Introduction
Osteosarcoma (OS), with an incidence of 4.4 per
million worldwide, is the most common type of primary malignant
bone tumor in children and adolescents, and accounts for 60% of all
malignant childhood bone tumors (1). A first major peak of morbidity occurs
in patients between 10 and 20 years of age, and the second, smaller
peak is observed in patients >50 years of age (1). OS usually occurs following rapid bone
growth, such as that observed in the proximal tibia, distal femur
and proximal humerus, and is characterized by the direct formation
of immature bone and osteoid tissue. The majority of OS tumors are
of high grade and result in pulmonary metastases. The five-year
overall survival rate is currently ~70% (2,3).
However, though significant advances have been made in OS treatment
strategies, patients exhibiting metastases or recurrent OS tumors
still have a poor prognosis, and account for 30–35% of all patients
with OS (2,4). The survival rates are even lower in
young patients with OS (18–30 years), due to the increased rates of
metastasis (5). Therefore, it is
important that novel OS targets and therapeutic approaches are
identified.
MicroRNAs (miRNA or miR) are a class of endogenously
expressed, non-coding small (~22 nucleotides) RNA molecules, which
exhibit a high degree of structure and function conservation in
metazoa (6–8). To date, a total of 450 miRNAs have
been found in mammalian cells; however, ≥1,000 miRNAs remain
uncharacterized (9,10). The biological functions of miRNAs
have yet to be fully elucidated, but previous studies have
demonstrated that they are involved in cell growth, apoptosis,
differentiation, and stress responses via the post-transcriptional
expression of target genes (11–13).
The miR-503 gene is located on human chromosome
Xq26.3 (9,14,15).
A previous study demonstrated that the expression of miR-503 was
suppressed in hepatocellular carcinoma (HCC) cells and primary
tumors (16). In addition,
overexpression of miR-503 inhibited tumor angiogenesis in
vivo and in vitro. A further study reported that miR-503
prevented angiogenesis in tumorigenesis, and demonstrated a novel
mechanism underlying hypoxia-induced basic fibroblast growth factor
2 (FGF2) and vascular endothelial growth factor A (VEGFA)
expression via hypoxia-inducible factor 1-α (HIF1-α)-mediated
inhibition of miR-503 (17).
miR-503 was also demonstrated to be a metastasis-associated miRNA,
which regulates the metastatic function of HCC cells (16). A recent study demonstrated that
miR-503 was downregulated in endometrial cancer cells, and
relatively high miR-503 expression levels resulted in longer
survival time (18). In addition,
miR-503 may act as a novel tumor suppressor gene in gastric cancer,
by inhibiting epithelial-mesenchymal transition (19).
To the best of our knowledge, no study to date has
investigated the role of miR-503 in OS cells. The present study
aimed to investigate the expression levels of miR-503 in OS cells,
as well as to assess the effects of miR-503 on OS cell
proliferation, apoptosis, migration and invasion. In addition, the
association between miR-503 and FGF2 expression was also
investigated.
Materials and methods
Tissue samples
Human OS and adjacent normal bone tissue samples
were harvested from patients undergoing surgery in the Orthopedic
Hospital of the General Hospital of PLA (Beijing, China) between
April and July 2013, and were diagnosed by an independent
pathologist. The patient cohort comprised 12 female and 8 male
patients and their average age was 23 years. None of the patients
had metastasis in the lung or any other organs at the time-point of
first diagnosis. None of the patients received preoperative
treatment, such as radiation therapy or chemotherapy. The present
study was approved by the Ethics Committee of the Orthopaedic
Hospital of the General Hospital of PLA. Written informed consent
was obtained from all the subjects of the present study.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the tissue samples and cell lines was
isolated using an RNA isolation kit (Ambion Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The integrity of the RNA was assessed by denaturing agarose gel
electrophoresis (Regobio, Shanghai, China). RT-qPCR was performed
using a TaqMan MicroRNA assay (Applied Biosystems Life
Technologies, Foster City, CA, USA) and a StepOnePlus real-time PCR
system (Applied Biosystems Life Technologies). All primers were
obtained from the TaqMan MicroRNA assays (Regobio). Small nuclear
U6 small nuclear RNA (Applied Biosystems Life Technologies) was
used as an internal control. The following primers were used for
reverse transcription: miR-503,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACCTGCAG-3′ and U6,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC-3′. The
corresponding PCR primers were as follows: Gene-specific forward
primers miR-503-Fwd, 5′-TGCGGTAGCAGCGGGAACAGTTC-3′ and U6-Fwd,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′, and a universal downstream primer,
5′-CCAGTGCAGGGTCCGAGGT-3′ (reverse). The RT-qPCR reaction system
contained 10 µl SYBR Premix Ex Taq™ II (2X), 0.8 µl
PCR forward primer (10 µM), 0.8 µl Uni0miR qPCR
Primer (10 µM), 0.4 µl ROX Peference Dye II (50X), 2
µl cDNA and 6 µl deionized H2O. The
thermocycling conditions were as follows: 95°C for 5 sec, 60°C for
34 sec and amplification for 40 cycles. The PCR products were
separated on a 2% agarose gel. Each experiment was conducted in
triplicate. Differences in gene expression levels, expressed as
fold changes, were calculated using the 2–ΔΔCt
method.
