Introduction
Lung cancer is the major cause of cancer-associated
mortality worldwide (1). Non-small
cell lung cancer (NSCLC), which accounts for ~85–90% of patients
with lung cancer, comprises three sub-types according to their
histological characteristics: Adenocarcinoma, squamous cell
carcinoma and large cell carcinoma (2,3).
Despite recent advances in treatments of NSCLC, they have only
yielded modest improvements in NSCLC patient outcomes, with the
overall five-year survival rate remaining at 15% (4). Therefore, it is required to discover
novel prognostic biomarkers as well as therapeutic targets for
NSCLC. Neuroepithelial transforming gene 1 (Net-1) is a 54-kDa
oncoprotein (5), which has also
been recognized as a Ras homolog family member A (RhoA) guanine
nucleotide exchange factor (GEF) (6), and which was initially identified in
a neuroepithelioma cell line (5).
Net-1 is a member of seven transmembrane four superfamily with two
distinct isoforms (Net-1 and Net-1A) and has a crucial role in cell
signal transduction, proliferation, migration and invasion; it is
also indicative of a poor prognosis of cancer patients (7–10).
Overexpression of Net-1 has been documented in a variety of human
cancer types, including hepatocellular carcinoma, breast cancer,
oesophageal adenocarcinoma, skin squamous cell carcinoma and
gastric adenocarcinoma (8,11–14).
Silencing of Net-1 by small interfering RNAs (siRNAs) was shown to
inhibit cancer cell motility, proliferation and extracellular
matrix invasion (8–10). A growing number of studies have
suggested that there may be cross-talk between the Net-1 and
transforming growth factor β signaling pathways in actin
cytoskeletal re-organization (15–17).
However, to the best of our knowledge, a comprehensive profiling of
Net-1 expression and function in advanced NSCLC has not been
performed.
The present study assessed the expression of Net-1
in NSCLC as well as normal adjacent lung tissues and performed
correlation analyses with various clinicopathological
characteristics in order to explore the p utilization of Net-1 as a
potential target for the development of therapeutic agents as well
as a novel tissue biomarker for human lung cancer.
Materials and methods
Clinical tissue samples
A total of 64 patients (16 women, 48 men) with
NSCLC, who underwent radical surgical resection at the Department
of Thoracic and Cardiovascular Surgery (The Third Xiangya Hospital,
Central South University, Changsha, China) from April 2009 to
October 2009, were enrolled in the present study. According to the
criteria of the World Health Organization (2004) (18), the 64 cases were divided into 29
squamous cell carcinomas, 32 adenocarci-nomas and 3 large-cell
carcinoma. All tumors were staged on the basis of the
tumor-nodes-metastasis (TNM) pathologic classification of the
International Association for the Study of Lung Cancer (19). Primary tumor tissues and their
corresponding adjacent normal tissues (5 cm from the margin of the
tumor) obtained from each surgical specimen were used for
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR), western blot and immunohistochemical analyses. The
protocol of the present study was approved by the Local Ethics
committee of the Third Xiangya Hospital, Central South University.
All patients provided written, informed consent, with separate
written, informed consent obtained for the optional provision of
tumour material for biomarker analyses, and from one patient for
use of lung tissues.
RNA extraction and RT-qPCR
RT-qPCR was performed as described previously
(20). Total RNA was extracted
from tissue specimens using TRIzol reagent (Invitrogen Life
Technologies, Inc., Carlsbad, CA, USA) according to the
manufacturer's instructions. RNA yield and purity were assayed
using a SmartSpec Plus Spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), comparing the A230/260 and A260/A280
ratios. First-strand cDNA synthesis (cDNA synthesis kit; Fermentas,
Burlington, ON, Canada) was performed using random hexamers on 1
µg of total RNA. The amplification was performed in a total
volume of 20 µl containing SYBR Premix Ex Taq (Tli RNaseH
Plus; cat no. DRR820S; Clontech Laboratories, Inc., Mountainview,
CA, USA). The qPCR protocol was as follows: Initial denaturation,
95°C for 1 min; primer-extension was performed in 40 cycles, each
cycle consisted of 95°C for 30 sec, 60°C for 20 sec, 72°C for 20
sec and final extension at 72°C for 10 min. The qPCR results were
quantified using a double standard curve. qPCR was performed using
the following primer sets: Net-1 forward,
5′-CTCTCCAGCCCAGTCTCACA-3′ and reverse, 5′-CCCTCACACTCTTCGTGCAG-3′)
and β-actin forward, 5′-GTCCACCTTCCAGCAGATGT-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCA-3′ (Sangon Biotech Co,. Ltd., Shanghai,
China). Amplifications was performed in 40 cycles. Each cycle
consisted of 30 sec at 95°C, 5 sec at 95°C and 20 sec at 60°C. mRNA
levels were determined using the comparative CT method. The
expression levels of each gene were normalized to those of β-actin,
which served as an endogenous internal control.
