Introduction
Multiple sclerosis (MS) is an inflammatory
autoimmune disease characterized by sporadic or multifocal
demyelination in the white matter of the central nervous system
(CNS), resulting in possible injury of the axons to differing
degrees. Experimental allergic encephalomyelitis (EAE) is an
inflammatory allergic disease affecting the CNS in a variety of
sensitive experimental animals, and this is often used as an animal
model of MS (1,2). Myelin oligodendrocyte glycoprotein
(MOG) has been widely used in previous studies as the inducing
antigen/immunogen for the EAE model. MOG is a transmembrane
glycopeptide of 26–28 kDa, which is expressed in the myelin sheath
membrane and on the outer surface of oligodendroglia cells
(3,4). Three identifiable epitopes exist in
the extracellular region, and MOG35-55 peptide is one of the
epitopes which causes encephalitis. MOG has been demonstrated to be
the sole autoantigen, not only to induce the T cell response, but
also to elicit the production of myelin sheath antibodies among the
constitutive proteins of the myelin sheath (5), results which revealed the advantage
of using MOG as the inducer for the establishment of the EAE model
and its pathological characteristics (6). The EAE model of C57BL/6 mice induced
by MOG approximates closely to human MS, with respect to the
pathological and clinical manifestations (7–9), and
therefore it is an ideal model to investigate the pathogenesis and
therapeutic strategies of MS (10,11).
The pathogenesis of MS remains to be fully
elucidated, however, the best-supported hypothesis is that MS is a
type of autoimmune disease of the myelin sheath in the CNS, in
which autoreactive CD4+Th1 lymphocytes, and the
cytokines they release, are the most important factors associated
with humoral immunity. The impairment in function of the CNS is
characterized by deletion of the myelin sheath, which is
predominantly mediated by interferon (IFN)-γ from activated T
cells. This induces differentiation of the Th1 cells and the immune
response targeted to the myelin sheath, leading to the development
of the disease. An imbalance in the Th1/Th2 cell response, and
either an inhibition of differentiation of the regulatory T cells
(Treg cells) or an impairment of their function, are also likely to
contribute towards the disease, even though autoreactive
CD4+Th1 lymphocytes and their released cytokines are
thought to be primarily responsible as the causative agents
(12–14). However, a number of biological
phenomena, which are encountered during studies of MS/EAE, may not
be explained by the predominant differentiation of the Th1 cells
and the inhibition of differentiation or functional impairment of
Treg cells under pathological conditions.
CD4+Th1 and CD8+T cells are
unable to directly attack the target cells, and they contribute to
inflammation and tissue injury predominantly by secreting
cytokines, which promote the activation and proliferation of
cytotoxic T lymphocytes (CTL), natural killer (NK) cells and
phagocytes, and mediate the immune response of the effector cells.
Th2 cells are also unable to cause direct injury of the target
cells, and these mediate the humoral immune response predominantly
by stimulating B lymphocytes to produce antibodies. Activated
CD8+T lymphocytes differentiate into CD8+
effector T cells and CTL. It is possible that oligodendroglia cells
are attacked by activated CTL and macrophages, since the expression
levels of the major histocompatibility complex (MHC) class I
molecules are upregulated in oligodendroglia cells during
inflammation. The immunocytes, which elicit direct myelin sheath
injury of the CNS, are possibly those among the CTL or macrophage
systems. The present study focused on an examination of the immune
response changes of CTL and mononuclear macrophages using an EAE
model, and aimed to ascertain which effector cells led to direct
myelin sheath injury of the CNS by comparing the roles of CTL and
mononuclear macrophages during the progression of EAE.
Materials and methods
Immunogens
Murine recombinant MOG35-55 peptide was artificially
synthesized (Sigma-Alrich, St. Louis, MO, USA; purity, >98%).
The amino acid sequence of the peptide was MEVGWYRSPFSRVVHLYRNGK,
with a molecular mass of 2,582.0 Daltons.
Experimental animals
A total of 80 healthy female wild-type C57BL/6 mice,
aged 8–10 weeks and weighing 18–22 g, were used in the present
study. The mice were provided by the Experimental Animal Center
(Wuhan University, Wuhan, China) and fed on nutritional foodstuff
in individual cages. The feeding environment was at room
temperature (18–25°C), with a relative humidity of 50–60%, and 12 h
day/night cycle lighting. The mice were numbered and grouped
following adaptive feeding for 1–2 weeks. The study was approved by
the Ethics Committee of Huazhong University of Science and
Technology (Wuhan, China).
