Introduction
The morbidity rates of prostate cancer are estimated
to be the highest of malignancies in American males, and is the
second leading cause of tumor-associated mortality among American
males, accounting for 27% (233,000 cases) of all newly diagnosed
cancers in males and 10% (29,480 cases) of all cancer-associated
mortality in American males in 2014 (1). Common treatment therapies include
radical prostatectomy, radiation or radiotherapy (external beam,
brachytherapy), chemotherapy and active surveillance (2). The majority of patients receive
hormone therapy in the early stage, however, a substantial
percentage of androgen-dependent types of cancer gradually become
resistant to castration, and ultimately progress to a more
aggressive and androgen-independent form (3,4).
There are also disadvantages to other treatments, which include
risks and injuries from surgery, systemic side effects following
radiation and chemotherapy, and anxiety during surveillance
(5,6). In addition, with prostate-specific
antigen screening and transrectal ultrasonography becoming
universal, and the development of magnetic resonance imaging and
transrectal ultrasound (US)-guided prostate biopsy, the accuracy of
diagnosis and localization has increased, increasing the number of
small-sized and low-grade lesions of prostate carcinoma being
diagnosed, particularly in young males (7). As a result, radiofrequency ablation
(RFA) has emerged as an invasive therapy between radical approaches
and active surveillance to manage small-sized, low-grade types of
prostate cancer (7). RFA energy
produces ionic or molecular friction and collision of particles,
and delivers high-frequency energy to target tissues, ultimately
inducing coagulative necrosis of tumor tissues (8,9). The
minimal temperature required for complete tissue destruction is
55°C (8).
Generally, RFA has been restricted to tumors
measuring <3 cm in diameter, as RFA cannot achieve complete
ablation for larger tumors (10).
This has often been attributed to limitations in uneven energy
deposition of RF heating in the tumor and the effects of blood flow
into and out of the tumor (11).
Vascular flow can cause heat dispersion through convection and
reduce the volume of thermally induced coagulation, which has been
referred to as the heat-sink effect (9). The heat-sink effect frequently leads
to treatment failure in patients, and this unwanted effect can be
accentuated when the tumor is hypervascular (9,12).
In addition, heat loss can causes variability in lesion volume and
shape (13). Furthermore, RFA
affects the tumor microenvironment, and incomplete RFA may induce
residual carcinoma cells to proliferate more rapidly and become
more aggressive (14).
In recent years there has been an increase in
investigations of a novel antivascular approach, which uses
low-frequency, low-intensity ultrasound-stimulated microbubbles
(USMB) to disrupt tumor vasculature (15–17).
Our previous study demonstrated that blood perfusion in human
prostate cancer xenografts in nude mice can be effectively arrested
for 2 h, which may be used to mitigate the effect of the heat sink.
In addition, USMB has the capacity to promote apoptosis, and
inhibit proliferation and angiogenesis in tumors (18,19),
which may assist in reducing the number of over-proliferating
residual carcinoma cells activated by incomplete thermal ablation.
Therefore, it is hypothesized that this technology to reduce tumor
blood flow may be used to enhance thermal ablation to treat
castration-resistant prostate cancer.
To the best of our knowledge, the effects of USMB on
RFA has not been previously reported. The present study
hypothesized that USMB prior to RFA may yield results similar to
those of surgery. A technique, which combines USMB prior to RFA may
offer promise for the treatment of relatively large or
hypervascular prostate tumors. In the present study, a subcutaneous
transplanted model of human prostate cancer in nude mice was used
to determine the involvement and effects of USMB in RFA.
Materials and methods
Cell lines and xenograft tumor model
The present study was approved by the Institutional
Review Board of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital (Shanghai, China). The PC3 human
androgen-independent prostate cancer cell line was obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in DMEM medium (GE Healthcare Life
Sciences, Logan, UT, USA), which was added with 10% fetal bovine
serum (Zhejiang Tianhang Biological Technology Co., Hangzhou,
China) in an incubator with 5% CO2 at 37°C. When The
cells reached 80% confluence, they were washed with
phosphate-buffered saline (PBS), trypsinized (trypsin: Ginuo
Biomedical Technology Co., Ltd., Hangzhou, China) and centrifuged
at 150 × g for 5 min at 25°C. Subsequently, the cells were
resuspended in PBS and the final viable cell solution was estimated
at a concentration of 1×107 cells per 100 µl.
A total of 50 male Balb-c nude mice (5–6 weeks old;
weight, 20–25 g) were purchased from Shanghai Super-B&K
Laboratory Animal Corp., Ltd. (Shanghai, China) and raised in the
Animal Laboratory of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital. The mice were housed in 10 cages (five mice per
cage) at a temperature of 25°C, under a 12-h light/dark cycle. The
mice were fed with sufficient chow and water for one week prior to
being injected with the PC3 prostate cancer cells. Each procedure,
including the establishing of the animal model and therapeutic
treatment, was performed under general anesthesia via an
intraperitoneal injection of 50 mg/kg of 1.5% pentobarbital sodium
(Merck Millipore, Darmstadt, Germany) and local sterilization. Each
mouse was subcutaneously inoculated with 1×107 PC3 cells
into the right flank. Subsequently, the mice were maintained under
specified pathogen-free conditions, and were observed for weight
gain, mental status and tumor size at 2-day intervals. Experiments
were initiated 18 days later, when the tumor size was ~8 mm in
maximum diameter, in 48 nude mice (two mice were excluded from the
experiment, as tumors had not established in them). Of these 48
mice, 45 were recruited for the subsequent experiment, as three
mice were excluded due to perfusion defects, observed by enhanced
ultrasonography (EUS), in the center of the tumors 18 days after
PC3 cell inoculation, which indicated necrosis.
Treatment procedures
The animals were randomly divided into three groups
of 15 animals: The USMB+RFA group was treated with combined therapy
(USMB followed by RFA), the RFA group was treated with RFA alone,
and the control group remained untreated. Following treatment, the
animals underwent EUS to evaluate the tumor and ablation volumes,
and the tumors were surgically excised for hematoxylin and eosin
(HE; Beijing Leagene Biotech Co., Ltd., Beijing, China) staining,
tumor apoptosis analysis, and the detection of cell proliferation
and angiogenesis, with five mice per group, at 1, 4 and 7 days
following treatment. Subsequently, the five mice in each group were
sacrificed by an overdose injection of sodium pentobarbital (150
mg/kg). Treatment with USMB alone was excluded from the present
study for three reasons. Firstly, USMB alone can inhibit tumor
growth, however, tumor growth occurs at a slower rate (18,19),
which was further confirmed in our previous studies (20), therefore the combination of USMB
with other therapies is suggested to treat cancer, rather than
alone. Secondly, RFA is able to achieve complete ablation of tumors
measuring <3 cm in diameter, however, eradication of larger
tumors and avoiding proliferation of residual tumor cells remains a
challenge. Finally, the present study aimed to investigated how to
enhance the curative effect of RFA, and hypothesized that USMB
prior to RFA may overcome the limitations of RFA.
