Introduction
Nasopharyngeal carcinoma (NPC) is the most common
type of nasopharyngeal malignant tumor in men and women in southern
China, with an incidence rate of 15–50/100,000 (1–4).
Etiological investigations indicated that the genesis of NPC is
highly complex, and that the Epstein-Barr virus as well as
environmental and genetic factors are correlated with the
development of NPC (5–7). Furthermore, patients present with
only few obvious symptoms in the early stage of NPC, and ~70% NPC
patients present with a locally advanced stage at the time of final
diagnosis (1,8,9). In
addition, it is known that NPC bears a substantial risk of invasion
and metastasis, which remain the leading cause of NPC-associated
mortality. Although concurrent chemotherapy and radiotherapy for
the treatment of NPC has improved the overall survival rate, local
relapse post-treatment and distant metastasis are common, to which
NPC patients eventually succumb (10–12).
Thus, there is an urgent requirement for effective and reliable
therapeutic methods to cure NPC.
High-mobility group box 1 (HMGB1) belongs to the
HMGB protein family, is associated with various biological
processes and has important roles in inflammation, immunity, cell
proliferation, cell migration, cell death, wound healing and
progression of malignant tumors (13,14).
Previous studies have revealed that HMGB1 is commonly overexpressed
in malignant tumor cells and that knockdown of HMGB1 markedly
inhibited cancer cell proliferation, invasion and metastasis
(14–16). In addition, a preliminary screening
study by our group found that HMGB1 is overexpressed in the HONE-1
NPC cell line, rendering it an ideal cell line for studies on the
roles of HMGB1 in NPC. The aim of the present study was to
determine the effects of HMGB1 knockdown on the proliferation,
migration and invasion of the HONE-1 cell line in order to explore
its suitability as a therapeutic target for treating NPC.
Materials and methods
Reagents and cell lines
The HONE-1 and 293T cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
Gibco-BRL (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA)
and fetal bovine serum (FBS) was purchased from Sijiqing Biotech.
Co. (Hangzhou, China). TRIzol reagent was purchased from Invitrogen
Life Technologies, Inc. and a Cell Counting kit-8 (CCK-8) was
purchased from Dojindo Biochem (Shanghai, China). An Annexin
V/fluorescein isothiocyanate (FITC) kit and Matrigel were purchased
from BD Biosciences (Franklin Lakes, NJ, USA). Crystal violet,
Giemsa, trypsinase, acrylamide, SDS and phosphate-buffered saline
(PBS) were purchased from JRDun Biotech (Shanghai, China).
Transwell well culture chambers were purchased from Corning
(Corning, NY, USA). A bicinchoninic acid (BCA) protein assay kit
was purchased from Thermo Fisher Scientific (Waltham, MA, USA) and
nitrocellulose filter membranes were purchased from Millipore
(Billerica, MA, USA). Antibodies against caspase-3 (cat. no.
Ab32351; rabbit monoclonal; 1:1,000), HMGB1 (cat. no. Ab79823;
rabbit monoclonal; 1:800) and advanced glycation end products
(RAGE; cat. no. Ab3611; rabbit polyclonal; 1:1,000) were purchased
from Abcam (Cambridge, MA, USA); monoclonal antibodies against
extracellular signal-regulated kinase (ERK)1/2 (cat. no. 9102;
rabbit polyclonal; 1:1,000), phosphorylated (p)-ERK1/2 (cat. no.
4376; rabbit IgG; 1:1,000) and GAPDH (cat. no. 5174; rabbit
monoclonal; 1:2,000) were purchased from CST Biotech (Shanghai,
China); monoclonal antibodies against B-cell lymphoma 2 (Bcl-2;
cat. no. Sc-492; rabbit polyclonal; 1:400) and Bcl-2-associated X
protein (Bax; cat. no. Sc-493; rabbit IgG; 1:500) were purchased
from Santa Cruz Biotechnology, Inc (Dallas, TX, USA).
Goat-anti-rabbit/rat horseradish peroxidase-conjugated secondary
antibodies were purchased from Beyotime Institute of Biotechnology
(Haimen, China).
Cell culture
HONE-1 cells were cultured in DMEM containing 15%
(v/v) heat-inactivated FBS, 100 mg/ml streptomycin (Corning
Incorporated, Corning, NY, USA) and 100 U/ml penicillin (Corning
Incorporated) at 37°C in a humidified atmosphere containing 5%
CO2.
Plasmid construction and transient
transfection
HMGB1-specific small interfering (si)RNA
overexpression vector was constructed for RNA interference as
previously described (17). The
resulting recombinant lentiviruses were transfected into HONE-1
cells to inhibit the expression of HMGB1. The siRNA target sequence
was as follows: 5′-TGGTGATGTTGCGAAGAAA-3′. HMGB1 expression in
untreated HONE-1 cells, cells treated with control vector (Mock
group), and HONE-1 cells with HMGB1 knockdown (HMGB1-KD) was
detected using real-time fluorogenic reverse-transcription
quantitative polymerase chain reaction (RT-qPCR) and western blot
analysis.
