Introduction
Non steroidal anti-inflammatory drugs (NSAIDs)
inhibit cyclooxygenase (COX) enzymes and are used extensively to
treat multiple illnesses (1).
There are several neurodegenerative disorders, in which concurrent
inflammatory stress occurs (1).
The importance of neuroinflammation in various neurodegenerative
conditions is supported by evidence from post mortem analyses,
accompanied by microglial activation and reactive astrocytes
(2–4), suggesting the importance of COX
enzymes. Several studies have suggested that anti-inflammatory
drugs, particularly NSAIDs, appear to be beneficial in slowing the
progression of neurodegenerative diseases, including Alzheimer's
disease (AD) (5–7), by inhibiting inflammatory responses
(8–10). NSAIDs exert their anti-inflammatory
effect by inhibiting COX isoforms (11). COX is a homodimer membrane
glycoprotein associated with a heme group involved in enzymatic
activities (12). Two important
isoforms of COX have been identified, COX-I and COX-II (13). Several studies have suggested the
beneficial role of COX-II in AD, as the expression and activity of
COX-II is increased in early stages of AD, determining the primary
protection of NSAIDs in preventing the earlier steps leading to
neurodegeneration (14).
There has been controversy regarding the role of
COX-I either as a protective or pro-inflammatory agent. COX-I is
prominently expressed in microglia (15). Microglial activation is reported
following aluminium (Al) administration (16), suggesting the involvement of COX-1
following Al-induced injury. COX-I is actively involved in
immunoregulation of central nervous system (1,17,18)
and its deletion reduces neuroinflammation and neuronal damage
induced by Aβ (19). However,
enhanced activity of COX-1 is reported as a source of oxidative
stress in Aβ-mediated neurotoxicity (13). Multiple studies (19–22)
have indicated the active involvement of COX-I in brain injury
induced by pro-inflammatory stimuli, including Aβ.
COX-II is expressed in the brain under normal
conditions (23), while it is an
inducible enzyme in other tissues and is expressed in response to
pro-inflammatory stimuli (24).
COX-II is prominently expressed in hippocampal and cortical
glutamatergic neurons (25), but
not in astrocytes and microglial cells (26), suggesting its distinctive role,
compared with COX-I. COX-II, which is predominantly present in
neurons, is important in regulating brain functions, including
synaptic plasticity (27,28), however, its specific role in the
hippocampus and cortex, which may be involved in cognitive
functions, remains to be elucidated. In AD, neuronal levels of
COX-II have been found to be elevated either in early stages
(15,29,30)
or decreased in later stages (31). An association between the induction
of COX-II and neuronal degeneration following stimulation of
glutamate seizures (32) and
spreading of depression waves (33) has also been reported, however, the
exact role remains to be elucidated.
Several evidence has supported the protective role
of NSAIDs, which inhibit COX-I and COX-II in diseases, including
AD, gastric cancer and colorectal cancer (34). Therefore, the balance between COX-I
and COX-II may be important to provide balance between the
inflammatory response and synaptic plasticity (23). The present study was performed to
investigate the distinctive role of COX enzymes in hippocampus- and
cortex-dependent cognitive function in Al-induced neurotoxicity. Al
is a widely used metal and is known as a neurotoxic agent (35). Al causes impaired
neurotransmission, oxidative stress (35) and increased lipid peroxidation
(36). Studies showed that Al is
responsible for the cognitive impairment (37,38).
Epidemiologically, there is an association between chronic Al
exposure and the incidence of AD (39), and furthermore, elevated levels of
Al have been reported in the brains of AD patients (40).
To understand the role of COX enzyme inhibition in
cognitive function, the present study administered mice with
piroxicam and celecoxib at specific doses to inhibit the COX
enzymes and to examine their contribution in hippocampal- and
cortex-dependent cognitive functions. This investigation aimed to
determine the distinct roles of COX-I and COX-II and examine the
effects of celecoxib and piroxicam on organizational behavior,
sociability, depression, anxiety and oxidative stress, which is a
hallmark of AlCl3-induced neurotoxicity.
Materials and methods
Drugs and chemicals
Aluminium Chloride hexa hydrate (cat. no AL0770) was
purchased from Scharlab (Barcelona, Spain). Celecoxib 100 mg
capsules (cat. no. 064C01) and piroxicam 20 mg capsules (cat. no.
12C018) were purchased from Getz Pharma Private Limited (Karachi,
Pakistan) and Global Pharmaceuticals (Chalfont, PA, USA),
respectively. 2,2-Diphenyl-1-picrylhydrazyl (DPPH; cat. no.