Cell lines and culture conditions
The human MG-63 OS cell line was purchased from the
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). The osteosarcoma cells were cultured in
RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS),
2.0 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin (all Regobio), and incubated at 37°C in a humidified
incubator supplemented with 5% CO2 and 95% air.
Cell transfection
Exponentially growing cells were seeded
(1.5×105 cells/well) into 12-well plates and incubated
for 3 or 24 h, followed by transfection with 30 nM miR-503
precursor or the negative control (Ambion; Thermo Fisher
Scientific, Waltham, MA, USA) with the X-treme GENE transfection
reagent (Roche Applied Science, Indianapolis, IN, USA) according to
the manufacturer's instructions. Transfection efficiency was
evaluated 48 h post-transfection.
Cell proliferation assay
A total of 24 h post-transfection, the cells were
trypsinized (Regobio), counted with a light microscope (CX4;
Olympus, Japan), and seeded at a density of 4×103
cells/well into 96-well plates. Following incubation in RPMI-1640
with 10% FBS and incubated at 37°C with 5% CO2 for 0–7
days, a cell proliferation assay was performed using a Cell
Counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). The solution absorbance was measured spectrophotometrically
at 450 nm using an MRX II absorbance reader (Dynex Technologies,
Inc., Chantilly, VA, USA). The experiments were performed in
triplicate in three independent experiments, and the data were
presented as the mean ± standard deviation (SD).
Cell apoptosis assay
A total of 48 h post-transfection, the MG63 cells
were harvested, resuspended, fixed, and finally resuspended in
staining solution containing 1 mg/ml RNase A (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China), 50 mg/ml propidium iodide (PI;
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), and 0.1% Triton
X-100 in phosphate-buffered saline. The stained cells were cultured
in 6-well plates (1×105 cells/well) to 70–80%
confluence. A PI/Annexin V-fluorescein isothiocyanate (FITC) assay
(cat. no. KGA108; Nanjing KeyGen Biotech Co., Ltd.) was used to
measure the number of apoptotic cells by flow cytometry. A total of
≤30,000 gated events were acquired from each sample. The results
were expressed as the percentage of apoptotic cells (PI and Annexin
V-FITC positive) in the gated cell population. The total apoptotic
rate was calculated as the early apoptotic rate plus the late
apoptotic rate. An Annexin V-PI/7-AAD Apoptosis Detection kit
(Nanjing KeyGen Biotech Co., Ltd.) was used to conduct the
apoptosis assay of lentivirus vector-transfected cells, as
described above. Each experiment was performed in triplicate, and
the data were presented as the mean ± SD.
Cell migration and invasion assays
A cell suspension of 0.2 ml RPMI-1640 medium
supplemented with 5% FBS was seeded into each well of the upper
Transwell chamber (8 µm pore size), and pre-coated with or
without Matrigel (Nanjing KeyGen Biotech Co., Ltd.). In the lower
chamber, 0.6 ml RPMI-1640 medium supplemented with 20% FBS was
added. Following incubation for 28 h at 37°C in a humidified
incubator with 5% CO2, the chambers were disassembled
and the membranes were stained with 2% crystal violet for 10 min
and placed on a glass slide. The number of cells penetrating the
membrane were counted under a light microscope (CX4; Olympus) in
ten random visual fields.
Western blot analysis
Protein samples were extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific) and then resolved on NuPAGE
4–12% Bis Tris gels (Invitrogen) and transferred to polyvinylidene
difluoride membranes (Roche Diagnostics, Basel, Switzerland). The
membranes were blocked with 5% skimmed milk/Tris-buffered saline
with Tween® 20, and probed with either polyclonal
anti-rat tubulin (1:1,000; cat. no. ab6161-100; Abcam, Cambridge,
UK), monoclonal anti-mouse β-actin (1:10,000; cat. no. ab6276-100;
Abcam), polyclonal anti-mouse neurophilin 2 (C-9; cat. no.
sc-13117; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or
polyclonal anti-goat deoxyhypusine hydroxylase (C-19; 1:1,000; cat.
no. sc-55157; Santa Cruz Biotechnology, Inc.) primary antibodies.