Animal grouping and establishment of the
EAE model
The 80 C57BL/6 mice were divided randomly into a
blank control group and the EAE group, with 40 mice in each group.
The control group animals were injected with 2 mg Complete™
Freund's adjuvant (Sigma-Aldrich, St. Louis, MO, USA)
subcutaneously at two points of the inguinal groove for each mouse
and 200 ng pertussis toxin (Sigma-Aldrich) was subsequently
injected into the abdominal cavity on the day of immunization and
48 h afterwards. Administration of the OG35-55 peptide and
immunological adjuvant were used to establish the chronic EAE
animal model in the EAE group: 0.2 ml mixed emulsion was injected
subcutaneously at two points of the inguinal groove for each mouse,
which contained 250 µg artificially synthesized MOG35-55
peptide and 2 mg Complete™ Freund's adjuvant (volume ratio, 1:1).
The mice were subsequently injected with 200 ng pertussis toxin
into the abdominal cavity on the day of immunization and 48 h
afterwards. The behavior and activities of the animals were
assessed daily prior to the completion of the experiment, on day
30, with the day of immunization being set as day 0. The severity
of clinical nervous symptoms of the EAE group mice were assessed
according to the Benson scoring standard (15). A total of eight mice were
sacrificed in the control and EAE groups on days 0, 7, 14, 21 and
30, and brain and spleen tissues were extracted for further
examination. The mice were anesthetized with 3 ml/kg chloral
hydrate (Sigma-Aldrich), following which the thoracic cavity was
opened in order to expose the heart. A needle was inserted from the
apex cordis to the aorta, and the right auricle was sectioned. A
total of 0.9% normal saline (NS; 300 ml) was perfused until the
perfusate was transparent, followed by perfusion of 50 ml 4%
paraformaldehyde (Servicebio Technology Co., Ltd, Wuhan,
China).
The scoring standard was applied as follows: 0
points, no symptoms; 1 point, tail inertia or postscript crouch
gait accompanied by powerful tail; 2 points, jump crouch gait
accompanied by tail inertia (ataxia); 2.5 points, ataxia
accompanied by partial paralysis of a single limb; 3 points,
complete paralysis of a single limb; 3.5 points, complete paralysis
of a single limb accompanied by partial paralysis of another limb;
4 points, complete paralysis of a couple of limbs; 4.5 points,
paralysis of four limbs; 5 points, death.
Histological examination
The abdominal skin of the experimental mice was cut
from the upper part along a curved line to each side following
anesthetization by injection of 10% chloral hydrate (3 ml/kg) into
the abdominal cavity. The peritoneum and costal bone on each side
was carefully incised to completely expose the thoracic cavity. A
perfusion needle was fixed, and inserted from the cardiac apex up
to the aorta. The right auricular appendix was cut and perfused
with ~300 ml 0.9% pre-cooled NS rapidly until clear fluid flowed
out. This was followed by initially rapid and then slow perfusion
of 500 ml 4% paraformaldehyde solution. An incision was made to the
scalp of the mice and the cranial bone was removed. The spleen was
removed from the abdominal cavity following careful extraction of
the whole brain tissue, and this was rinsed briefly with NS prior
to placement in 4% paraformaldehyde fixation solution. The tissue
was paraffin-embedded and sliced, followed by hematoxylin-eosin
(H&E; Sigma-Aldrich) staining, Luxol Fast Blue (LFB;
Sigma-Aldrich) staining and fluorescence immunohistochemistry
(IHC). Antibodies, together with their dilutions, were as follows:
Mouse anti-mouse IFN-γ monoclonal immunoglobulin (Ig)G (cat. no.
ab22543), 1:100; goat anti-mouse Iba-1 polyclonal IgG (cat. no.
ab107159), 1:100; rabbit anti-mouse CD8 polyclonal IgG (cat. no.
ab191905), 1:100; rabbit anti-mouse ki67 polyclonal IgG (cat. no.
ab15580), 1:100 (Abcam, Cambridge, UK).