101087701) and diethylether (cat. no. 676845) was obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Animals
Male Balb/c mice weighing 35–45 g were provided by
Amson Vaccines and Pharma, Ltd. (Islamabad, Pakistan). All
experiments performed complied with the rulings of the Institute of
Laboratory Animal Resources, Commission on Life Sciences, National
Research Council (1996) (41) and
the protocol was approved by the ethical committee for research on
animals (Internal Review Board, Atta-ur-Rahman School of Applied
Biosciences, National University of Sciences and Technology,
Islamabad, Pakistan). The animals were maintained in the animal
house (three mice/cage) at Atta-ur-Rahman School of Applied
Biosciences, National University of Sciences and Technology, under
controlled conditions (23–25°C; 10 h light/dark cycle), and house
separately according to the group to which they pertain. The
experimental mice were provided with access to distilled water and
a standard diet ad libitum.
Drug administration
In the present study a previously reported mouse
model was used (42) with certain
modifications. A total of four groups of animals were included, in
which treatment was performed in to the respective groups for a
duration of 30 days; I) Control group, 10 animals were provided
with distilled water and a standard diet; II)
AlCl3-induced neurotoxicity group: 10 animals were
administered with AlCl3 (250 mg/kg/day) dissolved in
distilled water; III) Celecoxib-treated group
(AlCl3+Cel), 10 animals were provided with
AlCl3 (250 mg/kg/day) dissolved in distilled water, and
celecoxib was provided in the feed at the dose of 15.6 mg/kg body
weight per day; IV) piroxicam-treated group (AlCl3+Pxm),
10 animals were provided with AlCl3 (250 mg/kg/day)
dissolved in distilled water and piroxicam was provided in the feed
at a dose of 12.5 mg/kg body weight per day. The administration
doses for the Al and the drugs were calculated based on the water
and diet consumption of the animals prior to initiation of the
experiments in the present study. None of treatment approaches
affected the water or food intake of the mice, or affected weight
changes in the groups of mice (data not shown).
Behavioral assessment
Morris water maze test for assessment
of spatial memory
The procedure for assessing spatial reference memory
was the same as that described previously (43) with modifications. On the 25th day
of treatment, the animals were subjected to a Morris water maze
test, which continued until the end of the experiment. The
experimental apparatus used was comprised of a circular water tank
filled with water, with an invisible platform placed below the
surface of the water. The temperature of the water was 21–23°C, and
the water was placed in an assessment room and clues external to
the maze were visible from pool for spatial orientation by mice.
These clues were maintained constant throughout the task. The pool
was divided into four equal quadrants. During spatial reference
memory training, the platform was always placed in the same spatial
location of the pool and the releasing positions of the mice were
changed in every trial. The mice received five trials per day for
consecutive five days. Each trial duration was 60 sec, with an
inter trial interval of 10 mins. The time taken by the mouse to
reach the platform was recorded.
Social preference test
The assessment of social preference used a
previously described method (44).
Two sessions of 10 min were performed, with 20 min gap between
them. In the first session, the test animal was exposed to a mouse,
which was confined to a small closed cage, while the second cage in
the testing box was empty. The mouse was allowed to interact with
the mouse and an empty cage. Following the first session, the
animal was returned back to its housing cage for 20 min. During the
second session, the stranger mouse was placed in the empty cage and
the test mouse was allowed to interact and the time of interaction
was recorded. The social novel preference was recorded and the
discrimination index (DI) for the two sessions was calculated;
which is the ratio between the time spent with mouse A (session I)
or stranger mouse (session II) and the total interaction time,
according to the following equation: DI = time spent with mouse A
or mouse B / total time of interaction.
Nesting behavior
Nesting behavior was assessed, as described earlier
(45) and the nest was scored from
0–5. Score 1, >90% cotton was untouched by mouse; score 2,
50–90% of cotton was torn up; score 3, mostly shredded cotton.;
score 4, completely shredded cotton only with one or 2 walls. Score
5, walls higher than mouse body height with perfect nest.
Assessment was performed in individual cages, normal bedding was
used and each cage was provided with 4 g of cotton for making a
nest. The mice were placed in these cages with cotton provided
overnight, and the results were assessed the following day.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for RNA expression
analysis
The protocol was adopted as explained earlier
(46) to examine the effect of COX
inhibitors on gene expression following treatment with respective
drugs. The animals were sacrificed by decapitation under
diethylether anesthesia, and their brains (50–100 mg; four
samples/group) were isolated to extract the hippocampus and cortex.
TRIzol was used to extract total RNA. The quality of the RNA was
assessed by running on agarose gel to obtain two ribosomal RNA
bands, and the quantity was determined using a spectrophotometer
(Optima SP300; Optima Inc., Tokyo, Japan). Equal quantities of RNA
were used (1 µg RNA in 40 µl of reaction mixture) for
RT into cDNA. cDNA (3 µl) was used for the PCR reactions
with at total reaction mixture (10 µM) containing MgCl2 (25
µM), dNTPs (10 µM) and Taq polymerase (0.625
U/25 µl) (Thermo Fisher Scientific, Inc.). The PCR
thermocycling (2720 Thermal Cycler; Applied Biosystems Life
Technologies, Foster City, CA, USA) was performed with the
following conditions: Initial denaturation for 95°C for 5 min,
followed by denaturation at 94°C for 30 s, annealing (temperatures
indicated in Table I) for 30 s,
and extension at 72°C for 30 s with the indicated number of cycles.