Protein isolation and western blot
analysis
Tumor tissues were extracted using
radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher
Scientific, Waltham, MA, USA). The protein concentration in all
samples was determined using a Bicinchoninic Acid Protein Assay kit
(cat no. 23227; Thermo Fisher Scientific). Protein lysates were
subjected to 10% SDS-PAGE followed by transfer onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). After blocking with 5% non-fat dry milk in Tris-buffered
saline containing Tween 20 (Sigma-Aldrich, St. Louis, MO, USA) for
60 min, the membrane was incubated with the primary antibody
overnight at 4°C. The following primary antibodies were used:
Rabbit anti-human NET-1 (cat no. 2500682; 1:1,000; Sigma-Aldrich)
and β-actin (cat no. sc47778; 1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Following three subsequent washing steps,
the membranes were incubated with peroxidase-conjugated polyclonal
rabbit anti-human secondary antibody (cat no. P0212; 1:2,000;
DakoCytomation, Glotstrup, Denmark) at room temperature for 2 h.
The blots were detected using an enhanced chemiluminescence
detection kit (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The immunoreactive bands were scanned
using an LAS-4000 imaging system (Fuji, Tokyo, Japan) to detect
protein expression levels. The results were analyzed using Image
Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) to
determine the relative band density ratio.
Immunohistochemical staining
Immunohistochemistry (IHC) was performed on
formalin-fixed paraffin-embedded (FFPE) histological sections. FFPE
specimens were sectioned at 4 µm onto charged slides and
heated for 30 min at 60°C. Slides were de-paraffinized in three
sequential baths of xylene for 3 min each, re-hydrated using a
graded series of ethanol for 5 min and then washed for 5 min in
distilled water. Following de-paraffinization, antigen retrieval
was performed using citrate buffer (0.1 mol/l, pH 6.0) in a
pressure cooker for 90 min. Slides were then cooled in water and
washed in phosphate-buffered saline (PBS). Intrinsic peroxidase
activity was blocked using 3% H2O2 (Beyotime
Institute of Biotechnology, Haimen, China) in methanol at room
temperature for 15 min. The slides were then rinsed three times
each with PBS. To block non-specific antibody binding, the sections
were incubated with 3% fetal bovine serum for 60 min at 37°C in a
humidified chamber. The slides were washed with PBS and then
incubated with rabbit anti-human NET-1 polyclonal antibody (cat no.
SAB2500682; 1:1,000; Sigma-Aldrich) overnight at 4°C. Following
incubation, sections were washed with PBS and subsequently
incubated with goat anti-rabbit monoclonal secondary antibody (cat.