Real-time fluorogenic RT-qPCR analysis of
HMGB1
HONE-1 cells were harvested and total RNA was
extracted using TRIzol according to the manufacturer's
instructions. Total RNA was used for cDNA synthesis of HMGB1 and
GAPDH by reverse transcription using an ABI-7300 real-time PCR
apparatus (Applied Biosystems, Thermo Fisher Scientific). All mRNA
primers were designed using Primer Premier 5.0 software (Premier
Biosoft, Palo Alto, CA, USA) and synthesized by JRDun Biotech.
Primers used in for PCR are listed in Table I. qPCR was performed according to
the manufacturer's instructions of the quantitative real-time
RT-PCR reaction kit (SYBR Green; Thermo Fisher Scientific).
Thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec, and 60°C for 45 sec. Gene
expression levels were quantified using the ΔΔCt method.
 | Table IPrimers used for quantitative
polymerase chain reaction analysis. |
Table I
Primers used for quantitative
polymerase chain reaction analysis.
| Gene | Primer sequence | Size (bp) |
|---|
| HMGB1 | F:
5′-TGATGTTGCGAAGAAACTG-3′ | 134 |
| R:
5′-GCTTTCCTTTAGCTCGATATG-3′ | |
| GAPDH | F:
5′-CACCCACTCCTCCACCTTTG-3′ | 110 |
| R:
5′-CCACCACCCTGTTGCTGTAG-3′ | |
Western blot analysis
HONE-1 cells were harvested and total protein was
extracted using radioimmunoprecipitation acid lysis buffer (JRDUN
Biotechechnology Co., Ltd., Shanghai, China). The protein
concentration was determined using the BCA protein assay kit
(Thermo Fisher Scientific). Equal amounts of protein (20 µg)
were separated by 10 or 15% SDS/PAGE, electrotransferred onto a
nitrocellulose filter membrane and probed with various primary
antibodies overnight at 4°C, followed by detection with
horseradish-peroxidase-conjugated secondary antibodies and
electrochemiluminescence development (Millipore) with X-ray film
(Kodak, Rochester, NY, USA) exposure. To normalize for protein
loading, antibodies directed against GAPDH were used, and the
proteins expression levels were expressed as a relative value to
that of GAPDH.
CCK-8 assay
Cells (5×103/100 µl) were seeded
in 96-well plates and cultured for 0, 12, 24 or 48 h at 37°C.
Subsequently, 100 µl serum-free DMEM containing 10% CCK-8
reagent (v/v) was added to each well, and cells were cultured for 1
h at 37°C. Finally, optical density values (OD) were determined at
450 nm using a 96-well plate reader (Multiskan MK3; Thermo Fisher
Scientific) (18).
Flow cytometric apoptosis assay
HONE-1 cells (5×104 per group treated as
above) were harvested, washed with PBS and stained using the
Annexin V/FITC kit according to the manufacturer's instructions.
Apoptosis was detected using a FACScalibur flow cytometer (BD
Biosciences). The percentage of cells in early apoptosis was the
population of Annexin V-positive and PI-negative cells, while the
percentage of cells in late apoptosis was resembled by Annexin
V-positive and PI-positive cells (19).
Wound-healing assay
A wound-healing assay was performed according to a
previously reported method with minor modifications (20,21).
HONE-1 cells were treated with control lentivirus (Mock group) or
HMGB1 siRNA lentivirus (HMGB1-KD), and seeded onto
35-mm2 petri dishes, and a sterile pipette (200
µl) was used to scratch the cell monolayer to generate a
line-shaped wound. Cells were washed once with cold PBS, and
incubated for 48 h. Migration of the cells was evaluated at 0, 24,
48 and 72 h.
Cell adhesion assay
A cell adhesion assay was performed in 12-well
plates according to a previous method with minor modification
(22,23). The wells were pre-coated with
fibronectin (BD Biosciences, Franklin Lakes, NJ, USA) overnight at
room temperature. HONE-1 cells were harvested and re-suspended in
DMEM containing 10% FBS. Subsequently 1×105 cells were
seeded into each well and incubated at 37°C for 1 h. The wells were
washed twice with warm PBS to remove the unattached cells, and the
attached cells were then stained with Giemsa for 10 min. The cells
were observed by using an optical Olympus IX50 microscope (Olympus,
Tokyo, Japan).