MB treatment
Albumin-coated MBs, also known as
perfluoropropane-albumin microsphere injection (RunKun
Pharmaceutical Co., Yueyang, China), were used for EUS and
therapeutic application. The MBs had a mean diameter of 3.4
µm, with 99% of the particles <10 µm in diameter
and an MB concentration of 6.5×108/ml. The MBs were
agitated gently for ~20 sec to produce a milky white suspension.
For EUS, a bolus injection of 0.10 ml MB was injected into the
caudal veins of the mice. To induce vessel blocking, a bolus
injection of 0.20 ml MB was administered, via the caudal veins, for
each treatment.
USMB treatment
The low frequency US equipment was manufactured by
the Shanghai Institute of Ultrasound in Medicine (Shanghai, China).
The diameter of the therapeutic US transducer was 20 mm, which
covered the entire tumor. The therapeutic parameters were
determined according to the orthogonal experimental design of our
previous study (21): Frequency,
20 kHz; acoustic intensity, 1 W/cm2; duty cycle, 40% (2
sec on/3 sec off); irradiation duration, 3 min. The intermittent
working mode allowed the MBs to refill following the destruction of
every MB. Following intraveneous injection of the MBs (0.20 ml),
the tumors were insonated percutaneously with low-frequency
low-intensity US for 3 min.
RFA procedure
RFA was performed using a 480-kHz VIVA RF generator
(Star Med Co., Ltd., Goyangsi, Korea). An 18-gauge internally
cooled electrode with a 5-mm-tip (Star Med Co., Ltd.) was inserted
percutaneously into the tumor. RFA was applied at 5 W in a
continuous mode for 12 sec. These RFA parameters were selected in
preliminary experiments to induce RF ablation without complete
destruction of the tumor, thereby permitting the evaluation of
ablation volumes and histological analysis of the peripheral
untreated tumor. The treatment duration and electrode tip
temperature were monitored throughout the RFA procedure.
Calculation of tumor and ablation
volume
A commercially available US imaging system (Mylab90;
Esaote SpA, Genoa, Italy), equipped with an LA522 high frequency
linear array probe (Esaote SpA), was used for EUS. Dual-frame
imaging, combining two-dimensional (2-D) and contrast modality was
performed using a low mechanical index (0.05). The depth, frequency
and other US conditions were the same during all EUS procedures.
The recorded diameters of the tumor and ablation lesion in the EUS
images were based on the consensus of two observers. The tumor and
ablation volumes were calculated according to the following
formula: Tumor or ablation volume (mm3) = (a ×
b2)/2, where a and b represent the longest and the
shortest diameters of the measured tumor (or volumes of ablation),
respectively. Tumor ablation was calculated using the following
formula: Rate of tumor ablation (%) = ablation volume / tumor
volume × 100%.
Sample collection and pathological
examination
The harvested tumor specimens were fixed in 10%
neutral formalin (Nanchang Yulu Experimental Equipment Co., Ltd.,
Nanchang, China) for 24 h, following which the tissues were
embedded in paraffin (Leica Microsystems GmbH, Wetzlar, Germany).
The largest sections (5-µm thick) were obtained for HE and
immunohistochemical staining to analyze tumor apoptosis,
proliferation and angiogenesis. A pathologist, blinded to the
experimental procedures, evaluated morphological tissue changes
under a light microscope (CX41; Olympus Corporation, Tokyo,
Japan).
Apoptosis of the tumor cells was determined using
terminal deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate nick-end labeling (TUNEL), using a commercially
available In situ Cell Death Detection kit (POD; Roche
Diagnostics GmbH, Mannheim, Germany). Slides were counterstained
with hematoxylin, and the total number of tumor cell nuclei and
TUNEL-positive cell nuclei were counted (magnification, ×400). The
apoptotic cells were those exhibiting DNA fragmentation and nuclei
stained brown or tan. At least five randomly-selected,
non-overlapping fields per tumor were analyzed, containing at least
500 nuclei for TUNEL staining. Apoptotic index (AI) was defined as
the ratio of TUNEL-positive tumor cell nuclei to the total tumor
cell nuclei, expressed as percentage.
Immunohistochemical quantification of the
proliferation of tumor cells was determined using rabbit anti-human
Ki-67 monoclonal antibody (1:500; cat. no. ab92742; Abcam,
Cambridge, UK). Antigen retrieval was performed by boiling in 10
mmol/L sodium citrate buffer for 15 min. The non-specific binding
sites were blocked with goat serum (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 30 min. The rabbit anti-Ki-67
antibody was applied to slides and incubated overnight at 4°C. The
slides were then incubated with goat anti-rabbit antibody (1:500;
cat. no.ZB-2301; OriGene Technologies, Beijing, China) for 30 min
at 37°C. The primary antibody was replaced by PBS, which served as
a negative control. The slides were counterstained with
hematoxylin, and the total number of tumor cell nuclei and
Ki-67-positive cell nuclei was counted (magnification, ×400).
Ki-67-positive cell nuclei were stained brown or tan. A total of
five randomly selected, non-overlapping fields were analyzed, with
at least 500 nuclei counted from each section for Ki-67 staining.
Proliferative index (PI) was defined as the ratio of Ki-67 positive
tumor cell nuclei to total tumor cell nuclei, expressed as
percentage.
Quantification of tumor angiogenesis in the tumor
tissue was determined using rabbit anti-mouse CD34 monoclonal
antibody (1:250; cat. no. ab81289; Abcam). Antigen retrieval was
performed by boiling in 10 mmol/L sodium citrate buffer for 15 min.
The non-specific binding sites were blocked with goat serum (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) for 30 min. The
rabbit anti-CD34 antibody was applied to slides and incubated
overnight at 4°C. The slides were then incubated with goat
anti-rabbit antibody (1:1,000; cat. no. ZB-2301; OriGene
Technologies) for 30 min at 37°C. The primary antibody was replaced
by PBS, which served as a negative control. The slides were
counterstained with hematoxylin. Blood vessels were stained brown.
CD34 staining of the blood vessels was observed under a microscope
(magnification, ×400). For each slide, microvessel density (MVD)
was calculated as the number of CD34-positive vessels in five
randomly-selected, non-overlapping fields.