no. M080825; 1:100; Zhong Shan Golden Bridge Biotechnology Co.,
Ltd) at 37°C for 30 min. Finally, specimens were stained with
3,3′-diaminobenzidine (Shanghai Mingrui Biological Technology Co.,
Ltd., Shanghai, China) and counterstained with hematoxylin
(Beyotime Institute of Biotechnology) at 37°C for 30 min. All
incubations were performed at room temperature. An experienced
pathologist evaluated and scored the immunohistochemical expression
using a light microscope (magnification, ×200). Net-1 expression
was quantified using customary scoring for intensity and the
percentage reactivity. Intensity of staining was graded as follows:
0, without stain; 1, straw yellow; 2, brown; 3, dark brown. The
extent of staining was evaluated as follows: 0, ≤5% positive cells;
1, 6–25% positive cells; 2, 26–50% positive cells; 3, 51–75%
positive cells; and 4, >75% positive cells. The final
immunoreactivity score was obtained by multiplying the intensity
and reactivity scores: IRS ≤2 was defined as negative, 2–5 point
was defined as weak positive (+); 5–9 points was defined as
moderate positive (++) and >9 points was defined as strong
positive (+++) (21).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Differences among the groups were determined by two-way
analysis of variance followed by Tukey's post-hoc test. Comparisons
between the two independent groups were performed using an unpaired
Student's t-test. All analyses were performed using SPSS
17.0 for Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Net-1 is overexpressed in NSCLC
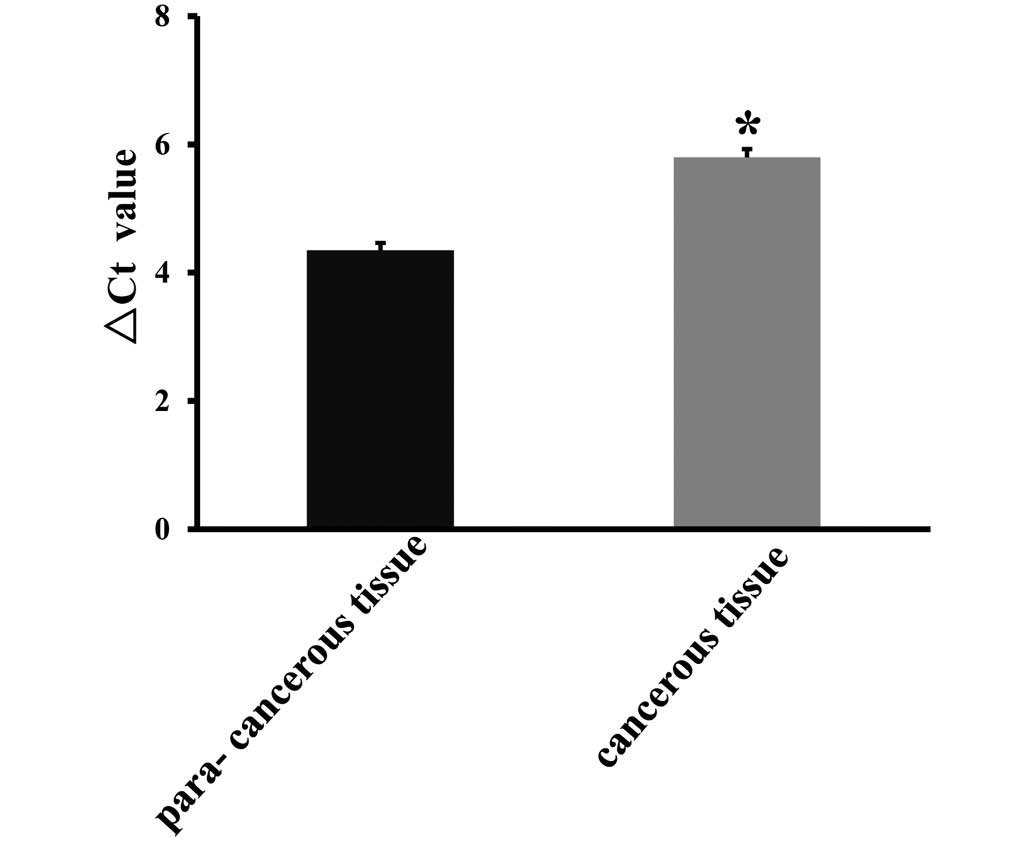
To investigate Net-1 expression in NSCLC, the
present study quantitatively examined the mRNA expression levels of
Net-1 in primary NSCLC tissues and their corresponding adjacent
non-cancerous tissues. As shown in Fig. 1, RT-qPCR analysis revealed that
NSCLC tissues exhibited higher levels of Net-1 expression compared
to those in the corresponding adjacent non-cancerous tissues
(P<0.05). In order to assess whether the differences in Net-1
expression between tumor and non-neoplastic samples are also
present at the protein level, the present study subsequently
examined the expression of Net-1 protein in lung cancers as well as
in their matched normal adjacent tissues using western blot
analysis. The expression levels of Net-1 protein in NSCLC tissues
were significantly higher than those in non-cancerous tissues,
which was in accordance with the mRNA expression levels of Net-1
detected by real-time RT-PCR. Representative blots are shown in
Fig. 2A and B. The finding that
Net-1 is overexpressed in NSCLC specimens compared with
non-cancerous tissues suggests the significance of Net-1 as a
potential prognostic biomarker.