In vitro invasion assay
The invasive abilities of HONE-1 cells were
evaluated according to a previously described method with minor
modifications (24). Cell invasion
assays were performed using Transwell culture chambers, which were
pre-coated with 80 ml Matrigel. The coated filters were thoroughly
washed with PBS and dried immediately prior to use. 0.75 ml DMEM
containing 10% FBS was placed in the lower chamber, while 0.5 ml
cell suspension (1×105/ml) in DMEM containing 1% FBS was
placed in the upper chamber followed by incubation for 48 h at 37°C
in an atmosphere containing 5% CO2. The number of the
cells which had transgressed through the Matrigel-coated filter was
evaluated by counting cells stained with 0.5% crystal violet
solution under an optical microscope (Olympus, Tokyo Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation, and statistical analyses were performed via a one-way
analysis of variance followed by post-hoc Dunnett's test. SPSS
software version 18.0 (International Business Machines, Armonk, NY,
USA) was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
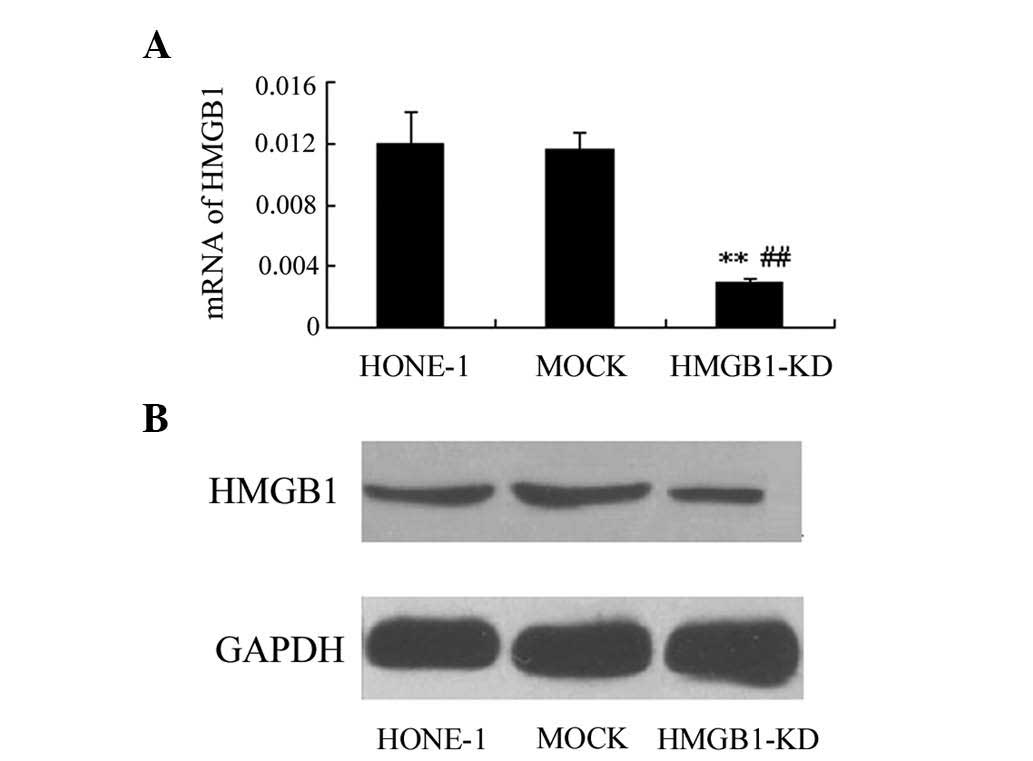
Knockdown of HMGB1 in HONE-1 cells
As shown in Fig.
1A, the expression of HMGB1 was significantly down-regulated in
HONE-1 cells transfected with HMGB1 siRNA expression plasmid,
compared to that in the untreated and mock-transfected groups
(P<0.01). Furthermore, western blot analysis demonstrated that
the protein expression of HMGB1 was obviously decreased after
transfection, which indicated the successful construction of HONE-1
cells with HMGB1 knockdown (Fig.
1B).
HMGB1 knockdown reduces the proliferation
of HONE-1 cells
HONE-1 cell proliferation was detected using the
CCK-8 assay after transfection. As shown in Table II, no significant difference in
proliferation was observed between the untreated and
mock-transfected HONE-1 cells (P>0.05). However, the
proliferation of HONE-1 cells with HMGB1 knockdown was
significantly inhibited at 12, 24 and 48 h (P<0.01), compared to
that of the untreated and mock-transfected HONE-1 cells. Therefore,
the results of the present study indicated that HMGB1 knockdown
significantly inhibited the proliferation of HONE-1 cells.
 | Table IIEffect of HMGB1 knockdown for
different time periods on the proliferation of HONE-1 cells. |
Table II
Effect of HMGB1 knockdown for
different time periods on the proliferation of HONE-1 cells.