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 19.0 (IBM SPSS, Armonk, NY, USA) was used to
analyze data. Repeated measures analysis of variance was used for
comparisons of differences in tumor volume, ablation volume,
ablation rate, AI, PI and MVD between the different time-points
within a single group, and a least significant difference (LSD)
test was used for multiple comparisons. One-way analysis of
variance was used for comparisons of differences in tumor volume,
AI, PI and MVD between groups at the same time-point, and the LSD
test was used for multiple comparisons. An independent sample
t-test was used for comparisons of differences in tumor
ablation volume and ablation rate between two treatment groups at
the same time-point. P<0.05 was considered to indicate a
statistically significant difference.
Results
Tumors were established in all animals following the
inoculation of PC-3 cells. All animals survived the procedures
until the designated time-point of euthanasia following the
procedure. The tumor and ablation volumes of RFA with or without
USMB were evaluated using ultrasonography. To assess differences in
histology between the three groups, tumor specimens were examined
using HE staining and immunohistochemical staining for apoptosis
(TUNEL assay), proliferation (Ki-67) and angiogenesis (MVD). HE
indicated necrosis in the center of the tumors from the treatment
groups, while no obvious necrosis was observed in the control
group. This confirmed the efficacy of the RFA alone and USMB+RFA
treatment methods.
Tumor and ablation volume
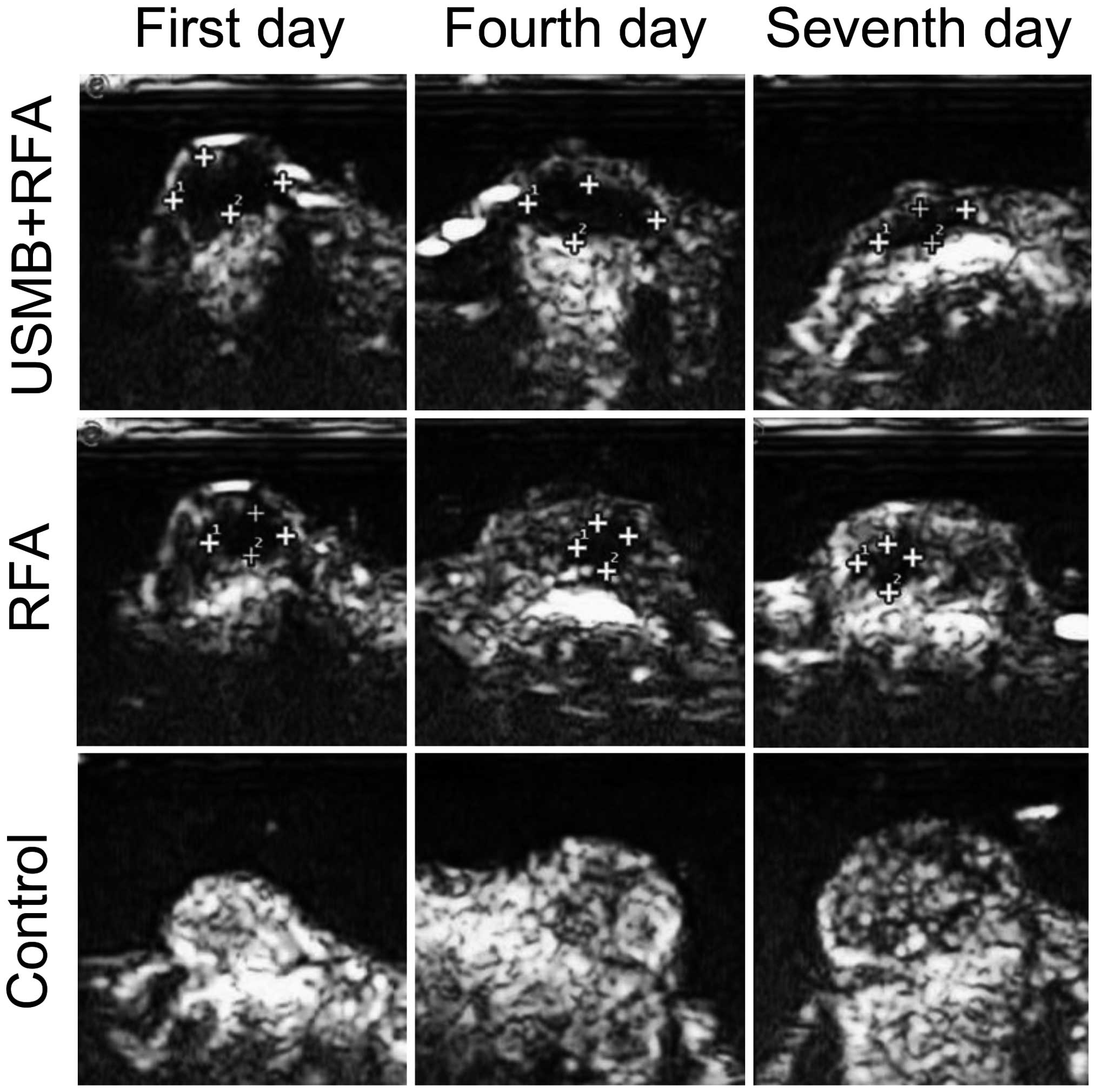
The implanted tumors were observed to be spherical,
elliptical or nodular in shape and were well-defined and
homogenously hypoechoic in the 2-D ultrasonography (Fig. 1). All xenografts demonstrated
marked and homogenous enhancement in EUS prior to treatment.
Following treatment, the regions of the ablation lesions in the two
treatment groups were readily distinguishable from the residual
tumor tissues, as perfusion defects were visible in the EUS.