Net-1 overexpression is associated with
poor clinical outcome of NSCLC
To further elucidate the biological and
clinicopathological significance of Net-1 in NSCLC, 64
paraffin-embedded NSCLC tissue specimens were examined by IHC
staining. The association of Net-1 expression and
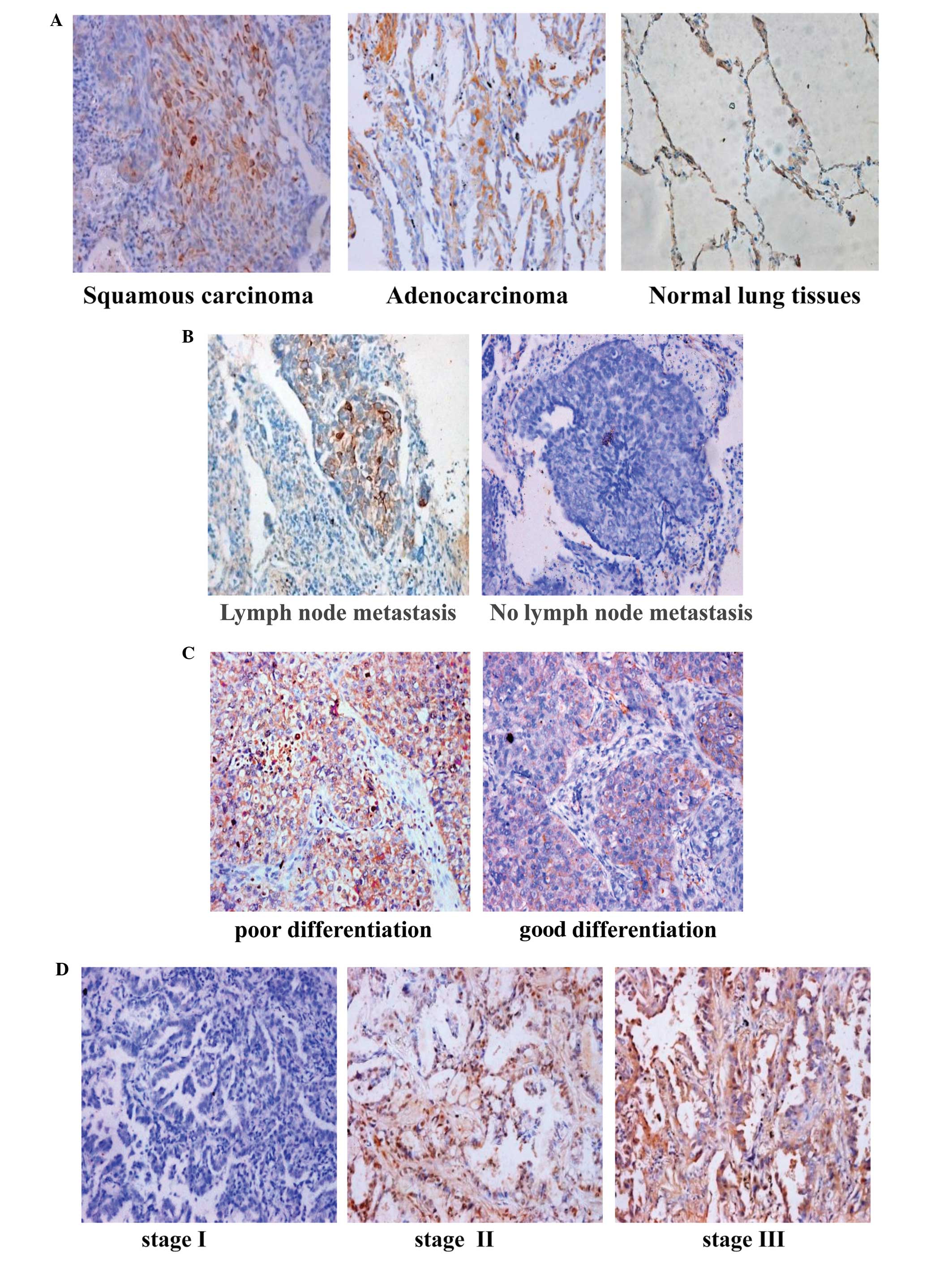
clinicopathological parameters is illustrated in Table I. As shown in Fig. 3A–D, positive immunostaining for
Net-1 was mostly observed in the tumor cells of NSCLC tissues. In
addition, Net-1 expression was significantly upregulated in primary
NSCLC tissues compared to that in their corresponding adjacent
non-cancerous tissues. Semi-quantitative IHC analysis indicated
that Net-1 staining in primary NSCLC was higher than that in
adjacent non-cancerous tissues. The Net-1 staining intensity
gradually increased in accordance with the clinical stages from
stages I–III (P<0.005) (Fig.
