
compared with normal esophageal epithelial cells. However, the role
of EMMPRIN in esophageal cancer remains to be elucidated. To the
best of our knowledge, the present study provides the first
evidence that EMMPRIN promotes the EMT of esophageal cancer in a
hypoxic environment, and that EMMPRIN is regulated by HIF-1α.
The biological functions of upregulated expression
of EMMPRIN were investigated under hypoxic conditions. Previous
studies demonstrated that EMMPRIN increased the metastasis ability
of cancer cells. In addition, previous reports demonstrated that
EMMPRIN accelerated tumor cell invasion by stimulating the
secretion of matrix metalloproteinase (7,8). The
present study also demonstrated that the number of invading cells
decreased following EMMPRIN suppression under hypoxic conditions.
These results suggested that EMMPRIN may have an important role in
hypoxia adaptation, promoting tumor cell survival and invasion. The
analysis of cellular metastasis levels demonstrated that EMMPRIN
increased esophageal cell migration and invasion under normoxic
conditions, and this increase was further apparent under hypoxic
conditions.
EMMPRIN promoted the EMT of cancer cells, including
breast and colon cancer cells (8).
However, in the present study, EMMPRIN revealed no induction of the
EMT in esophageal cells under normoxic conditions, as determined by
the absence of change in the expression levels of the EMT markers.
Notably, under hypoxic conditions, EMMPRIN markedly increased the
EMT in esophageal cells, and the expression levels of the
epithelial marker, E-cadherin, decreased, whereas those of
mesenchymal markers increased. These results indicated that
EMMPRIN, combined with hypoxic conditions, induced the EMT.
Bioinformatic analysis determined the existence of
eight putative hormone response elements (HREs) in the promoter
region of EMMPRIN (19). HIF-1
directly binds to a specific HRE located at position 130–133 on the
EMMPRIN promoter, and this may be involved in hypoxia-induced
transactivation of EMMPRIN (28,29).
The present study demonstrated that the expression of EMMPRIN was
markedly upregulated by HIF-1α, which is a transcription factor
with an important role in tumorigenic processes, including
metastasis and the EMT.
In conclusion, the present study demonstrated that
EMMPRIN promoted esophageal cancer migration and the EMT under
hypoxic conditions via the expression of HIF-1α. Further research
is required in order to investigate the molecular mechanism
underlying the regulation of the EMT in esophageal cancer cells,
and the association between EMMPRIN overexpression and hypoxic
conditions.
References
|
1
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–69. 2010.PubMed/NCBI
|
|
2
|
Hao JL, Cozzi PJ, Khatri A, Power CA and
Li Y: CD147/EMMPRIN and CD44 are potential therapeutic targets for
metastatic prostate cancer. Curr Cancer Drug Targets. 10:287–306.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanekura T and Chen X: CD147/basigin
promotes progression of malignant melanoma and other cancers. J
Dermatol Sci. 57:149–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toole BP and Slomiany MG: Hyaluronan, CD44
and Emmprin: Partners in cancer cell chemoresistance. Drug Resist
Updat. 11:110–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iacono KT, Brown AL, Greene MI and Saouaf
SJ: CD147 immunoglobulin superfamily receptor function and role in
pathology. Exp Mol Pathol. 83:283–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): Matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yurchenko V, Constant S and Bukrinsky M:
Dealing with the family: CD147 interactions with cyclophilins.
Immunology. 117:301–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
9
|
Muramatsu T and Miyauchi T: Basigin
(CD147): A multi-functional transmembrane protein involved in
reproduction, neural function, inflammation and tumor invasion.
Histol Histopathol. 18:981–987. 2003.PubMed/NCBI
|
|
10
|
Szubert S, Szpurek D, Moszynski R, Nowicki
M, Frankowski A, Sajdak S and Michalak S: Extracellular matrix
metalloproteinase inducer (EMMPRIN) expression correlates
positively with active angiogenesis and negatively with basic
fibroblast growth factor expression in epithelial ovarian cancer. J
Cancer Res Clin Oncol. 140:361–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Redzic JS, Kendrick AA, Bahmed K, Dahl KD,
Pearson CG, Robinson WA, Robinson SE, Graner MW and Eisenmesser EZ:
Extracellular vesicles secreted from cancer cell lines stimulate
secretion of MMP-9, IL-6, TGF-β1 and EMMPRIN. PLoS One.