Western blotting
The rat brains were harvested and homogenized at 4°C
using a Teflon glass homogenizer in 50 mM Tris-HCl (pH 7.4), 150 mM
NaCl, 10 mM NaF, 1 mM Na3VO4, 10 mM
β-mercaptoethanol, 5 mM EDTA, 2 mM benzamidine, 1.0 mM
phenylmethanesulfonyl fluoride, 5 mg/ml leupeptin, 5 mg/ml
aprotinin and 2 mg/ml pepstatin (Sigma-Aldrich). Three volumes of
the homogenized samples were subsequently added to one volume of
the extracting buffer (200 mM Tris-HCl, pH 7.6, 8% SDS, 40%
glycerol; Sigma-Aldrich). Protein concentrations were determined
using a bicinchoninic acid kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and between 9 and 11 mg/ml was used. A final
concentration of 10% mercaptoethanol and 0.05% bromophenol blue
(Sigma-Aldrich) were then added, and the samples were boiled in a
water bath for 10 min. The boiled samples were separated by 10%
SDS-PAGE (Sigma-Aldrich) and the separated proteins were
transferred onto nitrocellulose membranes (GE Healthcare Life
Sciences, Little Chalfont, UK). The membranes were subsequently
blocked with 5% nonfat milk dissolved in Tris-buffered saline
(TBS)-Tween-20 (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.2% Tween-20;
Sigma-Aldrich) for 0.5 h (16),
followed by an incubation with primary antibodies (1:200) at 25°C
for 0.5 h. The primary antibodies were as follows: Mouse anti-mouse
monoclonal IgG GAPDH (cat. no. ab9482), rabbit anti-mouse
polyclonal IgG MHC-I (cat. no. ab93364), rat anti-mouse monoclonal
IgG MHC-II (cat. no. ab139365) and rat anti-mouse monoclonal IgG
L-12 (cat. no. ab80682) (Abcam). The membranes were washed with
TBS-Tween-20, and then incubated with horseradish
peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies
(1:15,000) for 1 h at room temperature. After a final wash with
TBS-Tween-20, the grey scale of the blots was analyzed using an
Odyssey Infrared Imaging system (cat. no. 9120; LI-COR Biosciences,
Lincoln, NE, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR, mice tissue during all the experimental
stages was selected and the total RNA was extracted using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), according to the manufacturer's instructions. The cDNA (5
µg) was reverse-transcribed and synthesized using a Prime
Script First Strand cDNA Synthesis kit (cat. no. D6110A; Takara
Bio, Inc., Beijing, China) under the conditions of 37°C for 15 min
and 85°C for 5 sec. The gene products were subsequently amplified
by RT-qPCR (CFX Connect™ Optics Module; Bio-Rad Laboratories,
Inc.), according to the following method. The reaction system
comprised 5 µl SYBR Green mix (Sigma-Aldrich), 0.3 µl
the upstream and downstream primers, 1 µl cDNA and 3.4
µl RNase-free water. A total of 40 cycles were used in the
program (95°C for 3 min, 95°C for 10 sec, 60°C for 30 sec), and the
dissolving curve was examined at 65–90°C. The sequences of the
primers used were as follows: β-actin, upstream:
5′-CTGAGAGGGAAATCGTGCGT-3′ and downstream:
5′-CCACAGGATTCCATACCCAAGA-3′; Fas, upstream:
5′-CACCCTGACCCAGAATACCAAG-3′; and downstream:
5′-AGGCGATTTCTGGGACTTTGT-3′; perforin, upstream: 5′-
CACGCATGATCTGCTCTTCG-3′ and downstream:
5′-CGCTTCGGGTTCTGTTCTTC-3′.