3D). Furthermore, statistical analysis showed that high Net-1
expression was strongly associated with the differentiation degree
(P=0.021), clinical stage (P=0.023), lymph node metastasis
(P=0.005) and distant metastasis (P<0.005) of patients with
NSCLC, and this association is further summarized in Table II. However, no statistically
significant association between high Net-1 expression and other
clinicopathological variables, including gender, age, smoking
history, tumor size or histological type, was identified. These
results supported the hypothesis that Net-1 is involved in the
regulation of the invasive ability of NSCLC.
 | Table IAssociation between Net-1 protein
expression in tumors and various clinicopathological factors of 64
patients with non-small cell lung cancer. |
Table I
Association between Net-1 protein
expression in tumors and various clinicopathological factors of 64
patients with non-small cell lung cancer.
| Characteristic | Cases (n) | Net-1 protein
expression | P-value |
|---|
| Gender | | | 0.184 |
| Male | 15 | 2.86±1.54 | |
| Female | 5 | 1.87±0.55 | |
| Age, years | | | 0.322 |
| >60 | 8 | 3.01±1.90 | |
| ≤60 | 12 | 2.35±0.98 | |
| Smoking history | | | 0.184 |
| Smoker | 15 | 2.86±1.54 | |
| Non-smoker | 5 | 1.87±0.55 | |
| Tumor size | | | 0.790 |
| >3 cm | 15 | 2.56±1.42 | |
| ≤3 cm | 5 | 2.76±1.56 | |
| Histological
type | | | 0.351 |
| Scc | 15 | 2.44±1.16 | |
| Ac | 5 | 3.14±2.09 | |
| Tumor
differentiation | | | 0.031 |
| Moderate | 15 | 2.44±1.11 | |
| Poor | 5 | 3.29±2.40 | |
| TNM stage | | | 0.042 |
| I | 5 | 2.10±1.31 | |
| II | 13 | 2.62±1.52 | |
| III | 2 | 2.80±1.41 | |
| Lymph node
metastasis | | | 0.011 |
| Present | 8 | 2.73±1.11 | |
| Absent | 12 | 2.44±1.86 | |
 | Table IICorrelation between Net-1 and
clinicopathological characteristics of 64 patients with non-small
cell lung cancer. |
Table II
Correlation between Net-1 and
clinicopathological characteristics of 64 patients with non-small
cell lung cancer.
