Introduction
Colon cancer is one of the most common types of
gastrointestinal cancer, endangering human health (1). It was previously reported that
~650,000 individuals worldwide suffer mortality from colon cancer
annually, and the number of diagnoses and mortality continue to
increase annually (2,3). Despite the continual emergence of
early screening methods and novel chemotherapeutic strategies, the
survival rate of patients with colon cancer has failed to markedly
improved over the last 20 years (4). Therefore, an in-depth investigation
into the mechanisms underlying the pathogenesis of colon cancer is
crucial for the identification of effective treatment strategies,
and improvements in the survival rate of patients.
Biglycan is an important member of the small
leucine-rich proteoglycan family, which is expressed on the cell
surface and in the extracellular matrix (5). Previous studies indicated that a lack
of biglycan was associated with reduced bone mass and osteoporosis
(6,7). Previous reports also demonstrated
that the expression of biglycan was increased in multiple types of
tumor tissue, including liver (8),
ovarian (9), endometrial (10), pancreatic (11), gastric (12) and colon cancer (13). These findings suggest an important
role for biglycan in the development of cancer, however, the
underlying mechanism in colon cancer remains to be fully
elucidated.
The present study aimed to investigate the
expression of biglycan in HCT116 colon cancer cells using
biglycan-specific short hairpin (sh)RNA. A stably transfected cell
line was established through G418 screening, and the effects of
biglycan on colon cancer cell proliferation, migration, invasion
and apoptosis were investigated. Furthermore, how biglycan may be a
potential target for colon cancer gene therapy was discussed.
Materials and methods
Cell culture
Human HCT116 colon cancer cells were purchased from
the Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in McCoy's 5A medium
(Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal
bovine serum (FBS; GE Healthcare Life Sciences HyClone
Laboratories, Logan, UT, USA) at 37°C with 5% CO2. The
culture medium was changed every 2–3 days, and the cells were
digested with 0.25% trypsin (Beyotime Institute of Biotechnology,
Haimen, China) for passage when 80% confluence was reached.
Plasmid construction
The biglycan-specific shRNA plasmid was constructed
using the pGCsi-H1 plasmid (Shanghai GeneChem Co. Ltd.,, Shanghai,
China). The human biglycan mRNA sequence (NM_001711.4) was obtained
from the National Center for Biotechnology Information database
(http://www.ncbi.nlm.nih.gov/nuccore/NM_001711.4). A
total of four biglycan shRNA interference sequences, in addition to
a sequence of an identical length, which was not associated with
the human genome sequences (serving as the negative control), were
generated, according to the principles of shRNA design:
shRNA-biglycan-1,
5′-GATCCCCGATCTCCAAGATCCATGAGTTCAAGAGACTCATGGATCTTGGAGATCTTTTT-3′
(forward); shRNA-biglycan-2,
5′-GATCCCCGCTCTACATCTCCAAGAACTTCAAGAGAGTTCTTGGAGATGTAGAGCTTTTT-3′
(forward); shRNA-biglycan-3,
5′-GATCCCCGCTCAACTACCTGCGCATCTTCAAGAGAGATGCGCAGGTAGTTGAGCTTTTT-3′
(forward); and shRNA-biglycan-4,
5′-GATCCCCATCCAGGCCATCGAACTGGTTCAAGAGACCAGTTCGATGGCCTGGATTTTTT-3′
(forward). The shRNA fragments were subsequently ligated into the
pGCsi-H1 vector between the HindIII and BamHI sites.
The constructs obtained were verified through double restriction
enzyme digestion (Fermentas, Vilnius, Lithuania) and sequencing,
and these were termed shRNA-biglycan 1, 2, 3 and 4, or
shRNA-control.