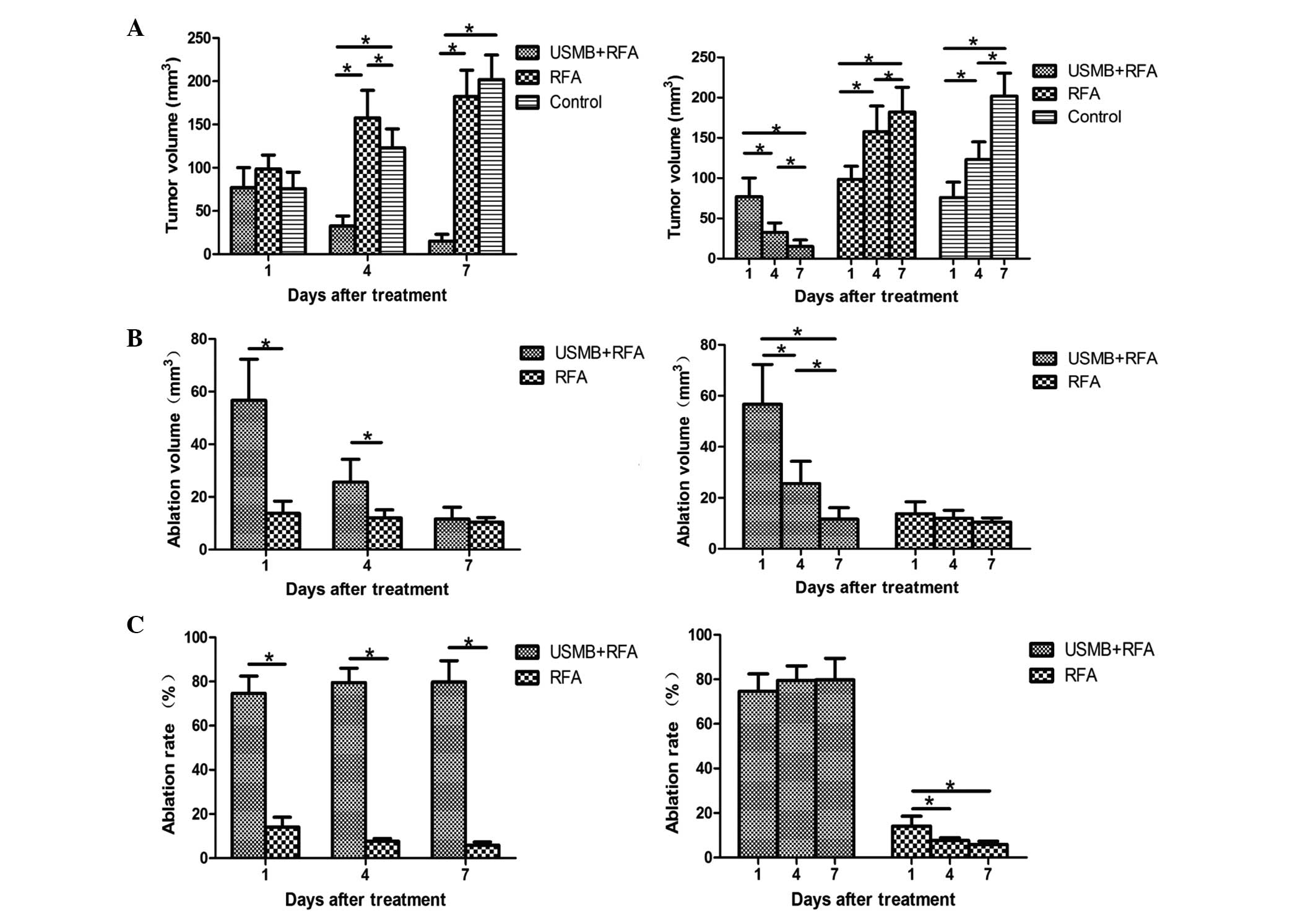
The tumor volumes in the days subsequent to
treatment are shown in Fig. 2A. No
significant differences in the three groups were observed 1 day
following treatments. Significant differences in tumor volumes were
observed among the three groups (P<0.05; Fig. 2A) at 4 days post-treatment. Tumor
volumes were reduced by treatment with USMB combined with RFA,
while the RFA group exhibited larger tumor volumes, compared with
the control group (P<0.001 and P=0.037, respectively; Fig. 2A). On day 7, the tumor volumes were
smaller in the USMB+RFA group, compared with the other two groups
(P<0.001; Fig. 2A). The tumors
treated with RFA were smaller than those in the control group,
although this difference was not significant (P=0.165; Fig. 2A). Only the combination of USMB and
RFA produced measurable tumor volume reduction, whereas tumor
volumes in the RFA group and control group increased over time
(P<0.05; Fig. 2A).
At 1 and 4 days post-treatment, a larger average
ablation lesion volume was observed in the USMB+RFA group, compared
with the RFA group (P=0.002 and P=0.022 at 1 and 4 days
post-treatment, respectively; Fig.
2B). In the two treatment groups, ablation volumes decreased
with time, although the differences were only significant in the
USMB+RFA group (Fig. 2B).
The ablation rates were higher in the USMB+RFA group
compared with the RFA group 1, 4 and 7 days following treatment