| Characteristic | Cases (n) | Patients with Net -1
expression score, n (%)
|
|---|
| – | + | ++ | +++ | P-value |
|---|
| Gender | | | | | | 0.141 |
| Male | 15 | 0 (0.0%) | 7 (46.7%) | 3 (20.0%) | 5 (33.3%) | |
| Female | 5 | 2 (40.0%) | 1 (20.0%) | 1 (20.0%) | 1 (20.0%) | |
| Age, years | | | | | | 0.920 |
| >60 | 8 | 1 (12.5%) | 3 (37.5%) | 1 (12.5%) | 3 (37.5%) | |
| ≤60 | 12 | 1 (8.3%) | 5 (41.7%) | 3 (25.0%) | 3 (25.0%) | |
| Smoking
history | | | | | | 0.141 |
| Smoker | 15 | 0 (0.0%) | 7 (46.7%) | 3 (20.0%) | 5 (33.3%) | |
| Non-smoker | 5 | 2 (40.0%) | 1 (20.0%) | 1 (20.0%) | 1 (20.0%) | |
| Tumor size | | | | | | 0.892 |
| >3 cm | 15 | 2 (13.3%) | 5 (33.3%) | 3 (20.0%) | 5 (33.3%) | |
| ≤3 cm | 5 | 0 (0.0%) | 3 (60.0%) | 1 (20.0%) | 1 (20.0%) | |
| Histological
type | | | | | | 0.237 |
| Scc | 15 | 0 (0.0%) | 8 (53.3%) | 2 (13.3%) | 5 (33.3%) | |
| Ac | 5 | 2 (40.0%) | 0 (0.0%) | 2 (40.0%) | 1 (20.0%) | |
| Tumor
differentiation | | | | | | 0.021 |
| Moderate | 15 | 2 (12.5%) | 8 (50.0%) | 3 (18.8%) | 3 (18.8%) | |
| Poor | 5 | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 3 (75.0%) | |
| TNM stage | | | | | | 0.023 |
| I | 5 | 0 (0.0%) | 4 (80.0%) | 1 (20.0%) | 0 (0.0%) | |
| II | 13 | 2 (15.4%) | 4 (30.8%) | 2 (15.4%) | 5 (38.5%) | |
| III | 2 | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 1 (50.0%) | |
| Lymph node
metastasis | | | | | | 0.005 |
| Present | 8 | 1 (12.5%) | 0 (0.0%) | 2 (25.0%) | 5 (62.5%) | |
| Absent | 12 | 1 (8.3%) | 8 (66.7%) | 2 (16.7%) | 1 (8.3%) | |
Discussion
The present study reported for the first time, to
the best of our knowledge, that Net-1 mRNA expression was
upregulated in NSCLC tissues compared to that in their
corresponding adjacent non-cancerous tissues. High-level Net-1
protein expression was also observed in 12 NSCLC patients,
suggesting that elevated expression of Net-1 may contribute to the
development of NSCLC. Clinical staging of cancer is based on the
TNM classification, which is used to determine the clinical outcome
and prognosis (22). By analyzing
the correlation between clinico-pathological variables of patients
and Net-1 protein expression, the present study found that high
Net-1 expression in NSCLC was significantly correlated with the
differentiation degree, clinical stage, lymph node metastasis and
distant metastasis. Multivariate analysis showed that Net-1 may be
used as an independent prognostic biomarker for patients with
NSCLC.
Previous studies supports the notion that Net-1 is
frequently overexpressed in various cancer types, including
hepatocellular carcinoma, gastric cancer, gliomas, cervical
carcinomas and breast cancer (10,23–26).
Using IHC staining, Lahiff et al (14) found that Net-1 expression is
markedly increased in invasive and metastatic adenocarcinoma of the
oesophagogastric junction. Murray et al (9) demonstrated that the migration and
invasion of AGS gastric cancer cells are inhibited by
Net-1-specific small interfering RNA. In addition, the study found
that knockdown of Net-1 resulted in the formation of round cells
and a loss of definition in the actin cytoskeleton (9). Zhang et al (13) further elucidated the role of NET-1
as a vital regulator of invasion and metastasis in the tumor, and
reported that overexpression of Net-1 increases the likelihood of
aggressive features in patients with squamous cell carcinoma of the
skin As a GEF specific for RhoA, Net-1 is able to mediate RhoA
activation. Activated Rho proteins are able to stimulate signaling
in multiple pathways by binding to target proteins, modulating
their activities and thereby regulating a range of biological
processes, including cell proliferation, apoptosis, differentiation
and cytoskeletal reorganization, signal transduction (6). Recently, Zhang et al (13) further elucidated the role of NET-1
as a vital regulator of invasion and metastasis in the tumor and
reported that overexpression of Net-1 greatly increases the
likelihood of the aggressive feature in human skin squamous cell
carcinoma patients. These studies suggested that Net-1 has vital
roles in the metastasis of malignant tumors. However, the
association between Net-1 and NSCLC has remained elusive. In order
to investigate the involvement of Net-1 in NSCLC, the present study
detected its mRNA and protein expression in 64 paired NSCLC tissues
by RT-qPCR and western blot analysis. Furthermore, Net-1 protein
expression in NSCLC tumor specimens was assessed by IHC and
subjected to a correlation analysis with regard to
clinicopathological features. The results showed that Net-1
expression was significantly elevated in tumor tissues compared to
that in their corresponding non-tumor tissues. In addition, the
results of the present study indicated that upregulation of Net-1
may be an important event in the development and progression of
NSCLC. The levels of Net-1 expression were strongly correlated with
the differentiation degree, clinical stage and lymph node
metastasis in the NSCLC patients assessed in the present study.