Cell transfection and stable cell line
screening
The HCT116 cells were seeded into six-well plates at
a density of 1.0×105 cells/well, and were cultured
overnight until 70–80% confluence was reached. The shRNA-biglycan
1, 2, 3 and 4 co nstructs or the shRNA control plasmid were
subsequently transfected into the cells using Lipofectamine™ 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. G418 (Invitrogen Life Technologies)
was added to the culture media at 24 h following transfection to
screen for stably transfected cells. G418-resistant individual
clones were retrieved at 4 weeks post-treatment. Following
confirmation of the expression of biglycan, the selected stable
clones were continuously cultured in G418-containing medium, and
expanded for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen Life Technologies). The RNA
was reverse-transcribed into cDNA using the first-strand cDNA
synthesis kit (Takara Bio, Inc., Dalian, China), according to the
manufacturer's instructions. The primer sequences were as follows:
Biglycan, upstream: 5′-GGTCTCCAGCACCTCTACGCC-3′ and downstream:
5′-AACACTCCCTTGGGCACCTT-3′; β-actin, upstream:
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and downstream:
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. The RT-qPCR reactions were
performed using an Exicycler™ 96 quantitative fluorescence analyzer
(Bioneer Corporation, Daejeon, Korea). The PCR reactions consisted
of an initial step at 95°C for 10 min; 40 cycles of 95°C for 10
sec, 58°C for 20 sec and 72°C for 30 sec; and a final step at 4°C
for 5 min.
Western blotting
The cells were lysed using radioimmunoprecipitation
lysis buffer (Beyotime Institute of Biotechnology), and the protein
concentration of each sample was measured using the bicinchoninic
acid method. Equal quantities of protein (40 µg) were separated by
13% SDS-PAGE (Solarbio Science and Technology Co., Ltd, Beijing,
China) for caspase-3, p21 and p27, or by 10% SDS-PAGE in all other
cases and subsequently transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Bedford, MA, USA). The membrane was
subsequently blocked with 5% non-fat dry milk at room temperature
for 1 h, followed by incubation with the following diluted primary
antibodies: Rabbit polyclonal anti-biglycan antibody (cat no.
sc-33788; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit polyclonal anti-caspase-3 antibody (cat no. wl01992a;
1:1,000), rabbit polyclonal anti-p27 antibody (cat no. wl01769;
1:1,000), rabbit polyclonal anti-cyclin A antibody (cat no.
wl01753; 1:200), rabbit polyclonal anti-p21 antibody (cat no.
wl0362; 1:100), rabbit polyclonal anti-cyclin D1 antibody (cat no.
wl01435a; 1:100) (all from Wanleibio, Shenyang, China), rabbit
polyclonal anti-p38 antibody (cat no. sc-7149; 1:100) and
anti-phosphorylated-p38 antibody (cat no. sc-101758; 1:100) (both
from Abcam, Cambridge, MA, USA) at 4°C overnight. The membrane was
subsequently incubated with horseradish peroxidase (HRP)-conjugated
goat anti-rabbit immunoglobulin G (cat no. A0208; 1:5,000; Beyotime
Institute of Biotechnology) at 37°C for 1 h. β-actin was used as an
internal loading control, for which HRP-conjugated monoclonal mouse
anti-β-actin antibody (cat no. KC-5A08; 1:10,000; Kangchen Biotech,
Shanghai, China) was used. An enhanced chemiluminescence substrate
(7 Sea Pharmtech, Shanghai, China) was added to the membrane in the
dark for image development, and the scanned images were
subsequently analyzed using Image J software (version 1.35;
National Institutes of Health, Bethesda, MD, USA).
Cell counting kit-8 (CCK-8) assay
The cells were counted and seeded into 96-well
plates (2×104 cells/well) and subsequently cultured as
before. Aliquots of 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) were added to the cell cultures at different time
points, and incubated at 37°C for 1 h. The absorbance at 450 nm was
measured for each well using a microplate reader (EL×800; Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Scratch wound assay
The cells of each group were seeded into 6-well
plates (1×105 cells/well). The cells, which had reached
80% confluence, were used for the assay. The culture medium was
discarded and a 200 µl pipette tip was used to perpendicularly
scratch a wound on the surface of the cell monolayer. The cells
were subsequently washed twice with serum-free culture media and
cultured in McCoy's 5A medium, containing 10% FBS. Images of the
cells were captured at 0, 12 and 24 h following the scratching
procedure using a phase-contrast microscope (Motic AE31; Motic
China, Xiamen, China), in order to calculate the distances that the
cells had migrated into the denuded area.
Transwell assay
Transwell chambers (Corning Life Sciences,
Tewksbury, MA, USA) coated with Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) were used for the cell invasion assay. A total of
5×104 cells were collected from each group and
resuspended in 200 µl serum-free cell culture medium. The cells
were subsequently seeded into the upper Transwell chamber, and 800
µl McCoy's 5A culture medium, containing 10% FBS, was added to the
lower chamber. Following a 24 h incubation, the cells in the upper
chamber were removed using a cotton bud, and the cells present on
the lower surface of the insert were fixed and stained with 0.5%
crystal violet dye (Amresco, Inc., Solon, OH, USA). Subsequently,
the cells were washed, images were captured and the number of cells
was quantified under an inverted microscope (Motic AE31; Motic
China).