This was followed by a final extension step at 72°C for 10 min.
Separation of the amplified PCR products was performed on a 2%
agarose gel (Merck Millipore, Karachi, Pakistan) with ethidium
bromide (Sigma-Aldrich) for staining. The quantification of each
PCR product band was determined using Image J 1.47 software
(National Institutes of Health, Bethesda, MD, USA). Actin was used
as a housekeeping gene to normalize the respective group of PCR
products.
 | Table IList of primers used in quantitative
polymerase chain reaction analysis. |
Table I
List of primers used in quantitative
polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) | Annealing temp
(°C) | Cycles (n) |
|---|
| Actin | Forward:
GCCTTCCTTCTTGGGTATGG
Reverse: CAGCTCAGTAACAGTCCGC | 55 | 32 |
| COX-1 | Forward:
CTACATCAGCTGGGAGTCCT
Reverse: CGTCCAGCACCTGGTACTTA | 55 | 35 |
| COX-2 | Forward:
CAGGTCATTGGTGGAGAGG
Reverse: CATGTTCCAGGAGGATGGAG | 54 | 35 |
Assessment of ex-vivo antioxidant
activity using a DPPH radical scavenging assay
The antioxidant activity in brain samples were
evaluated using a DPPH (Sigma-Aldrich) radical scavenging assay, as
described earlier (47) with
certain modifications. The control, AlCl3-treated,
celecoxib-treated and piroxicam-treated brain samples, with a 0.1
mg/ml protein concentration, were homogenized in 1 ml methanol.
Subsequently, 0.4 ml of 0.1 mM DPPH was added to the homogenized
brain tissue samples which were designated as test samples. Pure
DPPH solution was used as a control. The solutions were incubated
at 37°C for 30 min and the absorbance was measured at 517 nm using
an Optima SP300 spectrophotometer. The percentage DPPH inhibition
was calculated by using the following formula, and was normalized
to per/mg protein: DPHH inhibition (%) = (absorbance of control -
absorbance of test sample / absorbance of control) × 100.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean and the results were statistically analyzed using GraphPad
Prism software. One way analysis of variance was used followed by
Bonferroni's comparison test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of celecoxib and piroxicam on
learning and memory
The control, AlCl3-treated, celecoxib and
piroxicam treatment groups were investigated in a spatial reference
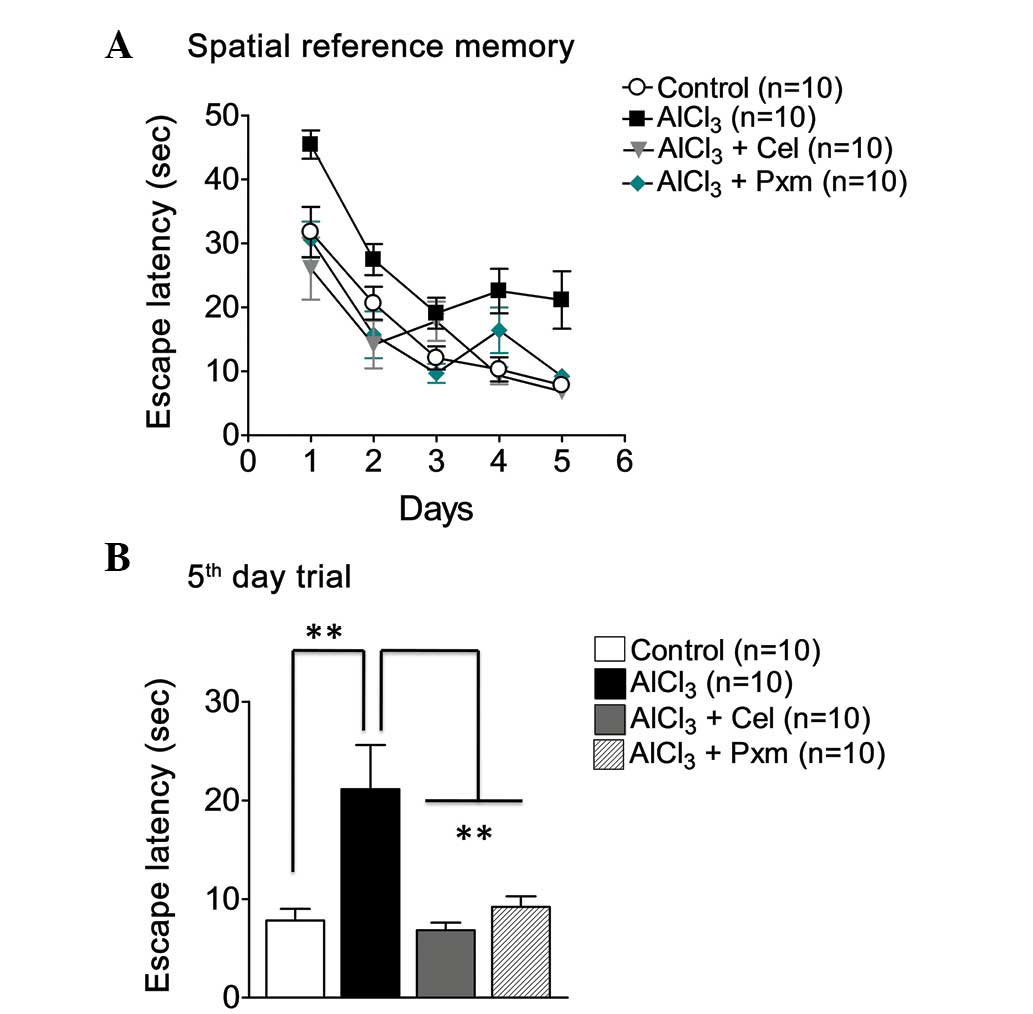
memory task using a Morris water test (Fig. 1A). The result of the trial on the
fifth day demonstrated a significant (P<0.01) improvement of
memory in the celecoxib-treated mice (6.84±0.76 sec) and
piroxicam-treated mice (9.20±1.08 sec), compared with the
AlCl3-treated mice (21.14±0.76 sec; Fig. 1B).