| Group | 0 h | 12 h | 24 h | 48 h |
|---|
| HONE-1 | 0.224±0.0042 | 0.304±0.0047 | 0.447±0.0035 | 0.703±0.006 |
| MOCK | 0.220±0.0025 | 0.307±0.0038 | 0.448±0.0032 | 0.700±0.004 |
| HMGB1-KD | 0.209±0.0026 | 0.262±0.0038a,b | 0.346±0.0046a,b | 0.451±0.006a,b |
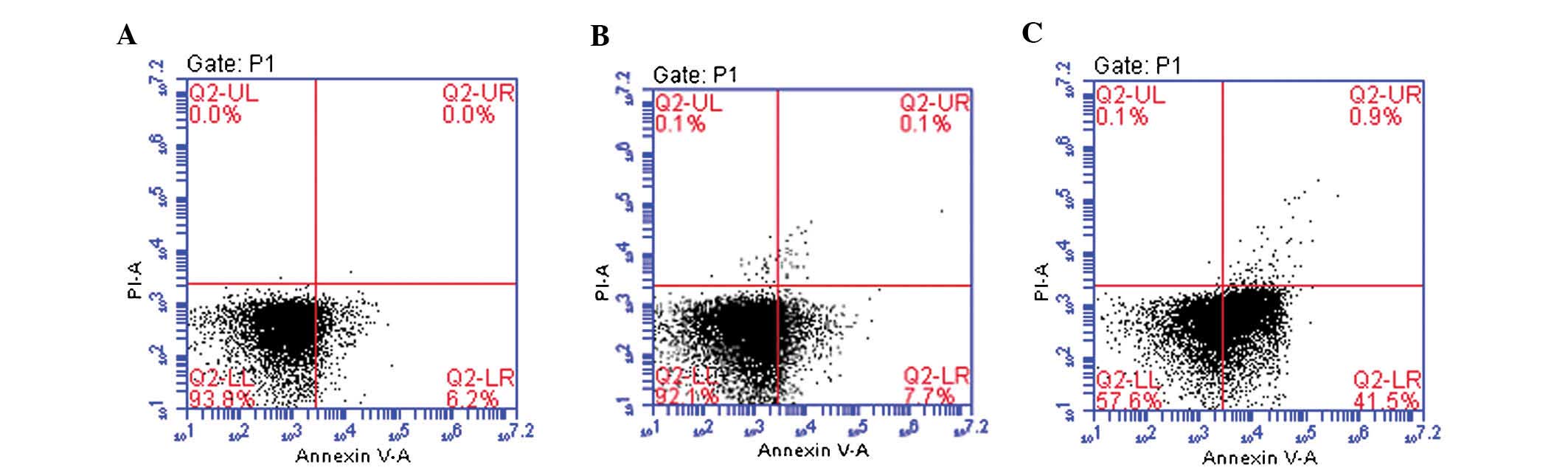
HMGB1 knockdown induces apoptosis of
HONE-1 cells
To assess whether the anti-proliferative effects of
HMGB1 knockdown on HONE-1 cells were due to induction of apoptosis,
flow cytometric analysis following staining with PI and Annexin
V-FITC was performed. As shown in Fig.
2, the apoptotic rate in the HMGB1-knockdown group was 42.4%,
which was significantly higher than that in untreated (6.2%) and in
the mock-transfected HONE-1 cells (7.8%). This result revealed that