Statistical analysis
SPSS 18.0 software (IBM, SPSS, Chicago, IL, USA) was
used for statistical analysis of the experimental data. The data
are expressed as the mean ± standard error of the mean. Student's
t-test was used to compare the differences between pairs of groups,
and one-way analysis of variance was used to compare several
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
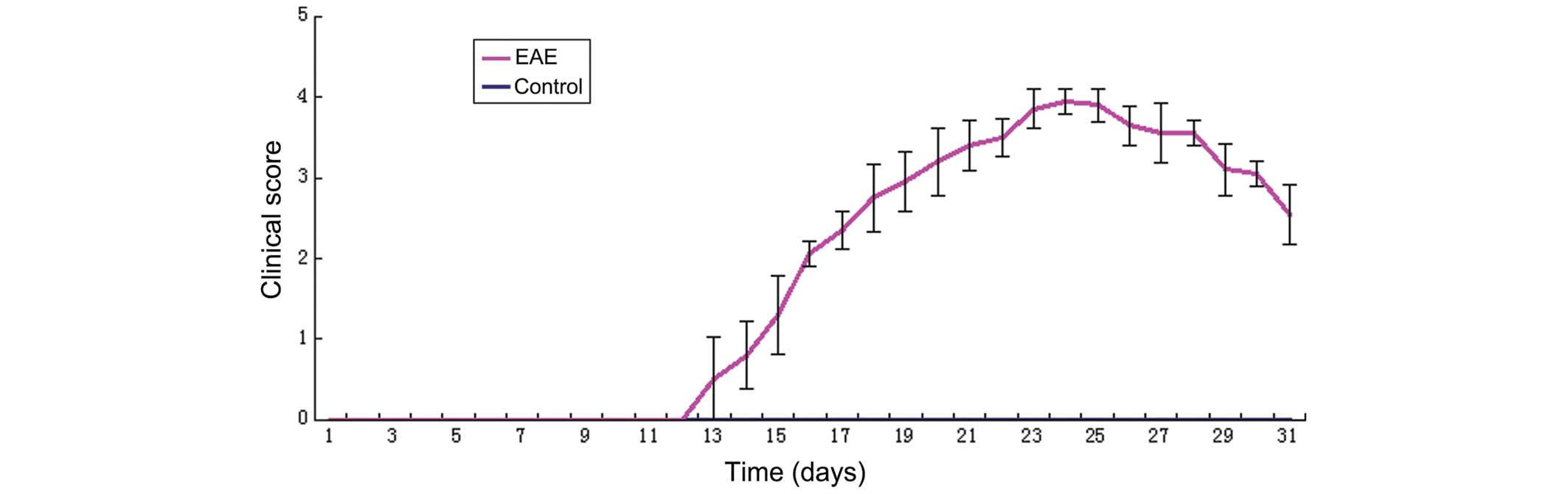
Establishment of the animal model
The EAE model of C57BL/6J mice was successfully
induced by injection of the MOG35-55 peptide and immunological
adjuvant. As shown in Fig. 1, the
experiment was terminated on day 30, and all mice in the control
group were free of the disease. The disease affected all mice in
the EAE model group, with the exception of those which were
sacrificed during the early stage. The mice in the EAE group were
free of the disease up to 11 days following immunization, at which
time certain mice were affected by the disease in rapid succession,
between days 12 and 14, with clinical scores of 1–2. The morbidity
reached its peak during days 20–24, with scores of 2.5–4.5. The
clinical symptoms of certain mice were alleviated following
survival for 3–4 days, and their scores declined. However, the
symptoms persisted and the disease was able to enter the chronic
stage.
Histological changes in the brain of the
experimental mice
The pathological condition of the mice was monitored
throughout the 30 day course of the experiment. The brain tissue in
each group appeared normal to the naked eye, however, a noticeable
level of congestion and edema were observed in the spleen of the
diseased mice in the EAE group. HE staining and LFB staining of the
mice brain in the control group revealed no abnormal change. In the
EAE group, a modest level of monocyte and lymphocyte infiltration
in the brain was observed, as the disease progressed. LFB staining
revealed a loosening of the white matter, discontinuity, breaking
and vacuole changes in the myelin sheath, and a white and flaky
deletion of the myelin sheath in certain regions, conforming to the
features of the chronic EAE mode (i.e. prominent myelin sheath
destruction and little apparent inflammatory cell
infiltration).
Fig. 2 shows the
results of the fluorescence IHC experiment of IFN-γ in the spleen
and brain of the experimental mice. A marginal quantity of green
IFN-γ fluorescence was observed by fluorescence IHC of IFN-γ in the
spleen of the control group, as revealed in Figs. 2Figure 3–4. In the EAE group, green fluorescence
was observed in the spleen on day 21 due to an abundant production
of IFN-γ by lymphocytes, indicating that the T lymphocytes were
activated. The noticeable fluorescence coloration remained visible
until day 30. However, no positive identification of IFN-γ was
measured by fluorescence IHC in the brain tissue, with the
exception of a very slight quantity of green IFN-γ fluorescence
around the ventricle on day 21 (data not shown).