Flow cytometric analysis
The effect of biglycan on the cell cycle progression
and cell apoptosis associated with colon cancer was measured by
flow cytometry. Briefly, the cells were trypsinized and fixed with
70% pre-cooled ethanol for 2 h, prior to the re-suspension of the
collected cells using staining solution containing 50 mg/ml
propidium iodide (PI) and 1 mg/ml RNAse A (Beyotime Institute of
Biotechnology) in 1 ml sodium citrate buffer. The cells were
subsequently analyzed for cell cycle distribution using a BD
FACSCalibur flow cytometer (BD Biosciences). Cellular apoptosis was
detected using the annexin V-fluorescein isothiocyanate (FITC)/PI
apoptosis detection kit (Nanjing KeyGen Biotech., Co., Ltd.,
Nanjing, China), according to the manufacturer's instructions.
Cells which had reached 80% confluence were treated with trypsin
and collected. Following washing with phosphate-buffered saline,
the cells were resuspended in 500 µl annexin V-binding buffer
(Nanjing KeyGen Biotech). Subsequently, 5 µl FITC-labeled annexin V
and 5 µl PI dye were added the cells, prior to an incubation at
room temperature in the dark for 15 min. The cells were analyzed by
flow cytometry to determine the degree of cellular apoptosis.
Statistical analysis
All experimental data are expressed as the mean ±
standard deviation. One-way analysis of variance was used for
paired comparisons between groups, and the Bonferroni post-hoc test
was used for multiple comparisons. Graphpad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA) was used for the data
analysis and for generating the graphs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of a stable HCT116 cell line
with biglycan silencing
To assess the role of biglycan in colon cancer
cells, biglycan-specific shRNA vectors were initially constructed,
which were subsequently transfected into HCT116 colon cancer cells.
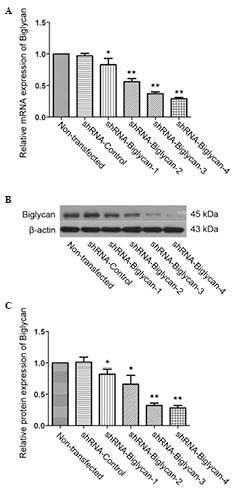
The mRNA expression levels of biglycan in each group of cells was
determined by RT-qPCR and western blot analysis. As shown in
Fig. 1, all the biglycan-specific
shRNA fragments downregulated the mRNA expression level of biglycan
in the transfected cells, compared with the shRNA-control group
(P<0.05 or P<0.01), among which shRNA-biglycan 4 exhibited
the greatest efficiency of interference (the percentage efficiency
values for the downregulation were 71.33±1.53% for biglycan mRNA,
and 72.33±3.51% for biglycan protein levels). On the basis of these
results, the cells which were stably transfected with
shRNA-biglycan 4 were selected for subsequent experiments.
Downregulation of biglycan inhibits the
proliferation of colon cancer cells and causes cell cycle
arrest
The effect of biglycan on the proliferation of colon
cancer cells was assessed using a CCK-8 assay. As shown in Fig. 2A, a significant decrease in cell
viability was observed between 2 and 3 days in the
shRNA-biglycan-transfected HCT116 cells, compared with that in the
shRNA-control-transfected or non-transfected cells (P<0.01). In
addition, the effect of biglycan on the progression of the cell
cycle was also detected by flow cytometry. The results revealed
that the downregulation of biglycan markedly affected the cell
cycle distribution (Fig. 2B and
C). The proportion of the cells in the G0/G1 phase was
significantly increased in the shRNA-biglycan group compared with
the shRNA-control or non-transfected groups (P<0.01), and the
proportion of cells in S phase was decreased in the shRNA-biglycan
group (P<0.01), which indicated that the cell cycle was arrested
in the G0/G1 phase following biglycan silencing. To further confirm
these results, the expression levels of the cell cycle markers,
cyclin A and D1, and the cyclin-dependent kinase inhibitors, p21
and p27, were subsequently determined (14). The results from western blot
analysis revealed that the downregulation of biglycan decreased the
protein expression levels of cyclins A and D1 compared with the
control cells, whereas the expression of p21 and p27 was increased
following biglycan inhibition (P<0.01; Fig. 2D and E). These results suggested
that the inhibition of biglycan expression arrested colon cancer
cells in the G0/G1 phase, and inhibited cell proliferation.