Based on these results, Net-1 is likely to be a tumor-promoting
molecule and may contribute to the pathogenesis and progression of
NSCLC. The results of the present study indicated that Net-1
expression is an independent prognostic factor for the outcome of
patients with advanced NSCLC. The findings of the present study not
only suggested that Net-1 may be a promising prognostic biomarker,
but also implied a possible link between the biological function of
Net-1 and the pathogenesis of NSCLC. Net-1 may therefore be an
important therapeutic target for NSCLC treatment. However, further
study is required in order to elucidate the molecular mechanism of
the involvement of Net-1 in the progression of NSCLC.
The present study had certain limitations. The
comprehensive mechanisms of Net-1 in NSCLC cells still remains to
be investigated. Furthermore, patient data regarding cancer
recurrence and mortality were not available for the cohort of the
present study. Furthermore, the present study was performed in a
retrospective manner on a relatively small number of cases. Thus,
the findings of the present study require confirmation by future
studies using a larger patient cohort and appropriate design.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that Net-1 is
overexpressed in NSCLC and correlated with the differentiation
degree, clinical stage and lymph node metastasis as well as
unfavorable prognosis of NSCLC patients. The results of the present
study provided novel insight into the function of Net-1 in the
development and progression of NSCLC and reported a potential
therapeutic target for this malignancy.
Abbreviations:
|
Net-1
|
neuroepithelial transforming gene
1
|
|
NSCLC
|
non-small cell lung cancer
|
Acknowledgments
The present study was supported by the National
Natural Scientific Foundation (grant no. 81472774) and the Research
Programme of the Science and Technology Department of Hunan
Province (grant nos. 2012FJ4076 and 2012TT2011).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong H, Han S, Yao H, Zhao H and Wang Y:
AP4 predicts poor prognosis in nonsmall cell lung cancer. Mol Med
Rep. 10:336–340. 2014.PubMed/NCBI
|
|
4
|
Reck M: What future opportunities may
immuno-oncology provide for improving the treatment of patients
with lung cancer? Ann Oncol. 23(Suppl 8): viii28–viii34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan AM, Takai S, Yamada K and Miki T:
Isolation of a novel oncogene, NET-1, from neuroepithelioma cells
by expression cDNA cloning. Oncogene. 12:1259–1266. 1996.PubMed/NCBI
|
|
6
|
Rossman KL, Der CJ and Sondek J: GEF means
go: Turning on RHO GTPases with guanine nucleotide-exchange
factors. Nat Rev Mol Cell Biol. 6:167–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yauch RL and Hemler ME: Specific
interactions among trans-membrane 4 superfamily (TM4SF) proteins
and phosphoinositide 4-kinase. Biochem J. 351:629–637. 2000.
View Article : Google Scholar
|
|
8
|
Leyden J, Murray D, Moss A, Arumuguma M,
Doyle E, McEntee G, O'Keane C, Doran P and MacMathuna P: Net-1 and
Myeov: Computationally identified mediators of gastric cancer. Br J
Cancer. 94:1204–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murray D, Horgan G, Macmathuna P and Doran
P: NET-1-mediated RhoA activation facilitates lysophosphatidic
acid-induced cell migration and invasion in gastric cancer. Br J
Cancer. 99:1322–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ecimovic P, Murray D, Doran P, McDonald J,
Lambert DG and Buggy DJ: Direct effect of morphine on breast cancer
cell function in vitro: Role of the NET-1 gene. Br J Anaesth.
107:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang GL, Chen L, Wei YZ, Zhou JM, Wu YY,
Zhang YX, Qin J and Zhu YY: The effect of NET-1 on the
proliferation, migration and endocytosis of the SMMC-7721 HCC cell
line. Oncol Rep. 27:1944–1952. 2012.PubMed/NCBI
|
|
12
|
Abba MC, Hu Y, Sun H, Drake JA, Gaddis S,
Baggerly K, Sahin A and Aldaz CM: Gene expression signature of
estrogen receptor alpha status in breast cancer. BMC Genomics.
6:372005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Wang J, Chen L, Wang G, Qin J, Xu
Y and Li X: Expression and function of NET-1 in human skin squamous
cell carcinoma. Arch Dermatol Res. 306:385–397. 2014. View Article : Google Scholar :
|
|
14
|
Lahiff C, Schilling C, Cathcart MC,
Mulligan N, Doran P, Muldoon C, Murray D, Pidgeon GP, Reynolds JV
and Macmathuna P: Prognostic significance of neuroepithelial
transforming gene 1 in adenocarcinoma of the oesophagogastric
junction. Br J Surg. 101:55–62. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papadimitriou E, Vasilaki E, Vorvis C,
Iliopoulos D, Moustakas A, Kardassis D and Stournaras C:
Differential regulation of the two RhoA-specific GEF isoforms
Net-1/Net-1A by TGF-β and miR-24: Role in epithelial- to
-mesenchymal transition. Oncogene. 31:2862–2875. 2012. View Article : Google Scholar
|
|
16
|
Lee J, Moon HJ, Lee JM and Joo CK: Smad3
regulates Rho signaling via NET1 in the transforming growth
factor-beta-induced epithelial-mesenchymal transition of human
retinal pigment epithelial cells. J Biol Chem. 285:26618–26627.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen X, Li J, Hu PP, Waddell D, Zhang J
and Wang XF: The activity of guanine exchange factor NET1 is
essential for transforming growth factor-beta-mediated stress fiber
formation. J Biol Chem. 276:15362–15368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Garg K, Franklin WA, Wistuba
II, Sabloff B, Noguchi M, Kakinuma R, Zakowski M, Ginsberg M,
Padera R, et al: Bronchioloalveolar carcinoma and lung
adeno-carcinoma: the clinical importance and research relevance of
the 2004 World Health Organization pathologic criteria. J Thorac
Oncol. 9:S13–9. 2006. View Article : Google Scholar
|
|
19
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society: international
multidisciplinary classification of lung adenocarcinoma: executive
summary. Proc Am Thorac Soc. 8:381–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin L, Li C, Xu Y, Wang L, Liu J, Wang D,
Hong C, Jiang Z, Ma Y, Chen Q and Yu F: Epigallocatechin gallate
promotes p53 accumulation and activity via the inhibition of
MDM2-mediated p53 ubiquitination in human lung cancer cells. Oncol
Rep. 29:1983–1990. 2013.PubMed/NCBI
|
|
21
|
Birner P, Oberhuber G, Stani J, Reithofer
C, Samonigg H, Hausmaninger H, Kubista E, Kwasny W,
Kandioler-Eckersberger D, Gnant M, et al: Evaluation of the United
States Food and Drug Administration-approved scoring and test
system of HER-2 protein expression in breast cancer. Clin Cancer
Res. 7:1669–1675. 2001.PubMed/NCBI
|
|
22
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennett G, Sadlier D, Doran PP, Macmathuna
P and Murray DW: A functional and transcriptomic analysis of NET-1
bioactivity in gastric cancer. BMC Cancer. 11:502011. View Article : Google Scholar
|
|
24
|
Shen SQ, Li K, Zhu N and Nakao A:
Expression and clinical significance of NET-1 and PCNA in
hepatocellular carcinoma. Med Oncol. 25:341–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tu Y, Lu J, Fu J, Cao Y, Fu G, Kang R,
Tian X and Wang B: Over-expression of neuroepithelial-transforming
protein 1 confers poor prognosis of patients with gliomas. Jpn J
Clin Oncol. 40:388–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wollscheid V, Kühne-Heid R, Stein I,
Jansen L, Köllner S, Schneider A and Dürst M: Identification of a
new proliferation-associated protein NET-1/C4.8 characteristic for
a subset of high-grade cervical intraepithelial neoplasia and
cervical carcinomas. Int J Cancer. 99:771–775. 2002. View Article : Google Scholar : PubMed/NCBI
|