HMGB1 knockdown significantly induced apoptosis in HONE-1
cells.
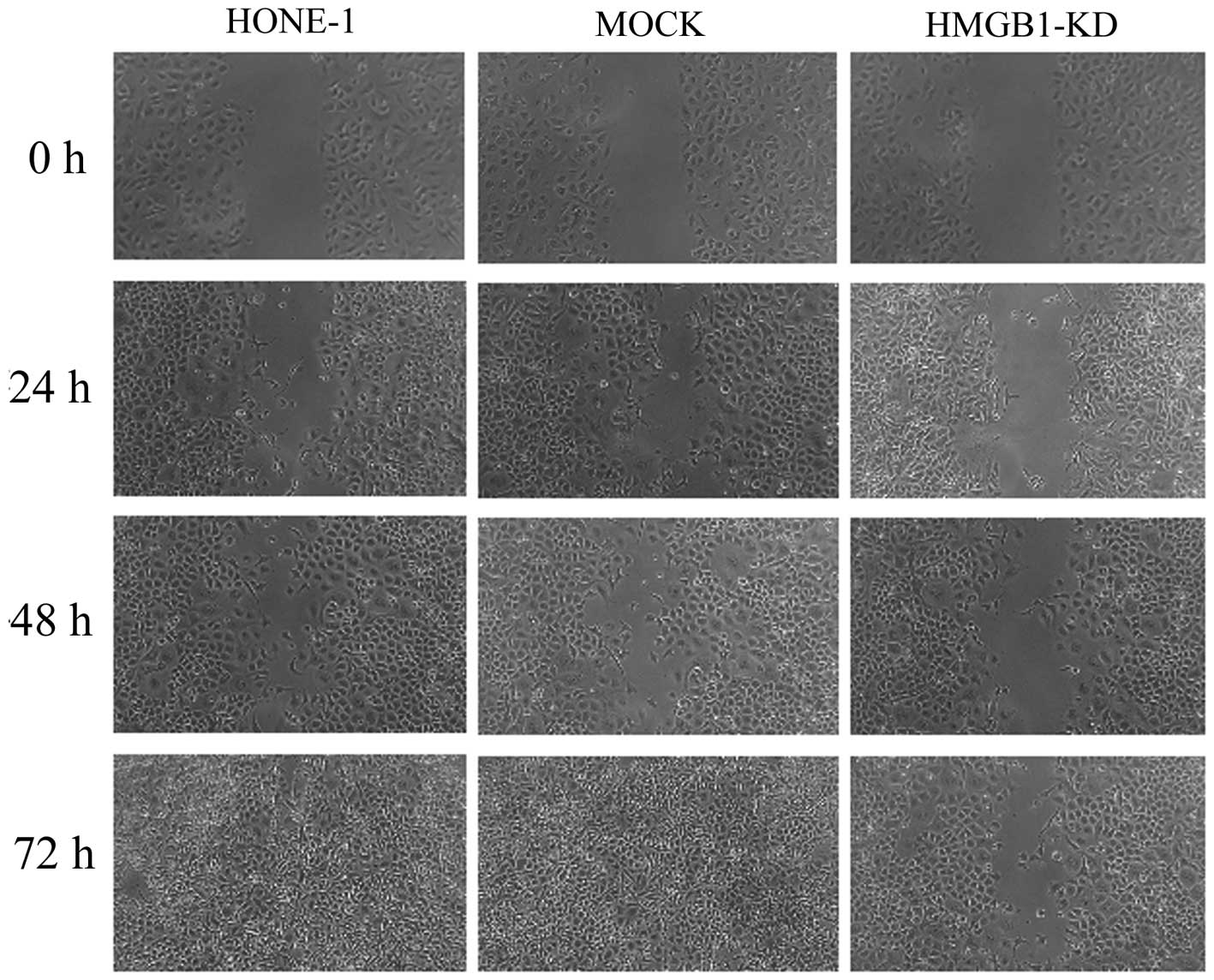
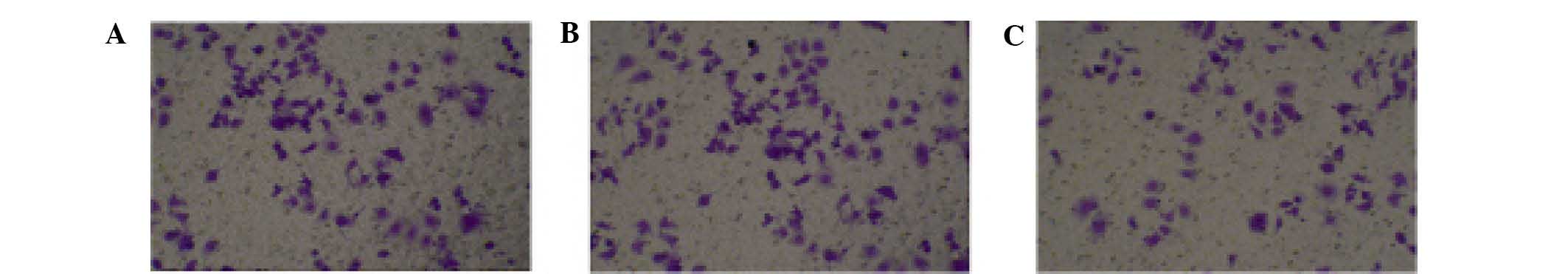
HMGB1 knockdown reduces the migratory,
invasive and adhesive capacities of HONE-1 cells
As demonstrated by the wound healing assay, HMGB1
knockdown effectively inhibited the migration of HONE-1 cells at
24, 48 and 72 h (Fig. 3). In the
untreated and mock-transfected HONE-1 cells, the wounds were
obviously smaller than those in the HMGB1 knockdown group at all of
the observed time-points and, by contrast to the HMGB1 knockdown
group, the wounds had disappeared in the untreated and
mock-transfected groups after 72 h. Furthermore, as indicated by
the Transwell assay, HMGB1 knockdown effectively inhibited the
invasion of HONE-1 cells (Fig. 4).