Double fluorescence IHC of Iba1 and ki-67 in the
brain of the experimental mice was subsequently performed (Fig. 3). No indication of proliferation of
the Iba1-positive cells in the control group was evident, and a
slight double fluorescence was visible on day 7 after immunization,
which remained apparent up to day 14. On conclusion of the
experiment at day 30, the proliferation reaction of Iba 1-positive
cells remained visible.
Subsequently, a double fluorescence IPC experiment
was performed with the anti-CD8 and anti-ki-67 antibodies in the
spleen of the experimental mice. As revealed in Fig. 4, a slight and sporadic
proliferation reaction of the CD8+ T lymphocytes was
visible on days 14 and 21 after immunization, whereas no
proliferation was observed during other stages, indicating that the
majority of the activated and proliferating lymphocytes were not
CD8+ T lymphocytes.
EAE altered the expression of the
associated proteins
As revealed in Fig.
5, the expression level of MHC I molecules in the brain tissue
of mice in the EAE group increased significantly on day 14, and the
higher levels of MHC I persisted until day 30 (P<0.05, compared
with the control group). The results indicated that the expression
of MHC I molecules increased with the onset of the disease, and the
increased levels were maintained at the higher level without a
decline. The expression levels of the MHC II molecules in mice in
the EAE group increased significantly by day 7 following the onset
of the disease, reached a peak on day 14, exhibited a decline on
day 21 and subsequently rose again on day 30, (P<0.05, compared
with the control group). The secretion of IL-12 in the brain
started to increase on day 7 and achieved its maximum level by day
21, maintaining a higher level up until day 30. The increases
compared with the control group were significant (P<0.05);
however, the level of IL-12 during the chronic stage of the disease
was lower compared with that during the peak stage (P<0.05).
EAE did no affect the mRNA expression
levels of Fas and perforin
Changes in the mRNA expression levels of Fas and
perforin were examined in the brain of the mice in the EAE and
control groups during the different stages of the experiment. The
differences identified were not significant (P>0.05; Fig. 6).
Discussion
It would appear that almost all the known
immunocytes are involved in processes associated with inflammation
and demyelination in the CNS, although the exact mechanisms of
various immunocytes and their secreted cytokines in MS development
remain to be fully elucidated (17–20).
Antigens themselves are not the direct causative agents of disease
during the immune response: Immunocytes, and molecules that are
activated by the antigen directly or indirectly, are the direct
cause. It is generally accepted that injury of the CNS, which is
characterized predominantly by demyelination, is mediated by IFN-γ,
a strong proinflammatory cytokine, which elicits the immune
response of myelin sheath by inducing differentiation of the Th1
cells. However, a study investigating IFN-γ and its receptor with
gene-knockout animals revealed that EAE still occurred, and that
the gene-knockout animals were more adversely affected by the
disease (21). This demonstrates
that MS/EAE may be caused by several types of pathological
mechanisms working in concert, and innate immunity, the specific
immune response and other regulatory mechanisms of the immune
response may be involved.
Cellular functions become diversified following
lymphocyte activation during the effector stage. T0
lymphocytes differentiate into CD4+ and CD8+
T lymphocytes and Treg cells. Each type of T lymphocyte then
further differentiates into the terminal immune effector cells. CTL
can attack the host cells directly, however, Th cells, Treg cells
and CD8+ T effector lymphocytes (together with B
lymphocytes, another type of humoral immunocyte) only regulate the
direction and extent of the immune response by secreting cytokines
or antibodies, which act further on macrophages and CTL, without
being able to attack the host cells directly. At present,
macrophages and CTL are known to cause direct injury of
oligodendroglia cells and the myelin sheath.
The macrophages, which are predominately featured in
the MS/EAE immune response are mononuclear macrophages and
neutrophils. In the present study, no infiltration and aggregation
of neutrophils was identified in the CNS, indicating that the major
pathogenic macrophages may be oligodendroglia cells in the CNS
and/or peripheral mononuclear macrophages. MHC molecules are not
constitutively expressed by oligodendroglia cells, although MHC I
molecules can be upregulated under pathological conditions,
including inflammation, whereas the expression of MHC II molecules
by oligodendroglia cells does not occur under any conditions. This
further demonstrated that CD4+ Th lymphocytes and Treg
cells do not directly attack oligodendroglia cells.