Detection was performed using horseradish peroxidase-conjugated
anti-rat immunoglobulin (Ig)G (1:10,000; cat. no. ab6734-1; Abcam),
anti-mouse IgG (1:10,000; cat. no. NA931 V; GE Healthcare Life
Sciences, Chalfont, UK) and anti-sheep/goat IgG (1:10,000; cat. no.
AB324P; EMD Millipore, Billerica, MA, USA) secondary antibodies,
using an Electro Chemiluminescence (ECL) Plus detection reagent and
an ECL-Hyperfilm (GE Healthcare Life Sciences).
Statistical analysis
Values are expressed as the mean ± standard
deviation, and statistical differences were compared between groups
using Student's t-tests. Data were analyzed with the SPSS 18.0
statistical software package (SPSS Inc., Chicago, IL). P<0.05
was considered to indicate a statistically significant
difference.
Results
miRNA-503 is downregulated in OS tissue
samples
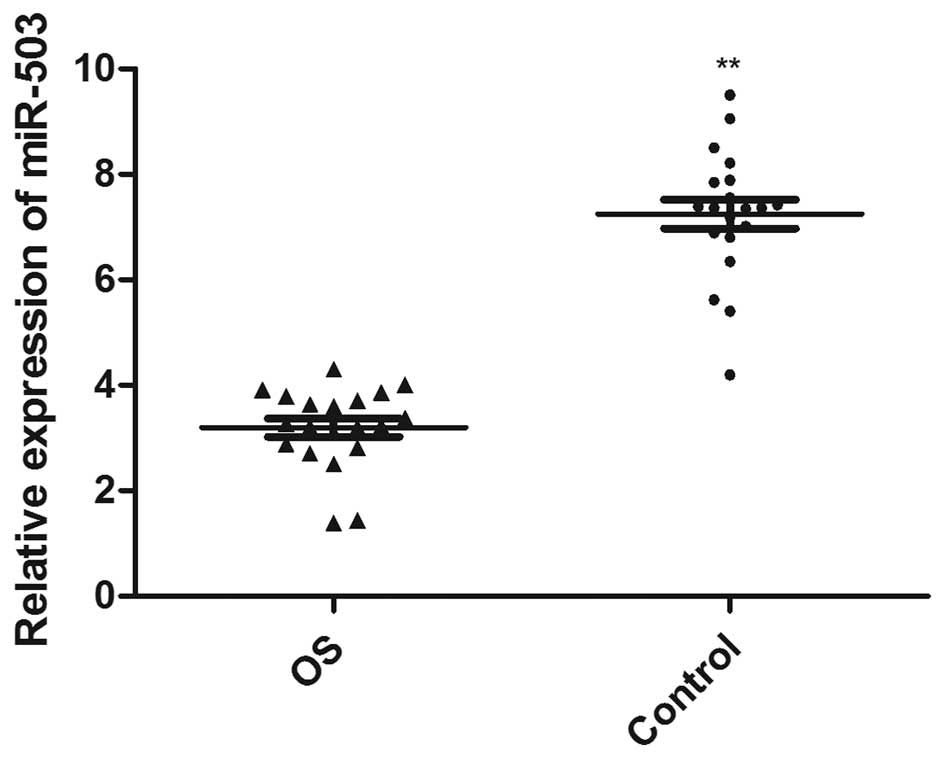
To analyze the miR-503 expression levels in OS
tissue samples, total RNA from the OS and adjacent normal bone
tissue samples of 20 patients with OS were extracted, and the
expression levels of miR-503 were detected. The expression levels
of miR-503 in the OS tissue samples were significantly decreased
(3.20±0.17), compared with those in normal tissue samples
(7.25±0.27; P<0.0001; Fig.
1).