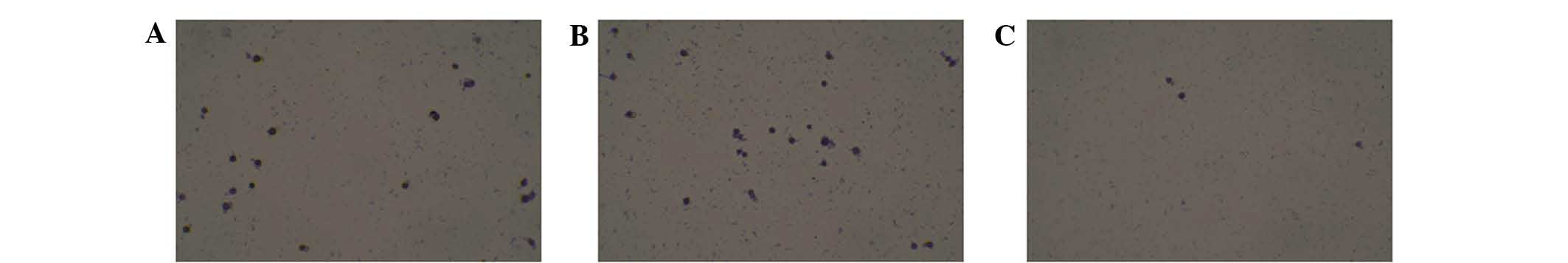
As shown in Fig. 5, there was no
obvious difference in cell adhesion between the untreated and
mock-transfected HONE-1 cells. However, in the HMGB1 knockdown
group, cell adhesion was significantly inhibited compared to that
in the untreated and mock-transfected HONE-1 cells. The combined
results of the wound healing, Transwell invasion and cell adhesion
assay indicated that HMGB1 knockdown significantly suppressed the
metastasis-forming capacity of HONE-1 cells.
HMGB1 knockdown enhances apoptosis
signaling in HONE-1 cells
The results of the present study indicated that
HMGB1 knockdown inhibited the proliferation, migration and invasion
of HONE-1 cells, while enhancing their apoptotic rate. To
investigate the possible underlying mechanisms, the expression of
apoptosis-associated signaling and effector proteins was determined
by western blot analysis. As shown in Fig. 6, there was no obviously difference
in the expression of Bcl-2, Bax, cleaved caspase-3, RAGE, p-ERK1/2
and ERK1/2 between the untreated and mock-transfected HONE-1 cells.
Western blotting was repeated three times and similar results were
obtained. However, in the HMGB1-knockdown group, the expression of
Bcl-2, RAGE and p-ERK1/2 was down-regulated, whereas the expression
of caspase-3 and Bax was obviously upregulated compared to that in
the untreated and mock-transfected HONE-1 cells. These results
indicated that the mechanisms of the inhibitory effects of HMGB1
knockdown on HONE-1 cells may be associated with the induction of
apoptosis via the HMGB1/RAGE pathway.
Discussion
To the best of our knowledge, the present study was
the first to indicate that HMGB1 knockdown inhibits the
proliferation, migration and invasion of HONE-1 human
nasopharyngeal carcinoma cells. In addition, the present study also
suggested that the underlying mechanisms of these inhibitory
effects may be mediated via induction of apoptosis and the
HMGB1/RAGE pathway. Previous studies have demonstrated that HMGB1
is able to promote tumor growth, migration and invasion, and that
overexpression of HMGB1 is commonly observed in various types of
malignant tumor tissue (13,15).
Therefore, downregulation of HMGB1 may be an obvious and feasible
approach for the treatment of cancer, including NPC. Lentiviral
vectors, which are among the most commonly used vectors in gene
therapy, are able to effectively transfect cells for the
establishment of stably transfected cell lines (25). In the present study, HMGB1
knockdown was successfully performed in HONE-1 cells by using the
lentiviral transfection method.
In the present study, in order to determine the
proliferation of HONE-1 cells, a CCK-8 assay was performed. The
CCK-8 assay is currently considered to be an advantageous
alternative to the MTT assay. The results of the present study
indicated that HMGB1 knockdown significantly inhibited the
proliferation of HONE-1 cells (18). Furthermore, flow cytometric
analysis demonstrated that the induction of apoptosis may be the
underlying reason for the anti-proliferative effects of HMGB1
knockdown on HONE-1 cells. A previous study reported that
overexpression of HMGB1 suppressed apoptosis of cancer cells via
inhibiting the activation of caspase-3 and -9 proteins (26). It is well known that caspase
proteins, cysteine-aspartic acid proteases, are the crucial
executioners of apoptosis. Caspase-3, one of the key cell death
proteases, is one of the most commonly activated cysteine protease
in the early stage of apoptosis, and is also considered as a marker
for cells undergoing apoptosis (27). Furthermore, Bcl-2 family proteins
also serve important roles in regulating intrinsic apoptosis
mediated by mitochondria and governing outer mitochondrial membrane
permeability and release of cytochrome C (28,29).
Bcl-2 is a well-known anti-apoptotic protein, whereas Bax is a
pro-apoptotic protein, with the ratio of Bcl-2 to Bax having a
crucial role in apoptosis induction (30). It has been reported that HMGB1 was
able to inhibit of Bax and suppress apoptosis induced by Bax in
mammalian cells (31). The present
study demonstrated that HMGB1 knockdown was able to downregulate
the expression of Bcl-2, while upregulating the expression of
caspase-3 and Bax. These results lead to the conclusion that HMGB1
knockdown promotes the induction of mitochondria-mediated
apoptosis.