CTL are restricted by MHC I molecules when killing
the target cells, and sensitized CTL cells are able to continuously
act on other target cells, which carry an identical antigen
following killing of the target cells, demonstrating that the
effects of CTL are self-propagating. A number of previous studies
revealed that the role of CTL in the occurrence and development of
MS/EAE is not negligible, however, it is controversial (22–25).
Additionally, it was revealed that CD8+ Treg cells
express specificity for their target organs, which may lead to the
direct killing of the activated immune response cells, or produce
cytokines, including TGF-β and IL-10, under the action of IFN-γ,
fulfilling the role of immune suppression (26). CTL elicit their effects
predominantly via the perforin and Fas/Fas L route. In the present
study, changes identified for Fas, perforin and IFN-γ during the
occurrence and development of chronic EAE were not significant, and
since they are associated with CTL function in the brain, no
appreciable changes were observed in the levels of these effector
molecules of CTL, which are capable of killing the target cells
directly. Nevertheless, the expression of the MHC I and MHC II
molecules, which are associated with the antigen-presenting
function of mononuclear macrophages, were upregulated, indicating
that there is also an increase in the expression levels of MHC I
molecules in the CNS, despite the evidence that T lymphocyte
activation is marked by IFN-γ production in spleen. These results
fail to demonstrate that CTL enhance the immune effect of MHC I
positive cells in the CNS via the perforin and Fas/Fas L route.
Mononuclear macrophages are a type of antigen
presenting cell (APC), which may also cause injury of the myelin
sheath in CNS by phagocytosis, dissolution, and even possibly
direct attack, in addition to the induction of the immune response
by presenting antigens. IL-12 is a type of cytokine specifically
produced by APC cells, including mononuclear macrophages and
dendritic cells. Activated microglia cells are one of the
predominant sources of IL-12 in the CNS. IL-12 may activate NK
cells in innate immunity, however, its major function, identical to
IFN-γ, is to promote the differentiation of Th0 cells into Th1
cells. In the present study, the expression levels of IL-12 changed
markedly in animals in the EAE group throughout the course of the
experiment, whereas the immunofluorescence studies revealed an
absence of any appreciable increase in IFN-γ secretion in the
brain, indicating that the degree of T lymphocyte activation was
not high. Given that the effects of IL-12 secreted by mononuclear
macrophages on the CNS are mediated earlier than those of IFN-γ,
and the effect of IL-12 in promoting Th cell differentiation and
regulating anti-body production of B lymphocytes is more marked
compared with IFN-γ, mononuclear macrophages exert a more important
role compared with CTL in the progression of EAE.
In conclusion, the effector cells causing direct
injury to the myelin sheath in the CNS may be mononuclear
macrophages other than CTL, including microglia cells, although it
was not determined whether the Iba 1-positive cells are mononuclear
macrophages migrating from peripheral tissues, or microglia cells
residing in the brain. A noticeable activation and proliferation of
mononuclear macrophages containing microglia cells during the
course of EAE, and the induced immune response fulfils a more
important role compared with CTL during the pathological process of
myelin sheath injury. Therefore, mononuclear macrophages are one of
the most important effector cells causing direct injury of the
myelin sheath in the CNS.
Acknowledgments
This study was supported by the National Program on
Key Basic Research Project of China (973 Program; no.
2012CB722401), and the National Natural Science Foundation of China
(nos. 81030051 and 21177046).
References
|
1
|
Bradl M and Linington C: Animal model of
demyelination. Brain Pathol. 6:303–311. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polman CH, Dijkstra CD, Sminia T and
Koetsier JC: Immunohistological analysis of macrophages in the
central nervous system of Lewis rats with experimental autoimmune
encephalomyelitis. J Neuroimmunol. 11:215–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendel 1, Keriero de Rosbo N and Ben-Nun
A: A myelin oligodendrocyte glycoprotein peptide induces typical
chronic experimental autoimmune encephalomyelitis in H-2b mice:
Fine specificity and T cell receptor V beta expression of
encephalitogenic T cells. Eur J Immunol. 25:1951–1959. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pál E, Yamamura T and Tabira T: Autonomic
regulation of experimental autoimmune encephalomyelitis in IL-4
knockout mice. J Neuroimmunol. 100:149–155. 1999. View Article : Google Scholar
|
|
5
|
Baumann N and Pham-Dinh D: Biology of
oligodendrocyte and myelin in the mammalian central nervous system.