(P<0.001; Fig. 2C). In the
USMB+RFA group, ablation rates increased with time, however, the
differences between the different time-points were not significant
(P>0.05; Fig. 2C). In the RFA
group, ablation rates decreased with time, and on day 1
post-treatment the rates were significantly higher, compared with
those on days 4 and 7 post-treatment (P=0.040 and P=0.016,
respectively; Fig. 2C).
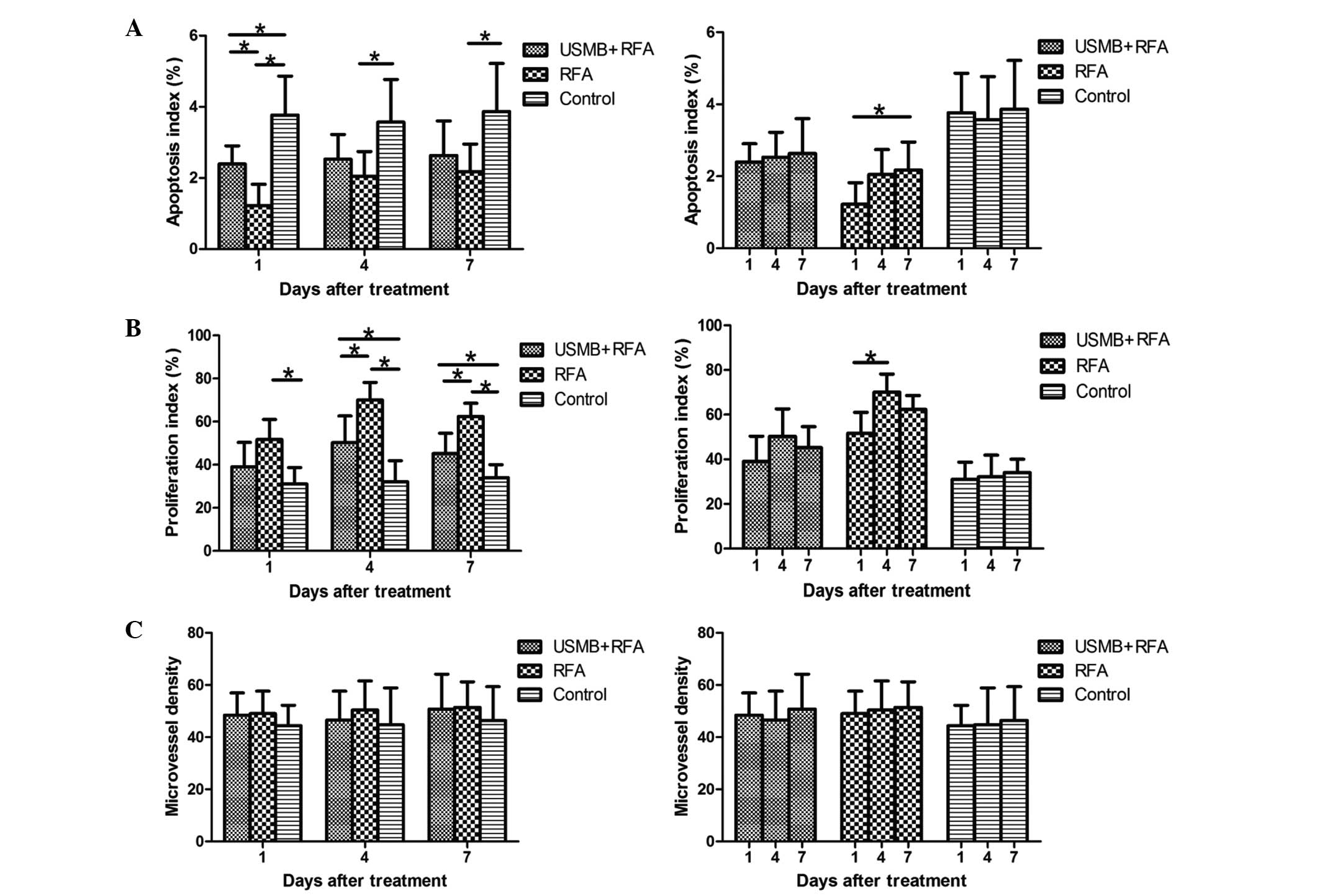
Tumor apoptosis
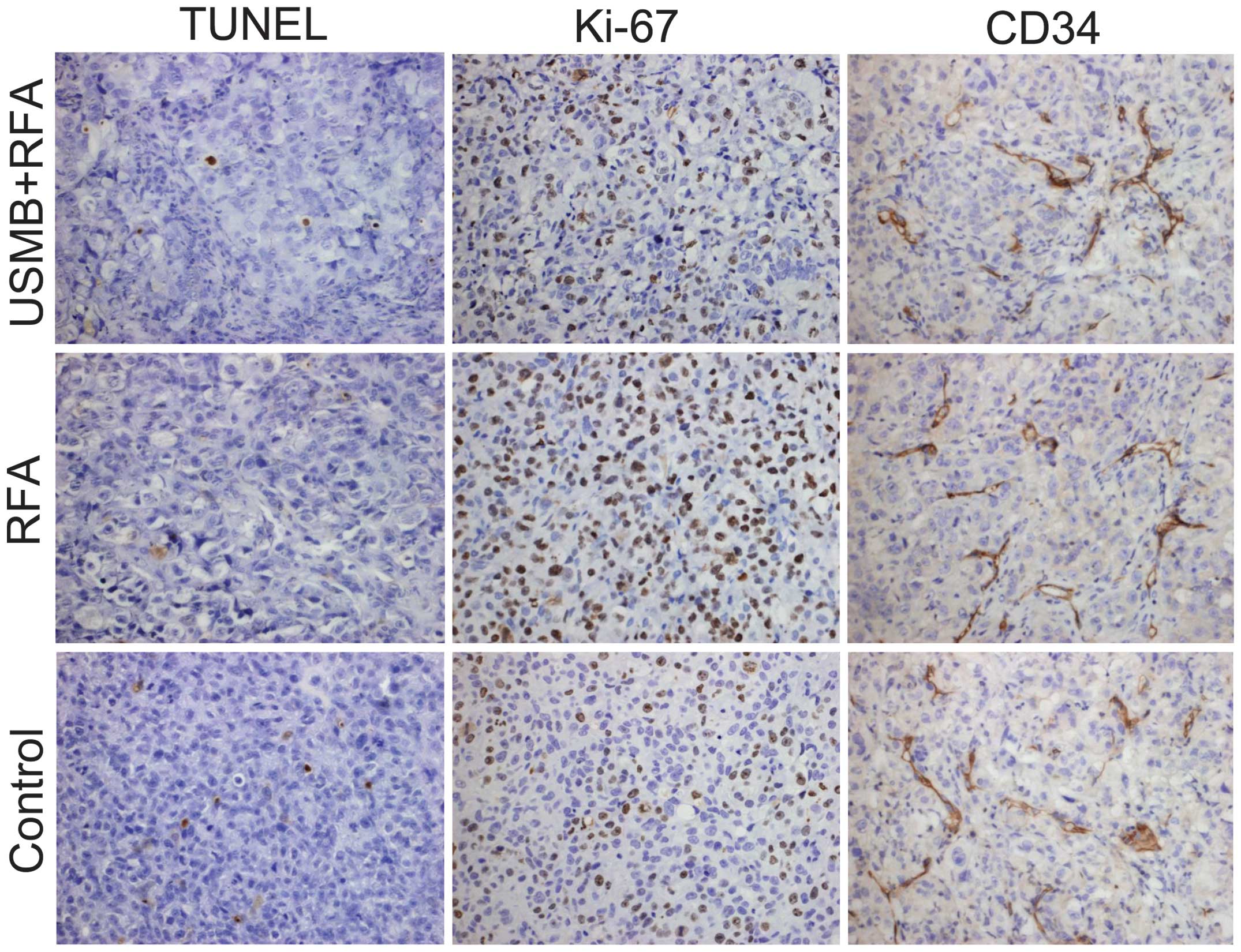
The present study performed TUNEL assays (Fig 3) of the subcutaneous tumors to
conform the apoptotic effects in the different groups. Treatment
with RFA alone and with USMB+RFA resulted in decreases in AI,
compared with the control (P<0.001 and P=0.016, respectively;
Fig. 4A) and AI decreased more
significantly in the RFA group, compared with the USMB+RFA group at
1 day post-treatment (P=0.034, Fig.
4A). At 4 and 7 days post-treatment, the AI was lower in the
RFA group, compared with the control (P=0.020 and P=0.027,
respectively; Fig. 4A) and the
USMB+RFA group, however, the differences between the RFA and
USMB+RFA groups were not significant (P=0.412 and P=0.506,
respectively; Fig. 4A). The AI of
the RFA group increased over time, however, only the difference
between days 1 and 7 was statistically significant (P=0.048;
Fig. 4A). The changes in AI in the
USMB+RFA and control groups over time were not significant
(P>0.05; Fig. 4A).