Effects of miR-503 overexpression on cell
growth
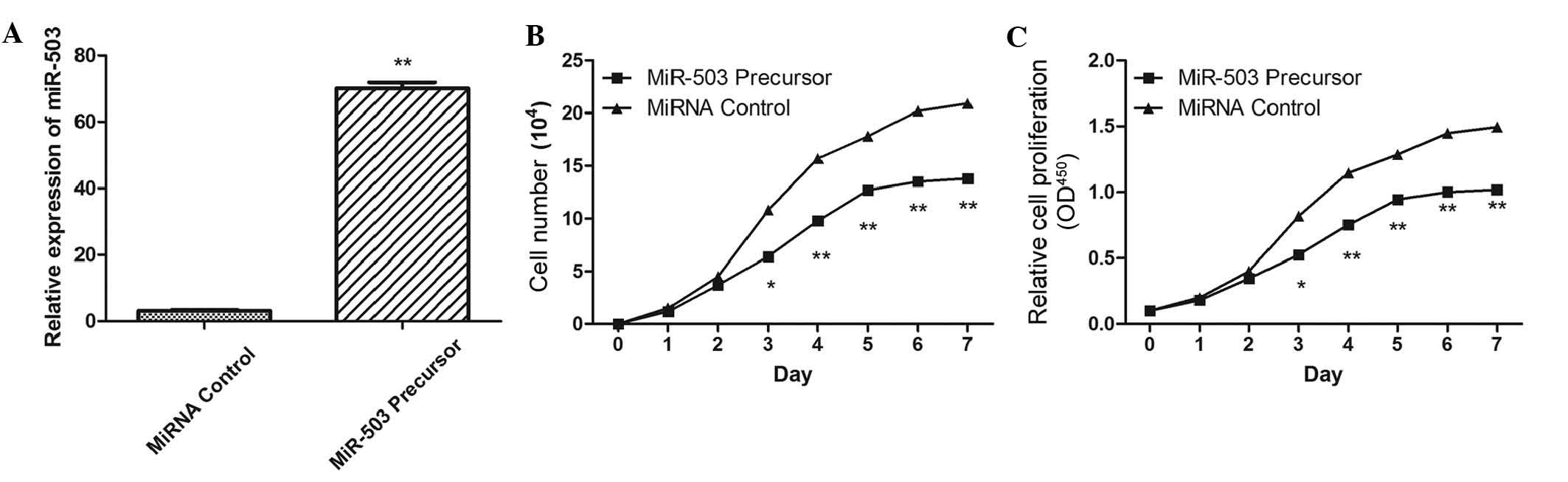
In order to assess the effects of miR-503 on OS cell
growth, the miR-503 precursor was transfected into the MG-63 cells,
and cell growth at various post-transfection time points was
examined. Transfection with miR-503 precursor upregulated miR-503
expression levels (Fig. 2A), and
significantly inhibited proliferation in cells post-transfection
(Fig. 2B and C) 3 days
post-transfection. To further explore the potential mechanism
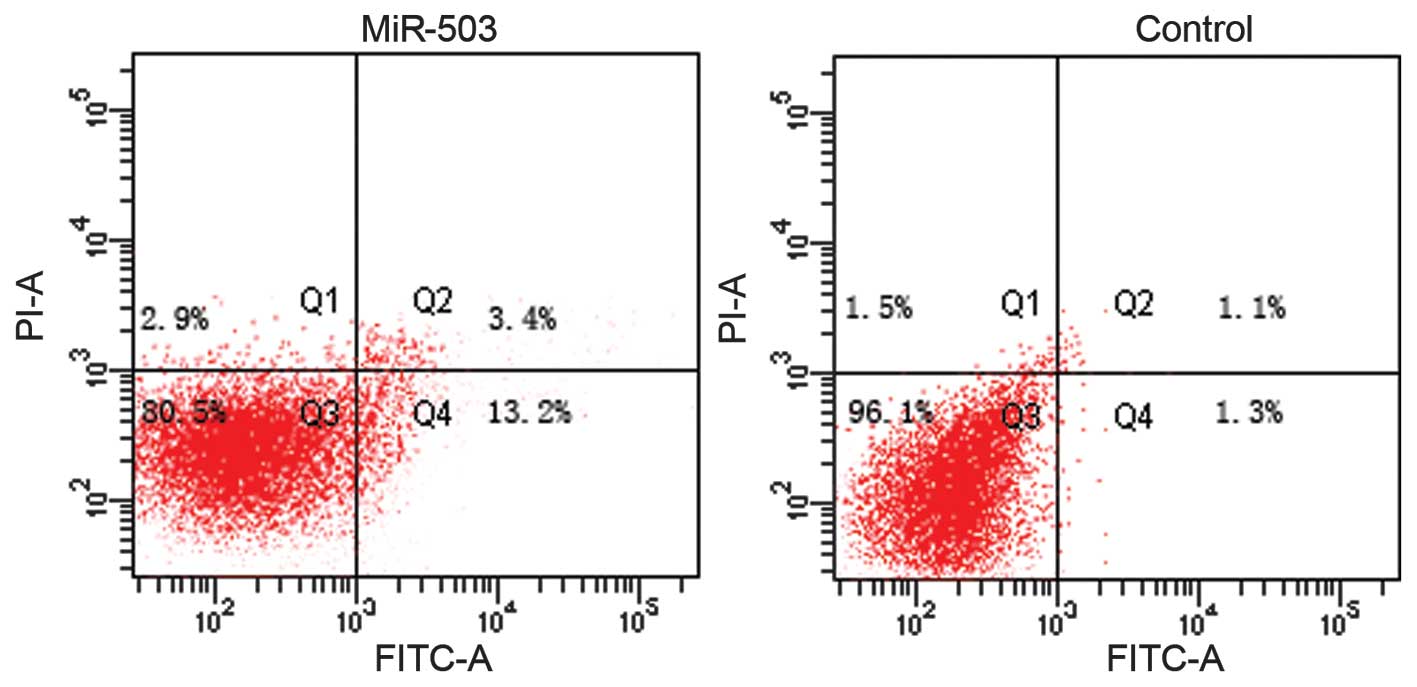
underlying the effects of miR-503 on cell growth, an apoptosis
assay was conducted. Overexpression of miR-503 significantly
induced cell apoptosis, as compared with negative controls
(Fig. 3; P<0.05).