In the early stages of tumor metastasis formation,
tumor cell invasion and reduction of the extracellular matrix (ECM)
are crucial steps, while tumor cell adhesion is another key stage
in metastasis formation (32). The
present study demonstrated that HMGB1 knockdown markedly inhibited
the invasion of HONE-1 cells in vitro; in addition, it was
demonstrated that HMGB1 knockdown obviously suppressed the adhesion
of HONE-1 cells to fibronectin. HMGB1 and RAGE can promote cell
proliferation as well as migration of malignant tumors (15). In addition, the proteolytic
degradation of the ECM is a critical step during tumor invasion,
and the activation of HMGB1/RAGE was shown to increase fibrinolysin
(24,33). Previous studies further indicated
that HMGB1 can bind to RAGE in the cell membrane and then induce
the activation of endocellular RAGE via through activation of ERK;
in addition, the HMGB1/RAGE pathway stimulates bioenergetics in a
p-ERK1/2-dependent process to sustain the enhanced tumor cell
growth and the resulting increased metabolic requirement of the
tumor (15,34,35).
The results of the present study showed that HMGB1 knockdown
downregulate the expression of RAGE and p-ERK1/2, which indicated
that HMGB1 knockdown inhibited the activation of HMGB1/RAGE
pathways.
In conclusion, the present study demonstrated that
HMGB1 knockdown suppressed the proliferation, migration and
invasion of the HONE-1 human nasopharyngeal carcinoma cell line,
and the possible underlying mechanisms may involve the induction of
mitochondria-mediated apoptosis and inhibition of HMGB1/RAGE
pathways.
References
|
1
|
Pan WL, Wong JH, Fang EF, Chan YS, Ng TB
and Cheung RC: Preferential cytotoxicity of the type I ribosome
inactivating protein alpha-momorcharin on human nasopharyngeal
carcinoma cells under normoxia and hypoxia. Biochem Pharmacol.
89:329–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: Molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie SH, Yu IT, Tse LA, Mang OW and Yue L:
Sex difference in the incidence of nasopharyngeal carcinoma in Hong
Kong 1983–2008: Suggestion of a potential protective role of
oestrogen. Eur J Cancer. 49:150–155. 2013. View Article : Google Scholar
|
|
4
|
Zeng J, Dai P, Ren L, Song B, Chen X, Wang
X, Wang J, Zhang T and Zhu W: Apoptosis-induced anti-tumor effect
of Curcuma kwangsiensis polysaccharides against human
nasopharyngeal carcinoma cells. Carbohydr Polym. 89:1067–1072.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hildesheim A and Levine PH: Etiology of
nasopharyngeal carcinoma: A review. Epidemiol Rev. 15:466–485.
1993.PubMed/NCBI
|
|
6
|
Jia WH and Qin HD: Non-viral environmental
risk factors for nasopharyngeal carcinoma: A systematic review.
Semin Cancer Biol. 22:117–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Q, Liu C, Yan C, Tao B, Li Z and Cai
Z: 5-aza-CdR induces the demethylation of Syk promoter in
nasopharyngeal carcinoma cell. Gene. 511:224–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsin CH, Wu BC, Chuang CY, Yang SF, Hsieh
YH, Ho HY, Lin HP, Chen MK and Lin CW: Selaginella tamariscina
extract suppresses TPA-induced invasion and metastasis through
inhibition of MMP-9 in human nasopharyngeal carcinoma HONE-1 cells.
BMC Complement Altern Med. 13:2342013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isobe K, Ito H, Shigematsu N, Kawada T,
Yasuda S, Hara R, Machida N, Takano H, Uchida Y, Uno T, et al:
Advanced nasopharyngeal carcinoma treated with chemotherapy and
radiotherapy: Distant metastasis and local recurrence. Int J Oncol.
12:1183–1187. 1998.PubMed/NCBI
|
|
10
|
He X, Ye M, Guo X, Pan Z, Zhang Z, He S
and Liu T: Treatment outcome of patients with stages I–II
nasopharyngeal carcinoma after late course accelerated
hyperfractionation radiotherapy alone. Oral Oncol. 48:1058–1063.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wei J, Kanada M, Yan L, Zhang Z,
Watanabe H and Terakaw S: Inhibition of store-operated Ca2+ entry
suppresses EGF-induced migration and eliminates extravasation from
vasculature in nasopharyngeal carcinoma cell. Cancer Lett.
336:390–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko YB, Kim BR, Nam SL, Yang JB, Park SY
and Rho SB: High-mobility group box 1 (HMGB1) protein regulates
tumor-associated cell migration through the interaction with BTB
domain. Cell Signal. 26:777–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang R, Tang D, Schapiro NE, Loux T,
Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT and Zeh HJ:
The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor
growth by regulating mitochondrial bioenergetics. Oncogene.