Immunohistochemical staining of cell
proliferation and angiogenesis
Immunohistochemical staining of Ki-67 in the
subcutaneous tumors (Fig. 3) was
performed to examine the proliferative effects in the different
groups. At 1 day post-treatment, the PI values were higher in the
control group, compared with the two treatment groups, although
only the difference with the RFA group was significant (P=0.005;
Fig. 4B). The PI of the tumors
treated with RFA were lower than those in the USMB+RFA group,
however, the difference was not significant (P=0.058; Fig. 4B). Significant differences in PI
were observed among the three groups 4 and 7 days post-treatment,
as PI was higher in the USMB+RFA group, compared with that in the
control, whereas the RFA exhibited a higher PI, compared with the
USMB+RFA group at 4 and 7 days post-treatment (P<0.05; Fig. 4B). The PI of the RFA group peaked 4
days following treatment, however only the difference between days
1 and 4 was statistically significant (P=0.013; Fig. 4B). The PI changes in the USMB+RFA
and control groups over time were not significant (Fig. 4B).
To determine the effects of treatment on tumor
angiogenesis, immunohistochemical staining of CD34 in the tumors
was performed to quantify MVD. No significant differences were
observed among the three groups throughout the experiment. The MVD
in the RFA group was higher than those in the USMB+RFA and control
groups at the three time-points, however, this was not significant
(P>0.05; Fig. 4C).
Discussion
Triggered by the increasing incidence of
small-volume and low-grade foci of prostate cancer, and in
investigating alternatives for patients unfit for radical
prostatectomy and surveillance, RFA has been considered a promising
treatment modality in animal experiments and clinical trials
(7,22,23).
However, RFA is susceptible to the heat-sink effect of flowing
blood, which affects the therapeutic effect of RFA, by decreasing
heat-induced coagulative necrosis, constituting a serious problem
often leading to local recurrences following ablation, particularly
in large and hypervascular tumors (9–12).
Thermal energy is conducted from the tip of an electrode to the
surrounding tissue and is attenuated during conduction, therefore,
cells at the edge of the tumor may survival sub-lethal injury
(14). In addition, tumor
perfusion and blood perfusion close to the tumor may affect the
volume and contour of the ablation lesion (13). Therefore, complete ablation is not
always achieved for large or hypervascular tumors, and RFA appears
to require re-ablations to achieve its desired effect.
To solve this problem, the combining of RFA with
various methods to relieve the heat-sink effect have provided
promising results (9,11,24).
These methods include pharmaceutical agents, transcatheter arterial
embolisation (TAE) and transcatheter arterial chemoembolization
(TACE). TAE and TACE have been used prior to RFA, which can lead to
a larger ablation volume and more predictable and reproducible
lesion shape, compared with the lesions created with RFA alone,
particularly in tumors with rich blood flow (9,13).
In addition, the size of the ablation lesion in the absence of
blood perfusion is not affected by the method of flow blockage
(13). However, it is difficult to
completely embolize the tumor-feeding vessels due to the complexity
of the blood supply and variation of blood vessel distribution
(25).
As tumor blood vessels are weaker in structure and
less competent than those in normal tissues (26), there is significant interest in
developing techniques that target this neovasculature in order to
reduce the heat-sink effect. In our previous study, blood perfusion
in human prostate cancer xenografts in nude mice was effectively
arrested for 2 h (unpublished data), which may be used to mitigate
the effect of heat-sink to enhance thermal ablation. In the present
study, as expected, ablation lesion volumes were significantly
larger when RFA was combined with USMB, compared with treatment
with RFA alone, although the differences were only significant 1
and 4 days post-treatment. As for tumor volume, the volumes in the
USMB+RFA group were smaller than those in the RFA group, although
the differences were only significant 4 and 7 days post-treatment.
Therefore, the ablation rates in the USMB+RFA group were
significantly higher than those in the RFA group throughout the
experiment.
The mechanism underlying the cessation of tumor
perfusion are predominantly vessel rupture, intravascular
thrombosis and oppression of surrounding edema. Tumor blood
perfusion can be blocked to different degrees using high intensity
focused US (HIFU) and low-frequency US (17,27,28).
With the presence of MBs, this vascular disruption effect can be
operated at low acoustic intensity, and overcomes the thermal
adverse effects of HIFU ablation (19). The antivascular effect is promising
and reproducible. It has been demonstrated that thermal, mechanical
(cavitation and other nonlinear mechanisms) and sonochemical
effects are likely to contribute to the antivascular activities
(26). These acoustic mechanisms
may act either directly damage vascular structure or indirectly by
inducing tissue response to the thermal, mechanical or
sono-chemical effects (26).
Inertial cavitation and its corresponding effects are considered to
be the dominant mechanisms underlying the microvessels damage
(17,29). Acoustic cavitation is defined as
the formation, growth, oscillation and collapse of bubbles under
the effect of an ultrasonic wave in a liquid medium (30). Acoustic cavitation is divided into
stable and transient cavitation. Bubbles pulsating over numerous
acoustic propagation cycles without collapse is stable cavitation,
whereas transient cavitation is intense and fast bubble expansion
over several acoustic wave cycles, leading to the eventual collapse
into smaller bubbles (31,32). In US, when bubbles pass through
blood vessels, they growth and contract in response to acoustic
waves in accordance with the changing acoustic pressure over time,
and these volumetric oscillations are important in therapeutic
action (33). In stable
cavitation, the oscillation of the bubbles can trigger the
surrounding fluid to flow close to the pulsating bubbles, termed
microstreaming (34).
Microstreaming imposes a large shear stress on nearby vascular
epithelial cells, and at high enough stresses, these endothelial
cells eventually detach from the vessel wall (35). In addition, in stable cavitation,
radiation forces can push bubbles in the direction of wave
propagation, which can exert an effect on the endothelium (36). Above a particular threshold, in the
transient cavitation stage, the bubble oscillation becomes so
poweful that the bubbles eventually collapse, inducing local high
temperatures and high pressures, and producing free radicals in the
surrounding blood (37). These
effects result in a shock wave. If the bubbles collapse close to a
blood vessel wall, the shock wave may create liquid jets that
impinge upon the vessel, creating poking holes on the vessel wall
(38). Therefore, the various
expansion, oscillation and collapse of the bubbles and the
resultant microsteaming, shock wave and fluid jets result in
microvessels distention, invagination and ultimately vessel rapture
(29,38–41).
The necessity of increasing ablation volume is also
induced by incomplete thermal ablation and may stimulate the
proliferation of residual tumor cells (14). Several studies have reported that
residual tumor cells following RFA may become more aggressive
(14,42,43).
In the present study, the proliferation-promoting effect of RFA was
elucidated by the tumor volume and histopathological findings,
compared with the control group. At 4 days post-treatment, the
tumor volumes in the RFA group were larger than those in the
control. There were fewer apoptotic cells and more proliferative
cells in the RFA group, compared with the control group 1, 4 and 7
days post-treatment. This hyperproliferation ma have been caused by
increased hypoxia, cytokines, heat-shock proteins and influx of
inflammatory cells at areas surrounding the ablated region
(14,44). In particular, hypoxia in residual
tumor tissues appears to be an important factor in
over-proliferation (43). Hypoxic
stress caused by RFA is less severe and prolonged, and residual
tumour cells tolerate this injury, as it leads to the activation of
cell signal transduction, which allow cells to undergo adaptive
changes promoting survival (43,44).