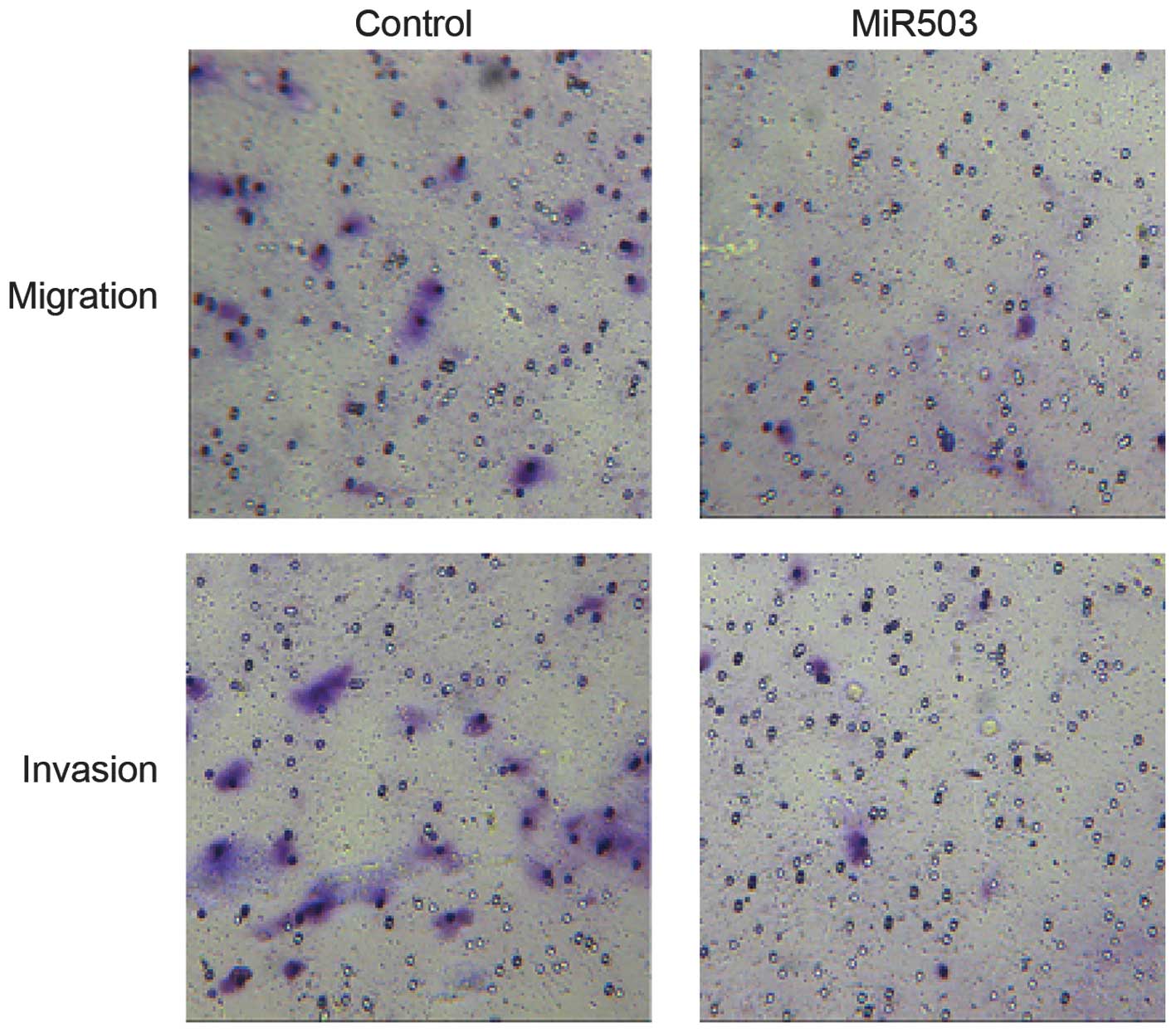
miR-503 inhibits MG-63 cell migration and
invasion
The potential role of miR-503 with regards to MG-63
cell migration and invasion was also investigated. MG-63 cells
transfected with miR-503 precursor demonstrated markedly decreased
migration and invasion levels, as compared with the negative
control (Fig. 4).