33:567–577. 2014. View Article : Google Scholar
|
|
16
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo J, Chen L, Luo N, Yang W, Qu X and
Cheng Z: Inhibition of TMEM45A suppresses proliferation, induces
cell cycle arrest and reduces cell invasion in human ovarian cancer
cells. Oncol Rep. 33:3124–3130. 2015.PubMed/NCBI
|
|
18
|
Hua YQ, Ouyang HQ, Chen Z, Meng ZQ, Luo
JM, Lin JH, Zhou ZH, Chen H, Wang K and Liu LM: Promoted cancer
growth by stimulating cell proliferation and decreasing apoptosis
using a lentivirus-based EphB2 RNAi in pancreatic carcinoma CFPAC-1
cells. Biomed Pharmacother. 65:123–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JG, Peng W, Yi J, Wu YB, Chen TQ, Wong
KH and Wu JZ: Chemical composition, antimicrobial activity against
Staphylococcus aureus and apro-apoptotic effect in SGC-7901 of the
essential oil from Toona sinensis (A. Juss) Roem leaves. J
Ethnopharmacol. 154:198–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arranz-Valsero I, Soriano-Romaní L,
García-Posadas L, López-García A and Diebold Y: IL-6 as a corneal
wound healing mediator in an in vitro scratch assay. Exp Eye Res.
125:183–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang XK, Yang YD, Tang SQ, et al:
Inhibitory Effect of Polysaccharides from Scutellaria barbata D.
Don on invasion and metastasis of 95-D cells lines via regulation
of C-MET and E-CAD expressions. Trop J Pharm Res. 12:517–522.
2013.
|
|
22
|
Lii CK, Lei YP, Yao HT, Hsieh YS, Tsai CW,
Liu KL and Chen HW: Chrysanthemum morifolium Ramat. Reduces the
oxidized LDL-induced expression of intercellular adhesion
molecule-1 and E-selectin in human umbilical vein endothelial
cells. J Ethnopharmacol. 128:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Chow VT and Jois SD: A novel, rapid
and sensitive heterotypic cell adhesion assay for CD2-CD58
interaction, and its application for testing inhibitory peptides. J
Immunol Methods. 291:39–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Z and Shulan Z: Inhibition effect of
Guizhi-Fuling-decoction on the invasion of human cervical cancer. J
Ethnopharmacol. 120:25–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubinson DA, Dillon CP, Kwiatkowski AV,
Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus
MT, et al: A lentivirus-based system to functionally silence genes
in primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Völp K, Brezniceanu ML, Bösser S, Brabletz
T, Kirchner T, Göttel D, Joos S and Zörnig M: Increased expression
of high mobility box1 (HMGBl) is associated with an elevated level
of the anti-apoptotic c-IAP2 protein inhuman colon carcinomas. Gut.
55:234–242. 2006. View Article : Google Scholar
|
|
27
|
Zhou S, Zheng T, Chen Y, Zhang J, Li L, Lu
F and Zhu JJ: Toward therapeutic effects evaluation of chronic
myeloid leukemia drug: Electrochemical platform for caspase-3
activity sensing. Biosens Bioelectron. 61:648–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MS, Ha JH, Yoon HS, Lee CK and Chi SW:
Structural basis for the conserved binding mechanism of
MDM2-inhibiting peptides and anti-apoptotic Bcl-2 family proteins.
Biochem Biophys Res Commun. 445:120–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghosh S, Bishayee K, Paul A, Mukherjee A,
Sikdar S, Chakraborty D, Boujedaini N and Khuda-Bukhsh AR:
Homeopathic mother tincture of Phytolacca decandra induces
apoptosis in skin melanoma cells by activating caspase-mediated
signaling via reactive oxygen species elevation. J Integr Med.
11:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family 'killer-proteins' and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Breznieeanu ML, Völp K, Bösser S, Solbach
C, Lichter P, Joos S and Zörnig M: HMGB1 inhibits cell death in
yeast and malnl Tlalian cells and is abundantly expressed in human
breast carcinoma. FASEB J. 17:1295–1297. 2003.
|
|
32
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taguchi A, Blood DC, del Toro G, Canet A,
Lee DC, Qu W, Tanji N, Lu Y, et al: Blockade of RAGE-amphoterin
signalling suppresses tumour growth and metastases. Nature.
405:354–360. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arumugam T, Simeone DM, Schmidt AM and
Logsdon CD: S100P stimulates cell proliferation and survival via
receptor for activated glycation end products (RAGE). J Biol Chem.
279:5059–5065. 2004. View Article : Google Scholar
|
|
35
|
Park JS, Arcaroli J, Yum HK, Yang H, Wang
H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ and Abraham
E: Activation of gene expression in human neutrophils by high
mobility group box 1 protein. Am J Physiol Cell Physiol.
284:C870–C879. 2003. View Article : Google Scholar : PubMed/NCBI
|