In the USMB+RFA group, fewer apoptotic cells were observed,
compared with the control, however, more apoptotic cells were
observed, compared with the RFA group 1 day following treatment. In
addition, there was more proliferative cells in the USMB+RFA group,
compared with the control, but fewer proliferative cells, compared
with the RFA group at 4 and 7 days post-treatment. These results
demonstrated that, although USMB+RFA reduced apoptosis and
stimulated proliferation in the residual carcinoma cells, it
assisted in reducing the degree of the proliferation-promoting
effect in RFA. The present study hypothesized that the combination
of USMB and RFA induced more lethal effect on the cells prior to
their initiation of adaptive changes, therefore, the number of
viable tumor cells in a hypoxic microenvironment decreased. In
addition, USMB itself has the capacity to promote apoptosis, and
inhibit proliferation and angiogenesis in tumors (18,19).
In the present study, the MVD in the RFA group was higher than that
in the USMB+RFA group at the three time-points, although without
statistical significance. The reason for this may be that tumor
angiogenesis requires longer than the 7 days following treatment.
As hyperproliferation following incomplete RFA can facilitate the
recurrence of tumors, USMB prior to RFA offers promise for use in
clinical practice.
The results of the present study suggested that the
benefit of combination therapy may be due to two aspects. USMB
prior to RFA may increase tumor ablation volume and ablation rate
by decreasing tumor blood flow and the vascular-mediated tissue
cooling effect. Secondly, USMB prior to RFA increases AI and reduce
PI in the residual carcinoma cells induced by RFA. Therefore, the
combined therapy is beneficial, compared with RFA alone, due to a
synergistic effect of the two types of therapy.
However, there were a number of limitations to the
present study. Firstly, the human prostate tumors were grown in
nude mice subcutaneously, however, the microenvironments within and
surrounding the tumors are different from those grown in clinical
cases. However, prostate cancer cells can only be grown in nude
mice in animals, and it enables accurate surgical removal of tumors
inoculated subcutaneously. Secondly, the present study used a
survival end point of only 7 days. Whether the therapeutic effects
change in the long term, and the effects on long-term survival
rates remain to be elucidated, but are difficult to determine in
this delicate model.
In conclusion, the present study demonstrated that
USMB prior to RFA produced larger volumes of ablation than RFA
alone, and increased AI and reduced PI in the residual carcinoma
cells induced by RFA. USMB prior to RFA is likely to have
significance in clinical application as a standard treatment in the
future, not only for prostate cancer, but for various types of
cancer, including hepatocellular carcinoma and renal carcinoma,
particularly for large, hypervascular tumors.
Acknowledgments
The authors would like to thank RunKun
Pharmaceutical Co. for providing the perfluoropropane-albumin
microsphere injection. The authors would also like to thank Star
Med Co., Ltd. for providing the RF generator and electrode. This
study was supported by the National Natural Science Foundation of
China (grant nos. 81271597 and 81401421).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fung-Kee-Fung SD, Porten SP, Meng MV and
Kuettel M: The role of active surveillance in the management of
prostate cancer. J Natl Compr Canc Netw. 11:183–187.
2013.PubMed/NCBI
|
|
3
|
Goldberg AA, Titorenko VI, Beach A and
Sanderson JT: Bile acids induce apoptosis selectively in
androgen-dependent and -independent prostate cancer cells. PeerJ.
1:e1222013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pienta KJ and Smith DC: Advances in
prostate cancer chemotherapy: A new era begins. CA Cancer J Clin.
55:300–318; quiz 323–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Penson DF: Quality of life after therapy
for localized prostate cancer. Cancer J. 13:318–326. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bul M, Zhu X, Valdagni R, Pickles T,
Kakehi Y, Rannikko A, Bjartell A, van der Schoot DK, Cornel EB,
Conti GN, et al: Active surveillance for low-risk prostate cancer
worldwide: the PRIAS study. Eur Urol. 63:597–603. 2013. View Article : Google Scholar
|
|
7
|
Bozzini G, Colin P, Nevoux P, Villers A,
Mordon S and Betrouni N: Focal therapy of prostate cancer: Energies
and procedures. Urol Oncol. 31:155–167. 2013. View Article : Google Scholar
|
|
8
|
Djavan B, Zlotta AR, Susani M, Heinz G,
Shariat S, Silverman DE, Schulman CC and Marberger M: Transperineal
radiofrequency interstitial tumor ablation of the prostate:
Correlation of magnetic resonance imaging with histopathologic
examination. Urology. 50:986–992. 1997. View Article : Google Scholar
|
|
9
|
Yoon SK, Choi JC, Cho JH, Oh JY, Nam KJ,
Jung SI, Kwon HC, Kim DC and Rha SH: Radiofrequency ablation of
renal VX2 tumors with and without renal artery occlusion in a
rabbit model: Feasibility, therapeutic efficacy and safety.
Cardiovasc Intervent Radiol. 32:1241–1246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morimoto M, Sugimori K, Shirato K, Kokawa
A, Tomita N, Saito T, Tanaka N, Nozawa A, Hara M, Sekihara H, et
al: Treatment of hepatocellular carcinoma with radiofrequency
ablation: Radiologic-histologic correlation during follow-up
periods. Hepatology. 35:1467–1475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mostafa EM, Ganguli S, Faintuch S, Mertyna
P and Goldberg SN: Optimal strategies for combining transcatheter
arterial chemoembolization and radiofrequency ablation in rabbit
VX2 hepatic tumors. J Vasc Interv Radiol. 19:1740–1748. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee IJ, Kim YI, Kim KW, Kim DH, Ryoo I,
Lee MW and Chung JW: Radiofrequency ablation combined with
transcatheter arterial embolisation in rabbit liver: investigation
of the ablation zone according to the time interval between the two
therapies. Br J Radiol. 85:e987–e994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang I, Mikityansky I, Wray-Cahen D,
Pritchard WF, Karanian JW and Wood BJ: Effects of perfusion on
radiofrequency ablation in swine kidneys. Radiology. 231:500–505.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kroeze SG, van Melick HH, Nijkamp MW,
Kruse FK, Kruijssen LW, van Diest PJ, Bosch JL and Jans JJ:
Incomplete thermal ablation stimulates proliferation of residual
renal carcinoma cells in a translational murine model. BJU Int.
110:E281–E286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang JH, Brayman AA, Reidy MA, Matula TJ,
Kimmey MB and Crum LA: Vascular effects induced by combined 1-MHz
ultrasound and microbubble contrast agent treatments in vivo.