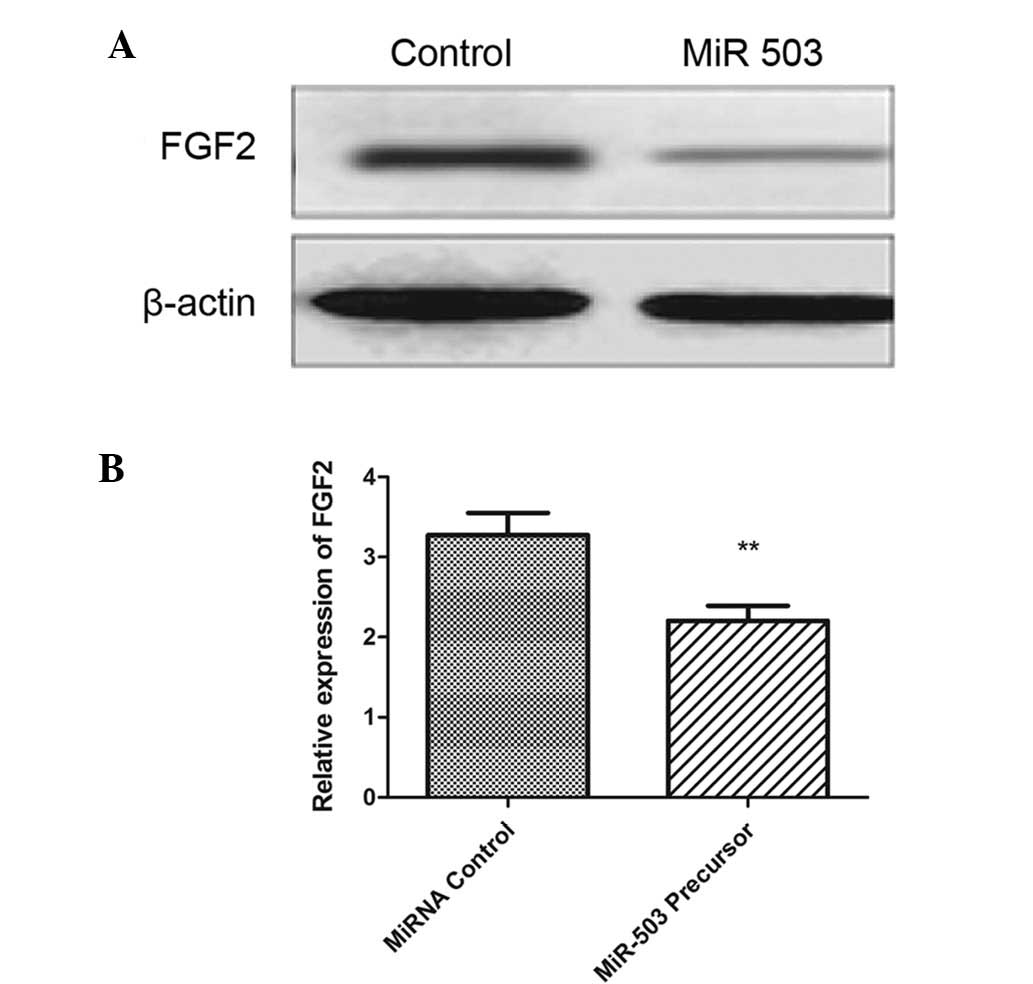
miR-503 downregulates FGF2 in OS
cells
The protein expression levels of FGF2 were also
quantified by western blotting in the MG-63 cells transfected with
miR-503 precursor. The protein expression levels of FGF2 were
significantly decreased in MG-63 cells following transfection with
miR-503 precursor (Fig. 5A and B;
P<0.001). These results suggest that miR-503 inhibits FGF2
translation in OS cancer cells.