Physiol Rev. 81:871–927. 2001.PubMed/NCBI
|
|
6
|
Wang H, Munger KL, Reindl M, O'Reilly EJ,
Levin LI, Berger T and Ascherio A: Myelin oligodendrocyte
glycoprotein antibodies and multiple sclerosis in healthy young
adults. Neurology. 71:1142–1146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roscoe WA, Welsh ME, Carter DE and Karlik
SJ: VEGF and angiogenesis in acute and chronic MOG((35-55)) peptide
induced EAE. J Neuroimmunol. 209:6–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berard JL, Wolak K, Fournier S and David
S: Characterization of relapsing-remitting and chronic forms of
experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia.
58:434–445. 2010.
|
|
9
|
Slavin A, Ewing C, Liu J, Ichikawa M,
Slavin J and Bernard CC: Induction of a multiple sclerosis-like
disease in mice with an immunodominant epitope of myelin
oligodendrocyte glycoprotein. Autoimmunity. 28:109–120. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanamori M, Kawaguchi T, Nigro JM,
Feuerstein BG, Berger MS, Miele L and Pieper RO: Contribution of
Notch signaling activation to human glioblastoma multiforme. J
Neurosurg. 106:417–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao P and Segal BM: Experimental
autoimmune encephalomyelitis. Methods Mol Med. 102:363–375.
2004.PubMed/NCBI
|
|
12
|
Steinman L: Antigen-specific therapy of
multiple sclerosis: The long-sought magic bullet.
Neurotherapeutics. 4:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oukka M: Interplay between pathogenic Th17
and regulatory T cells. Ann Rheum Dis. 66(Suppl 3): iii87–iii90.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edwards LJ, Robins RA and Constantinescu
CS: Th17/Th1 phenotype in demyelinating disease. Cytokine.
50:19–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benson JM, Stuckman SS, Cox KL, Wardrop
RM, Gienapp IE, Cross AH, Trotter JL and Whitacre CC: Oral
administration of myelin basic protein is superior to myelin in
suppressing established relapsing experimental autoimmune
encephalomyelitis. J Immunol. 162:6247541999.
|
|
16
|
Qu N, Zhou XY, Han L, Wang L, Xu JX, Zhang
T, Chu J, Chen Q, Wang JZ, Zhang Q and Tian Q: Combination of PPT
with LiCl treatment prevented bilateral ovariectomy-induced
hippocampal-dependent cognition deficit in rats. Mol Neurobiol. Dec
23;Epub ahead of print. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lehuen A, Diana J, Zaccone P and Cooke A:
Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol.
10:501–513. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lisak RP: Neurodegeneration in multiple
sclerosis: Defining the problem. Neurology. 68:S5–S12; discussion
S43-S54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goverman J: Autoimmune T cell responses in
the central nervous system. Nat Rev Immunol. 9:393–407. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller AH, Maletic V and Raison CL:
Inflammation and its discontents: The role of cytokines in the
pathophysiology of major depression. Biol Psychiatry. 65:732–741.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy AC, Lalor SJ, Lynch MA and Mills
KH: Infiltration of Th1 and Th17 cells and activation of microglia
in the CNS during the course of experimental autoimmune
encephalomyelitis. Brain Behav Immun. 24:641–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friese MA and Fugger L: Pathogenic CD8(+)
T cells in multiple sclerosis. Ann Neurol. 66:132–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fletcher JM, Lalor SJ, Sweeney CM, Tubridy
N and Mills KH: T cells in multiple sclerosis and experimental
autoimmune encephalomyelitis. Clin Exp Immunol. 162:1–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saxena A, Martin-Blondel G, Mars LT and
Liblau RS: Role of CD8 T cell subsets in the pathogenesis of
multiple sclerosis. FEBS Lett. 585:3758–3763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobottka B, Harrer MD, Ziegler U, Fischer
K, Wiendl H, Hünig T, Becher B and Goebels N: Collateral bystander
damage by myelin-directed CD8+ T cells causes axonal loss. Am J
Pathol. 175:1160–1166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niederkorn JY: Emerging concepts in CD8(+)
T regulatory cells. Curr Opin Immunol. 20:327–331. 2008. View Article : Google Scholar : PubMed/NCBI
|