Downregulation of biglycan suppresses the
migratory and invasive properties of colon cancer cells
Previous studies demonstrated that biglycan exerts a
distinct role in different tumor entities (15–17).
To further investigate the underlying mechanism of biglycan in
colon cancer, the effect of biglycan on colon cancer cell migration
and invasion was determined. A scratch wound assay revealed that
biglycan knockdown markedly reduced the spontaneous migration
distance of shRNA-biglycan-transfected HCT116 cells compared with
that of the shRNA-control or non-transfected cells at 24 h after
the scratching of the cell surface (P<0.01; Fig. 3A and B). Additionally, the effect
of biglycan downregulation on the invasive ability of the cells was
determined using a Transwell invasion assay. As shown in Fig. 3C and D, the number of invasive
cells in the shRNA-biglycan group was significantly lower compared
with that in the control group (P<0.01). These results indicated
that the downregulation of biglycan expression inhibited the
migration and invasive capability of the colon cancer cells.
Downregulation of biglycan activates the
p38 signaling pathway and induces apoptosis in colon cancer
cells
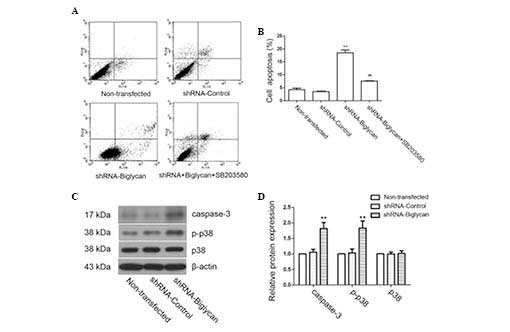
The effect of biglycan on the apoptosis of colon
cancer cells was investigated by flow cytometry. As shown in
Fig. 4A and B, the proportion of
apoptotic cells in the shRNA-biglycan group (18.45±1.09%) was
significantly higher compared with that in the
shRNA-control-transfected group (3.51%±0.16) or the non-transfected
group (4.24±0.61%), indicating that the downregulation of biglycan
induced colon cancer cell apoptosis. Western blot analysis also
revealed that the expression of the proapoptotic caspase-3 protein
was markedly increased following the downregulation of biglycan
(Fig. 4C and D), which was
consistent with the flow cytometry results. Furthermore, a previous
study demonstrated that the p38 signaling pathway has an important
role in cell apoptosis (18). In
the present study, we identified that the p38 signaling pathway was
activated following biglycan knockdown; the addition of the p38
signal inhibitor, SB203580, effectively reversed the increased
apoptotic cell number compared with the shRNA-biglycan group
(7.56±0.18%; P<0.01; Fig. 4A and
B). These results indicated that biglycan may exert a role in
regulating apoptosis in the colon cancer cells, specifically by
inhibiting apoptosis via the regulation of the p38 MAPK signaling
pathway.
Discussion
Previous studies demonstrated that biglycan exerts
an important role in the mechanisms underlying the pathogenesis and
progression of cancer, and abnormal expression levels of biglycan
is usually indicative of a poor prognosis. For example, the
expression levels of biglycan were significantly upregulated in
esophageal squamous cell carcinoma (19), gastric (20), pancreatic (11) and colon (13) cancer; whereas decreased expression
levels of biglycan were identified in human abdominal aortic
aneurysms (21). This body of
evidence indicates that biglycan may exert its distinct properties
in different tumor entities. In the present study, the shRNA
interference-mediated knockdown of biglycan markedly inhibited the
proliferation of colon cancer cells, caused cell cycle arrest at
the G0/G1 phase, suppressed the migratory and invasive properties
of the HCT116 cells, and induced cell apoptosis. These results
indicated that biglycan exerts an important role in the
tumorigenesis and progression of colon cancer.
It is well established that the primary hallmarks of
malignant tumors include uncontrolled tumor cell growth, dispersion
and metastasis. The migration and invasion of the tumor cells is
associated with the aggressive potential of cancer (22). Previous studies demonstrated that
the extracellular matrix and the basement membrane were disrupted
during tumorigenesis and metastasis (23,24).
Biglycan is an important component of the extracellular matrix.