Effect of celecoxib and piroxicam on
social behavior
Social affiliation and social novelty preference
assessments were performed to examine the effect on sociability and
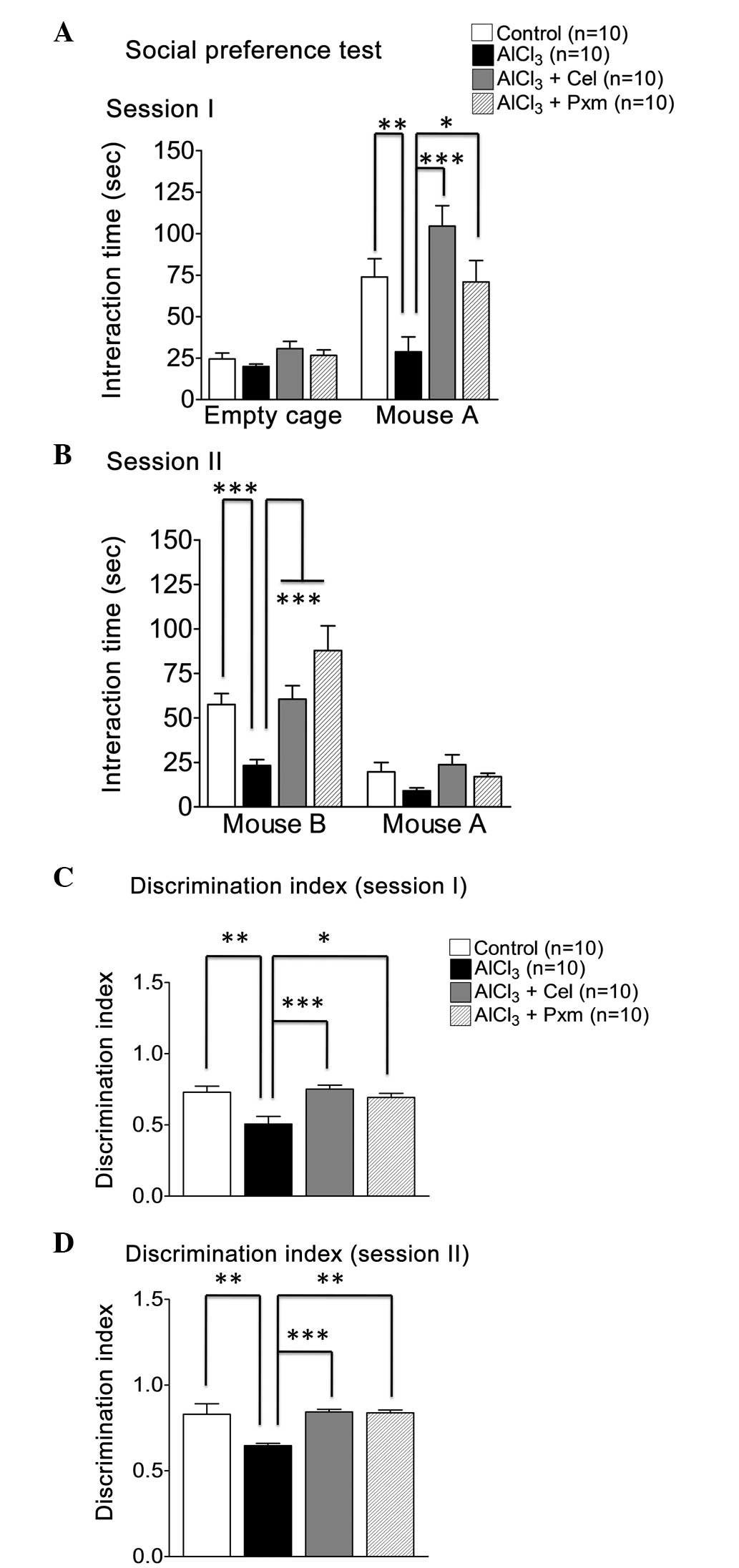
preferences for social novelty. The comparison revealed that, in
session I, the mice in the AlCl3-treated group spent
less time (28.8±8.97 sec) with the familiar mouse (mouse A),
compared with the control group (73.9±10.97 sec), however, the mice
in the control group spent less time in the empty cage (Fig. 2A). During session I, the mice in
the celecoxib and piroxicam treatment groups exhibited elevated
social interaction, spending a longer duration with mouse A
(104.5±12.29 sec and 70.90±12.84 sec, respectively; Fig. 2A).