8:e712252013. View Article : Google Scholar
|
|
12
|
Grass GD, Tolliver LB, Bratoeva M and
Toole BP: CD147, CD44, and the epidermal growth factor receptor
(EGFR) signaling pathway cooperate to regulate breast epithelial
cell invasiveness. J Biol Chem. 288:26089–26104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papadimitropoulou A and Mamalaki A: The
glycosylated IgII extracellular domain of EMMPRIN is implicated in
the induction of MMP-2. Mol Cell Biochem. 379:107–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao SH, Wang Y, Wen L, Zhai ZB, Ai ZH,
Yao NL, Wang L, Liu WC, Chen BL, Li Y, et al: Basigin-2 is the
predominant basigin isoform that promotes tumor cell migration and
invasion and correlates with poor prognosis in epithelial ovarian
cancer. J Transl Med. 11:92–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang MJ, Kim HP, Lee KS, Yoo YD, Kwon YT,
Kim KM, Kim TY and Yi EC: Proteomic analysis reveals that
CD147/EMMPRIN confers chemoresistance in cancer stem cell-like
cells. Proteomics. 13:1714–1725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M, Li Y and Zhang W: EMMPRIN/CD147 expression is
associated with disease-free survival of patients with colorectal
cancer. Med Oncol. 30:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai L, Guinea MC, Slomiany MG, Bratoeva M,
Grass GD, Tolliver LB, Maria BL and Toole BP: CD147-dependent
heterogeneity in malignant and chemoresistant properties of cancer
cells. Am J Pathol. 182:577–585. 2013. View Article : Google Scholar :
|
|
18
|
Hibino T, Sakaguchi M, Miyamoto S,
Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R and
Huh NH: S100A9 is a novel ligand of EMMPRIN that promotes melanoma
metastasis. Cancer Res. 73:172–183. 2013. View Article : Google Scholar
|
|
19
|
Ke X, Fei F, Chen Y, Xu L, Zhang Z, Huang
Q, Zhang H, Yang H, Chen Z and Xing J: Hypoxia upregulates CD147
through a combined effect of HIF-1α and Sp1 to promote glycolysis
and tumor progression in epithelial solid tumors. Carcinogenesis.
33:1598–1607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou
T and Rosenthal EL: CD147 and AGR2 expression promote cellular
proliferation and metastasis of head and neck squamous cell
carcinoma. Exp Cell Res. 318:1788–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura K, Kodama J, Hongo A and
Hiramatsu Y: Role of emmprin in endometrial cancer. BMC Cancer.
12:191–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grass GD, Bratoeva M and Toole BP:
Regulation of invadopodia formation and activity by CD147. J Cell
Sci. 125:777–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui HY, Guo T, Wang SJ, Zhao P, Dong ZS,
Zhang Y, Jiang JL, Chen ZN and Yu XL: Dimerization is essential for
HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem
Biophys Res Commun. 419:517–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han M, Trotta P, Coleman C and Linask KK:
MCT-4, A511/Basigin and EF5 expression patterns during early chick
cardiomyogenesis indicate cardiac cell differentiation occurs in a
hypoxic environment. Dev Dyn. 235:124–131. 2006. View Article : Google Scholar
|
|
25
|
Boulos S, Meloni BP, Arthur PG, Majda B,
Bojarski C and Knuckey NW: Evidence that intracellular cyclophilin
A and cyclophilin A/CD147 receptor-mediated ERK1/2 signalling can
protect neurons against in vitro oxidative and ischemic injury.
Neurobiol Dis. 25:54–64. 2007. View Article : Google Scholar
|
|
26
|
Philip B, Ito K, Moreno-Sánchez R and
Ralph SJ: HIF expression and the role of hypoxic microenvironments
within primary tumours as protective sites driving cancer stem cell
renewal and metastatic progression. Carcinogenesis. 34:1699–1707.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai YP and Wu KJ: Hypoxia-regulated
target genes implicated in tumor metastasis. J Biomed Sci.
19:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Le Floch R, Chiche J, Marchiq I, Naiken T,
Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP and Pouysségur J:
CD147 subunit of lactate/H+ symporters MCT1 and
hypoxia-inducible MCT4 is critical for energetics and growth of
glycolytic tumors. Proc Natl Acad Sci USA. 108:16663–16668. 2011.
View Article : Google Scholar
|
|
29
|
Xia X, Lemieux ME, Li W, Carroll JS, Brown
M, Liu XS and Kung AL: Integrative analysis of HIF binding and
transactivation reveals its role in maintaining histone methylation
homeostasis. Proc Natl Acad Sci USA. 106:4260–4265. 2009.
View Article : Google Scholar : PubMed/NCBI
|