Mounting evidence supports the hypothesis that biglycan fulfils an
oncogenic role in gastric cancer, and gastric cancer cells
exhibited increased motility and invasive properties upon biglycan
overexpression (17). In the
present study, the shRNA-mediated knockdown of biglycan markedly
reduced the spontaneous migration distance and the number of
invasive colon cancer cells. Furthermore, it was observed that the
shRNA-biglycan-transfected HCT116 cells exhibited a decreased rate
of cell growth compared with that of the shRNA-control-transfected
or non-transfected cells. The cell cycle progression analysis
further revealed that the decreased expression of biglycan notably
affected the cell cycle distribution. The proportion of cells in
the G0/G1 phase was significantly increased, and the expression
levels of the cell cycle-associated proteins, cyclins A and D1,
were down-regulated, whereas the levels of their inhibitory
proteins, p21 and p27, were upregulated. These results were
consistent with previously published results, which demonstrated
that biglycan may promote cell proliferation and the migratory
abilities of cells (25,26). Furthermore, these results
indirectly support our previous findings that biglycan
overexpression promoted xenograft colon tumor growth, and that the
upregulation of biglycan was associated with the malignancy in
human colorectal cancer (13,27).
The results of the present study indicated that biglycan has an
oncogenic role in the colon cancer process, which is associated
with regulating the proliferation, migration and invasion of colon
cancer cells.
Deregulated proliferation and inhibition of
apoptosis promotes tumorigenesis. As reported previously, the p38
signaling pathway occupies a central role in the regulation of
tumor cell proliferation and apoptosis (28). Multiple evidence supports the
hypothesis that the p38 signaling pathway is activated in colon
cancer cells and induces apoptosis. Furthermore, the activation of
the p38 signaling pathway by biglycan has been demonstrated in
multiple cell types (29,30). To further investigate the mechanism
of action of biglycan in regulating cell apoptosis, the effect of
the p38 signal inhibitor, SB203580, was assessed in the present
study. The results revealed that the knockdown of biglycan caused a
significant increase in apoptotic cell numbers, in addition to the
activation of the p38 signaling pathway. However, SB203580
effectively reversed the increase induced by biglycan silencing.
These findings indicated that the inhibition of biglycan may induce
cell apoptosis and that biglycan regulates the p38 signaling
pathway by exerting an antiapoptotic effect in tumorigenesis.
In conclusion, the results in the present study
demonstrated that decreased expression levels of biglycan inhibited
cell proliferation, arrested the cell cycle at the G0/G1 phase, and
suppressed the invasion and migration of colon cancer cells.
Furthermore, the shRNA-mediated knockdown of biglycan induced
apoptosis in colon cancer cells, mediated, at least in part, by an
activation of the p38 signaling pathway. Therefore, biglycan may
represent a target for gene therapy in the treatment of colon
cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81201968), the
Natural Science Foundation of Liaoning Province (no. 201102111) and
the Doctoral Starting Foundation of Liaoning Province (no.
20091045).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PloS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wadhwa S, Embree MC, Bi Y and Young MF:
Regulation, regulatory activities and function of biglycan. Crit
Rev Eukaryot Gene Expr. 14:301–315. 2004. View Article : Google Scholar
|
|
6
|
Ameye L, Aria D, Jepsen K, Oldberg A, Xu T
and Young MF: Abnormal collagen fibrils in tendons of
biglycan/fibro-modulin-deficient mice lead to gait impairment,
ectopic ossification and osteoarthritis. FASEB J. 16:673–680. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young MF, Bi Y, Ameye L and Chen XD:
Biglycan knockout mice: New models for musculoskeletal diseases.
Glycoconj J. 19:257–262. 2002. View Article : Google Scholar
|
|
8
|
Nishino R, Honda M, Yamashita T, Takatori
H, Minato H, Zen Y, Sasaki M, Takamura H, Horimoto K, Ohta T, et
al: Identification of novel candidate tumour marker genes for
intrahepatic cholangiocarcinoma. J Hepatol. 49:207–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan S, Cheng L, White JT, Lu W, Utleg AG,
Yan X, Urban ND, Drescher CW, Hood L and Lin B: Quantitative
proteomics analysis integrated with microarray data reveals that
extracellular matrix proteins, catenins and p53 binding protein 1
are important for chemotherapy response in ovarian cancers. OMICS.