In session II, the time spent with the stranger
mouse (mouse B), compared with mouse A was calculated. The control
group spent significantly (P<0.001) more time (57.5±6.18 sec)
with mouse B, compared with mouse A, compared with the
AlCl3-treated group (23.2±3.31 sec), which demonstrated
lack of social novelty preference (Fig. 2B). The celecoxib (60.5±7.52 sec)
and piroxicam treatment groups (87.80±13.89 sec) exhibited a
significant social novelty preference (Fig. 2B).
In determining the DI of the mice in session I,
piroxicam (0.7±0.02) exhibited significantly better effects than
the celecoxib group (0.75±0.03) when the two drug treatment groups
were compared with the AlCl3-treated group (0.50±0.5;
Fig. 2C).
The DI calculated of the mice in session II
indicated that the control group demonstrated better social novelty
preference (0.83±0.06), compared with the AlCl3-treated
group (0.64±0.01), which was noted to exhibit a deficit in social
novelty preference (Fig. 2D).
Treatment with celecoxib (0.84±0.01) and piroxicam (0.83±0.01)
rescued social novelty in the diseased mice (Fig. 2D).
Effect of celecoxib and piroxicam on
nesting behavior
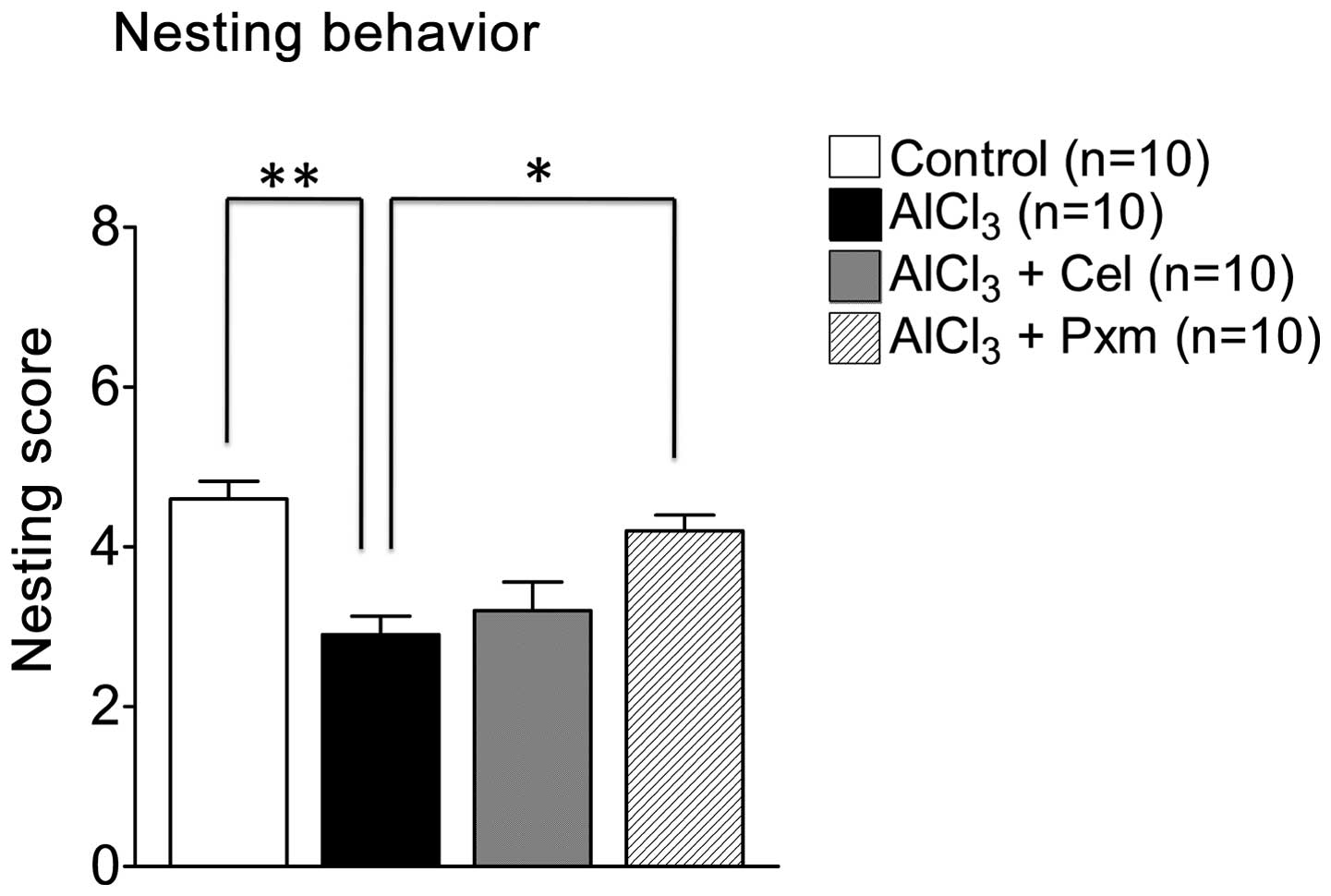
Nesting behavior was assessed to determine the
organizational and daily activities of living in mice. As shown in
Fig. 3, the nest score of the
AlCl3-treated group (2.9±0.23) declined, compared with
the control group (4.6±0.22). Piroxicam was effective and improved
nesting score (4.2±0.2), whereas celecoxib (3.10±0.43) was not
effective (Fig. 3).