Ultrasound Med Biol. 31:553–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wood AK, Bunte RM, Price HE, Deitz MS,
Tsai JH, Lee WM and Sehgal CM: The disruption of murine tumor
neovasculature by low-intensity ultrasound-comparison between 1-
and 3-MHz sonication frequencies. Acad Radiol. 15:1133–1141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Gao S, Zhao Y, Li P, Liu J, Li P,
Tan K and Xie F: Disruption of tumor neovasculature by microbubble
enhanced ultrasound: A potential new physical therapy of
anti-angiogenesis. Ultrasound Med Biol. 38:253–261. 2012.
View Article : Google Scholar
|
|
18
|
Shen ZY, Shen E, Diao XH, Bai WK, Zeng MX,
Luan YY, Nan SL, Lin YD, Wei C, Chen L, et al: Inhibitory effects
of subcutaneous tumors in nude mice mediated by low-frequency
ultrasound and microbubbles. Oncol Lett. 7:1385–1390.
2014.PubMed/NCBI
|
|
19
|
Wang Y, Hu B, Diao X and Zhang J:
Antitumor effect of micro-bubbles enhanced by low frequency
ultrasound cavitation on prostate carcinoma xenografts in nude
mice. Exp Ther Med. 3:187–191. 2012.PubMed/NCBI
|
|
20
|
Yang Y, Bai W, Chen Y, Nan S, Lin Y and Hu
B: Low-frequency low-intensity ultrasound mediated microvessel
disruption combined with docetaxel to treat prostate carcinoma
xenografts in nude mice: A new type of chemoembolization. Oncol
Lett. In press.
|
|
21
|
Yang Y, Bai W, Chen Y, Lin Y and Hu B:
Optimization of low-frequency low-intensity ultrasound mediated
microvessel disruption on prostate cancer xenografts in nude mice
by orthogonal experimental design. Oncol Lett. In press.
|
|
22
|
Djavan B, Zlotta AR, Susani M, Heinz G,
Shariat S, Silverman DE, Schulman CC and Marberger M: Transperineal
radiofrequency interstitial tumor ablation of the prostate:
correlation of magnetic resonance imaging with histopathologic
examination. Urology. 50:986–983. 1997. View Article : Google Scholar
|
|
23
|
McGahan JP, Griffey SM, Budenz RW and
Brock JM: Percutaneous ultrasound-guided radiofrequency
electrocautery ablation of prostate tissue in dogs. Acad Radiol.
2:61–65. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakasone Y, Ikeda O, Kawanaka K, Yokoyama
K and Yamashita Y: Radiofrequency ablation in a porcine kidney
model: Effect of occlusion of the arterial blood supply on ablation
temperature, coagulation diameter and histology. Acta Radiol.
53:852–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan X, Zhou G, Zheng C, Liang H, Liang B,
Song S and Feng G: Heat shock protein 70 expression and effect of
combined trans-catheter arterial embolization and radiofrequency
ablation in the rabbit VX2 liver tumour model. Clin Radiol.
69:186–193. 2014. View Article : Google Scholar
|
|
26
|
Levenback BJ, Sehgal CM and Wood AK:
Modeling of thermal effects in antivascular ultrasound therapy. J
Acoust Soc Am. 131:540–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu
H and Wang ZB: Tumor vessel destruction resulting from
high-intensity focused ultrasound in patients with solid
malignancies. Ultrasound Med Biol. 28:535–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wood AK, Ansaloni S, Ziemer LS, Lee WM,
Feldman MD and Sehgal CM: The antivascular action of physiotherapy
ultrasound on murine tumors. Ultrasound Med Biol. 31:1403–1410.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Brayman AA, Bailey MR and Matula
TJ: Blood vessel rupture by cavitation. Urol Res. 38:321–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmadi F, McLoughlin IV, Chauhan S and
ter-Haar G: Bio-effects and safety of low-intensity, low-frequency
ultrasonic exposure. Prog Biophys Mol Biol. 108:119–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polat BE, Hart D, Langer R and
Blankschtein D: Ultrasound-mediated transdermal drug delivery:
Mechanisms, scope and emerging trends. J Control Release.
152:330–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carvell KJ and Bigelow TA: Dependence of
optimal seed bubble size on pressure amplitude at therapeutic
pressure levels. Ultrasonics. 51:115–122. 2011. View Article : Google Scholar
|
|
33
|
Stride EP and Coussios CC: Cavitation and
contrast: The use of bubbles in ultrasound imaging and therapy.
Proc Inst Mech Eng H. 224:171–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hosseinkhah N and Hynynen K: A
three-dimensional model of an ultrasound contrast agent gas bubble
and its mechanical effects on microvessels. Phys Med Biol.
57:785–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
VanBavel E: Effects of shear stress on
endothelial cells: Possible relevance for ultrasound applications.
Prog Biophys Mol Biol. 93:374–383. 2007. View Article : Google Scholar
|
|
36
|
Dayton P, Klibanov A, Brandenburger G and
Ferrara K: Acoustic radiation force in vivo: A mechanism to assist
targeting of microbubbles. Ultrasound Med Biol. 25:1195–1201. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basta G, Venneri L, Lazzerini G, Pasanisi
E, Pianelli M, Vesentini N, Del Turco S, Kusmic C and Picano E: In
vitro modulation of intracellular oxidative stress of endothelial
cells by diagnostic cardiac ultrasound. Cardiovasc Res. 58:156–161.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Brayman AA, Kreider W, Bailey MR
and Matula TJ: Observations of translation and jetting of
ultrasound-activated microbubbles in mesenteric microvessels.
Ultrasound Med Biol. 37:2139–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coralic V and Colonius T: Shock-induced
collapse of a bubble inside a deformable vessel. Eur J Mech B
Fluids. 40:64–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H, Kreider W, Brayman AA, Bailey MR
and Matula TJ: Blood vessel deformations on microsecond time scales
by ultrasonic cavitation. Phys Rev Lett. 106:0343012011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao F, Xiong C and Xiong Y: Constrained
oscillation of a bubble subjected to shock wave in microvessel.
Prog Nat Sci. 19:1109–1117. 2009. View Article : Google Scholar
|
|
42
|
Ke S, Ding XM, Kong J, Gao J, Wang SH,
Cheng Y and Sun WB: Low temperature of radiofrequency ablation at
the target sites can facilitate rapid progression of residual
hepatic VX2 carcinoma. J Transl Med. 8:732010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nijkamp MW, van der Bilt JD, de Bruijn MT,
Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O and Borel Rinkes
IH: Accelerated perinecrotic outgrowth of colorectal liver
metastases following radiofrequency ablation is a hypoxia-driven
phenomenon. Ann Surg. 249:814–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|