Discussion
The present study demonstrated that miR-503
expression was involved in the inhibition of cellular proliferation
and the induction of OC cell apoptosis. In addition, miR-503 was
able to inhibit the migration and invasion of OS cells, which
suggested it had an important role in the metastasis of OS.
Furthermore, the anticancer effects of miR-503 may be mostly due to
FGF2 inhibition.
miRNAs are able to silence target genes either by
direct degradation or by inhibiting their translation. Increasing
evidence suggests that miRNAs may function as oncogenes or tumor
suppressors in human cancer (20–22),
which demonstrates their potential role as promising molecular
targets for cancer therapy.
miR-503 is differentially expressed in various types
of cancer (23). miR-503 is
upregulated in human parathyroid carcinomas (24). Additionally, elevated miR-503
expression was associated with shorter survival rate in patients
with adrenocortical carcinoma (25). Furthermore, miR-503 induced
G1 phase arrest by targeting an overlapping set of
cell-cycle regulators during monocyte differentiation into
macrophages (26). miR-503 was
also induced during myogenesis, and promoted cell-cycle arrest via
cell division cycle 25A degradation (27). However, in other types of cancer,
such as oral cancer and non-metastatic prostate cancer xenografts,
miR-503 expression was downregulated (28,29).
Previous studies also revealed that miR-503 was able to silence
cyclin D1, which is implicated in a variety of cancer types,
thereby reducing S-phase cell populations and inhibiting cell
growth (18,30). Furthermore, a previous study
demonstrated that miR-503 acted as a cell cycle regulator, and is
involved in cell adhesion, angiogenesis and cell migration
(31). The regulation of miR-503
expression has also been demonstrated to be important in drug
resistance and metastatic traits (32). In the present study, the expression
levels of miR-503 were significantly decreased in OS tissue
samples, compared with normal tissue samples, and may share a
similar mechanism of tumor promotion.
FGF2 is one of the most important regulators of
angiogenesis (33,34). The present study demonstrated that
FGF2 expression is downregulated by miR-503. The results are
concordant with those of Kim et al (35), who demonstrated that miR-503
targets FGF2, and has a role in pulmonary arterial hypertension. A
previous study reported that endothelial miR-15a shares similar
seed sequences with miR-503, and was able to negatively regulate
angiogenesis by inhibiting FGF2 and VEGFA expression (36). Furthermore, several studies have
reported the anti-angiogenesis effects of miR-503 in tumorigenesis,
and provide a novel mechanism for hypoxia-induced FGF2 and VEGFA
expression via HIF1α-mediated inhibition of miR-503 (16,17).
In conclusion, the present study demonstrated that
miR-503 expression was involved in the inhibition of cellular
proliferation, and in the induction of OS cell apoptosis. In
addition, miR-503 was able to inhibit the migration and invasion of
OS cells, which may be regulated by the inhibition of FGF2
expression.
References
|
1
|
Tsunemi T, Nagoya S, Kaya M, Kawaguchi S,
Wada T, Yamashita T and Ishii S: Postoperative progression of
pulmonary metastasis in osteosarcoma. Clin Orthop Relat Res.
159–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers PA, Gorlick R, Heller G, Casper E,
Lane J, Huvos AG and Healey JH: Intensification of preoperative
chemotherapy for osteogenic sarcoma: results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol. 16:2452–2458.
1998.PubMed/NCBI
|
|
4
|
Bacci G, Briccoli A, Longhi A, Ferrari S,
Mercuri M, Faggioli F, Versari M and Picci P: Treatment and outcome
of recurrent osteosarcoma: Experience at Rizzoli in 235 patients
initially treated with neoadjuvant chemotherapy. Acta Oncol.
44:748–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janeway KA, Barkauskas DA, Krailo MD,
Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R and
Marina N: Outcome for adolescent and young adult patients with
osteosarcoma: A report from the children's oncology group. Cancer.
118:4597–4605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berezikov E, Guryev V, van de Belt J,
Wienholds E, Plasterk RH and Cuppen E: Phylogenetic shadowing and
computational identification of human microRNA genes. Cell.