Effect of celecoxib and piroxicam on gene
expression
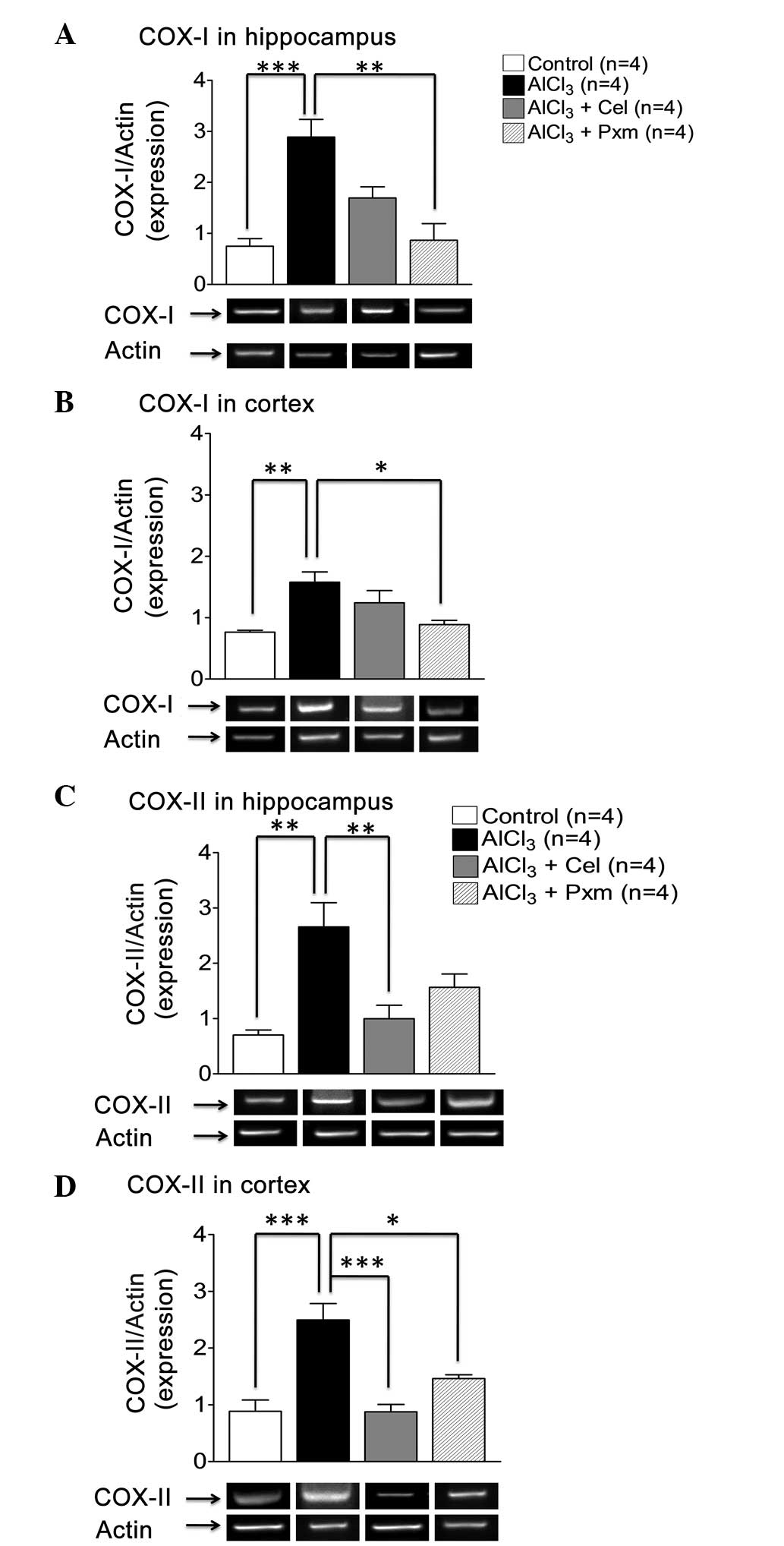
In the present study, RT-qPCR analysis was performed
to examine the effect of drug treatment on gene expression. In the
hippo-campus, there was an increase in the levels of COX-I
(2.8±0.34) in the AlCl3-treated group, compared with the
control group (0.74±0.15), however, only piroxicam treatment
decreased the expression of COX-I significantly (0.9±0.32),
whereas, celecoxib was not effective (Fig. 4A).