13:345–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Li W, Li X, Tai Y, Lü Q, Yang N and
Jiang J: Expression and significance of biglycan in endometrial
cancer. Arch Gynecol Obstet. 289:649–655. 2014. View Article : Google Scholar
|
|
11
|
Aprile G, Avellini C, Reni M, Mazzer M,
Foltran L, Rossi D, Cereda S, Iaiza E, Fasola G and Piga A:
Biglycan expression and clinical outcome in patients with
pancreatic adenocarcinoma. Tumour Biol. 34:131–137. 2013.
View Article : Google Scholar
|
|
12
|
Hu L, Duan YT, Li JF, Su LP, Yan M, Zhu
ZG, Liu BY and Yang QM: Biglycan enhances gastric cancer invasion
by activating FAK signaling pathway. Oncotarget. 5:1885–1896. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu X, Ma Y, Xiao J, Zheng H, Song C, Gong
Y and Xing X: Up-regulated biglycan expression correlates with the
malignancy in human colorectal cancers. Clin Exp Med. 12:195–199.
2012. View Article : Google Scholar
|
|
14
|
Weber CK, Sommer G, Michl P, Fensterer H,
Weimer M, Gansauge F, Leder G, Adler G and Gress TM: Biglycan is
overexpressed in pancreatic cancer and induces G1-arrest in
pancreatic cancer cell lines. Gastroenterology. 121:657–667. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Recktenwald CV, Leisz S, Steven A, Mimura
K, Müller A, Wulfänger J, Kiessling R and Seliger B:
HER-2/neu-mediated down-regulation of biglycan associated with
altered growth properties. J Biol Chem. 287:24320–24329. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makatsori E, Lamari FN, Theocharis AD,
Anagnostides S, Hjerpe A, Tsegenidis T and Karamanos NK: Large
matrix proteoglycans, versican and perlecan, are expressed and
secreted by human leukemic monocytes. Anticancer Res. 23:3303–3309.
2003.PubMed/NCBI
|
|
17
|
Hu L, Duan YT, Li JF, Su LP, Yan M, Zhu
ZG, Liu BY and Yang QM: Biglycan enhances gastric cancer invasion
by activating FAK signaling pathway. Oncotarget. 5:1885–1896. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu YH, Yang F, Zhang SS, Zeng TT, Xie X
and Guan XY: High expression of biglycan is associated with poor
prognosis in patients with esophageal squamous cell carcinoma. Int
J Clin Exp Pathol. 6:2497–2505. 2013.PubMed/NCBI
|
|
20
|
Wang B, Li GX, Zhang SG, Wang Q, Wen YG,
Tang HM, Zhou CZ, Xing AY, Fan JW, Yan DW, et al: Biglycan
expression correlates with aggressiveness and poor prognosis of
gastric cancer. Exp Biol Med (Maywood). 236:1247–1253. 2011.
View Article : Google Scholar
|
|
21
|
Theocharis AD and Karamanos NK: Decreased
biglycan expression and differential decorin localization in human
abdominal aortic aneurysms. Atherosclerosis. 165:221–230. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu-Hirota R, Sasamura H, Kuroda M,
Kobayashi E, Hayashi M and Saruta T: Extracellular matrix
glycoprotein biglycan enhances vascular smooth muscle cell
proliferation and migration. Circ Res. 94:1067–1074. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto K, Ohga N, Hida Y, Maishi N,
Kawamoto T, Kitayama K, Akiyama K, Osawa T, Kondoh M, Matsuda K, et
al: Biglycan is a specific marker and an autocrine angiogenic
factor of tumour endothelial cells. Br J Cancer. 106:1214–1223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xing X, Gu X, Ma T and Ye H: Biglycan
up-regulated vascular endothelial growth factor (VEGF) expression
and promoted angiogenesis in colon cancer. Tumor Biol.
36:1773–1780. 2015. View Article : Google Scholar
|
|
28
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Babelova A, Moreth K, Tsalastra-Greul W,
Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter
J, Schaefer RM, Gröne HJ and Schaefer L: Biglycan, a danger signal
that activates the NLRP3 inflammasome via toll-like and P2X
receptors. J Biol Chem. 284:24035–24048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schaefer L, Babelova A, Kiss E, Hausser
HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte
M, et al: The matrix component biglycan is proinflammatory and
signals through Toll-like receptors 4 and 2 in macrophages. J Clin
Invest. 115:2223–2233. 2005. View
Article : Google Scholar : PubMed/NCBI
|