120:21–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wienholds E and Plasterk RH: MicroRNA
function in animal development. FEBS Lett. 579:5911–5922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sewer A, Paul N, Landgraf P, Aravin A,
Pfeffer S, Brownstein MJ, Tuschl T, van Nimwegen E and Zavolan M:
Identification of clustered microRNAs using an ab initio prediction
method. BMC bioinformatics. 6:2672005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caporali A, Meloni M, Vollenkle C, Bonci
D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH,
et al: Deregulation of microRNA-503 contributes to diabetes
mellitus-induced impairment of endothelial function and reparative
angiogenesis after limb ischemia. Circulation. 123:282–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou B, Ma R, Si W, Li S, Xu Y, Tu X and
Wang Q: MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor
angiogenesis and growth. Cancer Lett. 333:159–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J and Wang W: Analysis of microRNA
expression profiling identifies microRNA-503 regulates metastatic
function in hepa-tocellular cancer cell. J Surg Oncol. 104:278–283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y and Yin
LR: MicroRNA-503 suppresses proliferation and cell-cycle
progression of endometrioid endometrial cancer by negatively
regulating cyclin D1. FEBS J. 280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
microRNA-503 inhibits gastric cancer cell growth and
epithelial-to-mesenchymal transition. Oncol Lett. 7:1233–1238.
2014.PubMed/NCBI
|
|
22
|
Sarver AL, Li L and Subramanian S:
MicroRNA miR-183 functions as an oncogene by targeting the
transcription factor EGR1 and promoting tumor cell migration.
Cancer Res. 70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen
J, Su F, Yao H and Song E: Up-regulation of miR-21 mediates
resistance to trastuzumab therapy for breast cancer. J Biol Chem.
286:19127–19137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh SS, Yoo JY, Nuovo GJ, Jeon YJ, Kim S,
Lee TJ, Kim T, Bakàcs A, Alder H, Kaur B, et al: MicroRNAs/TP53
feedback circuitry in glioblastoma multiforme. Proc Natl Acad Sci
USA. 109:5316–5321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao JJ, Yang J, Lin J, Yao N, Zhu Y,
Zheng J, Xu J, Cheng JQ, Lin JY and Ma X: Identification of miRNAs
associated with tumorigenesis of retinoblastoma by miRNA microarray
analysis. Childs Nerv Syst. 25:13–20. 2009. View Article : Google Scholar
|
|
26
|
Corbetta S, Vaira V, Guarnieri V,
Scillitani A, Eller-Vainicher C, Ferrero S, Vicentini L, Chiodini
I, Bisceglia M, Beck-Peccoz P, et al: Differential expression of
microRNAs in human parathyroid carcinomas compared with normal
parathyroid tissue. Endocr Relat Cancer. 17:135–146. 2010.
View Article : Google Scholar
|
|
27
|
Ozata DM, Caramuta S, Velazquez-Fernandez
D, Akçakaya P, Xie H, Höög A, Zedenius J, Bäckdahl M, Larsson C and
Lui WO: The role of microRNA deregulation in the pathogenesis of
adre-nocortical carcinoma. Endocr Relat Cancer. 18:643–655. 2011.
View Article : Google Scholar
|
|
28
|
Forrest AR, Kanamori-Katayama M, Tomaru Y,
Lassmann T, Ninomiya N, Takahashi Y, de Hoon MJ, Kubosaki A, Kaiho
A, Suzuki M, et al: Induction of microRNAs, mir-155, mir-222,
mir-424 and mir-503, promotes monocytic differentiation through
combinatorial regulation. Leukemia. 24:460–466. 2010. View Article : Google Scholar
|
|
29
|
Sarkar S, Dey BK and Dutta A: MiR-322/424
and -503 are induced during muscle differentiation and promote cell
cycle quiescence and differentiation by down-regulation of Cdc25A.
Mol Biol Cell. 21:2138–2149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu YC, Chen YJ, Wang HM, Tsai CY, Chen WH,
Huang YC, Fan KH, Tsai CN, Huang SF, Kang CJ, et al: Oncogenic
function and early detection potential of miRNA-10b in oral cancer
as identified by microRNA profiling. Cancer Prev Res (Phila).
5:665–674. 2012. View Article : Google Scholar
|
|
31
|
Watahiki A and Wang Y, Morris J, Dennis K,
O'Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PloS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Q, Feng MG and Mo YY: Systematic
validation of predicted microRNAs for cyclin D1. BMC Cancer.
9:1942009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cross MJ and Claesson-Welsh L: FGF and
VEGF function in angiogenesis: signalling pathways, biological
responses and therapeutic inhibition. Trends Pharmacol Sci.
22:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu
X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, et al: An
endothelial apelin-FGF link mediated by miR-424 and miR-503 is
disrupted in pulmonary arterial hypertension. Nat Med. 19:74–82.
2013. View
Article : Google Scholar :
|
|
36
|
Yin KJ, Olsen K, Hamblin M, Zhang J,
Schwendeman SP and Chen YE: Vascular endothelial cell-specific
microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol
Chem. 287:27055–27064. 2012. View Article : Google Scholar : PubMed/NCBI
|