In the cortex, a significant (P<0.01) increase in
the level of COX-I (1.57±0.16) was observed in the
AlCl3-treated group, compared with the control group
(0.8±0.03). Piroxicam treatment resulted in significant (P<0.05)
downregulation in the expression of COX-I (0.9±0.06), compared with
the AlCl3-treated group. Celecoxib treatment (1.24±0.2)
was not found to be effective (Fig.
4B).
In the hippocampus, upregulation in the levels of
COX-II (2.65±0.43) were observed in the AlCl3-treated
group, compared with the control group (0.7±0.09; Fig. 4C), exhibiting inflammatory stress.
Celecoxib treatment resulted in significant (P<0.01)
downregulation in the levels of COX-II (1±0.24), indicating its
selective effect on the gene expression of COX-II, whereas
piroxicam treatment was not effective (1.60±0.23; Fig. 4C).
In the cortex, the levels of COX-II were elevated in
the AlCl3-treated group (2.4±0.30), compared with the
control group (0.90±0.20). The celecoxib and piroxicam treatment
groups exhibited downregulated levels of COX-II (0.90±0.13 and
1.46±0.06, respectively), compared with the
AlCl3-treated group (Fig.
4D).
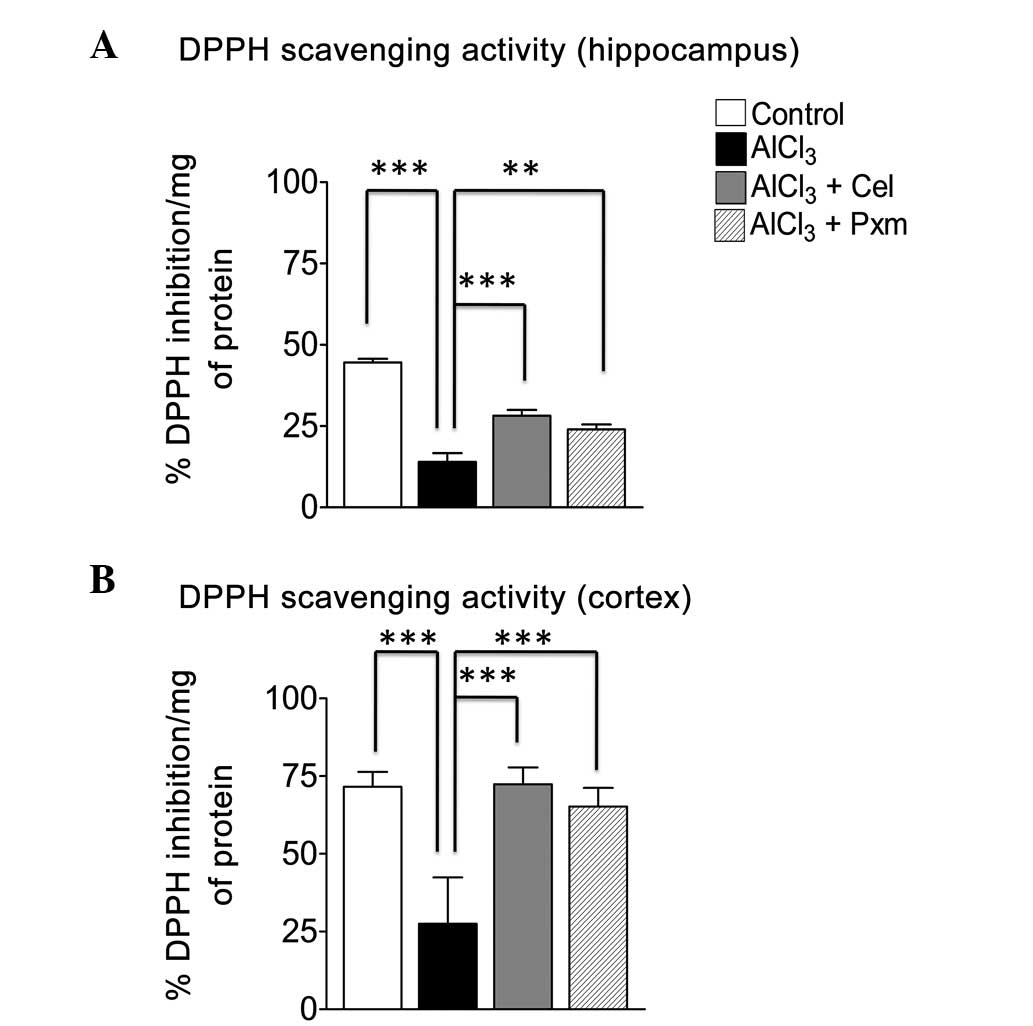
Ex-vivo DPPH assay
To investigate the effect of celecoxib and Piroxicam
on oxidative stress, a DPPH assay was performed in the hippocampus
and cortex of the brain tissues of the mice in the treatment
groups. The results demonstrated that the AlCl3-treated
group exhibited a substantial load of free radicals and a decreased
percentage of DPPH inhibition (14±2.7%) in the hippocampus, also
indicative of decreased endogenous anti-oxidants, compared with the
control (44.6±1.07%; Fig. 5A). The
celecoxib-treated group (28.2±1.8%) exhibited a significant
(P<0.001) increase in the percentage inhibition of free radicals
in the hippocampus, whereas the piroxicam-treated group was less
effective (24±1.51%; P<0.01; Fig.
5A).
In the cortex, the AlCl3-treated group
(27.51±14.87%) exhibited increased oxidative stress resulting in
free radical production, compared with the control group
(71.54±4.85%; Fig. 5B). Celecoxib
treatment led to the effective inhibition of the free radicals
(72.4±5.4) induced by AlCl3. Similarly, piroxicam
treatment led to increased free radical scavenging activity
(65.2±6.02%), compared with the AlCl3-treated group
(Fig. 5B).
Discussion
The present study attempted to identify which COX
enzyme inhibition is predominantly responsible for the improvement
in hippocampal- and cortex-dependent cognitive function in the
AlCl3-treated mice model to determine the role of NSAIDs
in neurodegenerative disorders. The present study demonstrated the
significant effect of celecoxib and piroxicam on learning and
memory, determined using the Morris water maze test. The two drugs
exhibited similar efficacy in the Morris water maze, which is a
hippocampus-dependent memory task. These results are concordance
with those of earlier studies, which reported that selective COX-II
inhibition restores memory function in APP-overexpressing
transgenic mice (48) and
selective COX-I inhibition promotes learning (11). Treatment with celecoxib and
piroxicam demonstrated memory enhancing effects by decreasing the
expression levels of the COX-I and II isoforms in mice, suggesting
that decreased expression levels may have decreased inflammation
and increased memory. Another possible reason for the enhanced
memory in the COX-II inhibitor-treated group is the inhibition of
overexpressed COX-II in the hippocampal neurons, resulting in
improved memory. COX-1 inhibition may improve memory through
decreasing inflammation, however, the exact mechanism remains to be
elucidated.
Social affiliation and social novelty preference are
amygdala- and cortex-dependent behaviors (49). The present study suggested that
celecoxib exhibited improved effects on social affiliation (session
I), whereas piroxicam exhibited a more marked effect on social
novel preference. It has been revealed that COX-II inhibition is
beneficial in suppressing the stress induced by elevated COX-II
enzyme in rat brain (50).
Similarly, the role of piroxicam in novel social preference is a
novel finding. The present study investigated, for the first time,
the effect of the two COX inhibitors in an AlCl3-induced
neurotoxicity mouse model, and demonstrated that COX inhibitors
assist in improving social recognition memory, suggesting their
potential role in neurodegenerative conditions accompanied with
social memory problems. Further investigations are required to
determine the importance of the effect, and to investigate the
mechanism through which they act to improve these symptoms in
neurodegeneration.
Nest building is a common behavior in mice and is
associated with the maintenance of body temperature (51). It is a prefrontal cortex- and
hippocampus-based behavior (52),
and it has been reported that damage in the medial prefrontal
cortex and hippocampus leads to the reduction in nesting material
consumption and disturbs the quality of the nest (52,53).
The present study revealed that piroxicam improved the quality of
the nest and reversed Al-induced impairment, whereas, celecoxib
failed to produce a significant effect. These are novel findings
and suggest an additional pharmacological role of piroxicam,
however, the exact underlying mechanism remains to be
elucidated.
In the present study, the levels of COX-I and COX-II
were elevated in the hippocampus and cortex in the
AlCl3-treated group, and piroxicam reduced the
expression levels of COX-I in hippocampus and cortex, which may be
its underlying mechanism in improving cognitive functions. This
drug has not been investigated previously for its effect on gene
expression in the AlCl3-treated mouse model. Other COX-I
inhibitors have been investigated and have offered protection
against mild to moderate cognitive impairment in patients with
neuro-degenerative disease (54).
Celecoxib treatment also led to reduced expression levels of COX-II
in the hippocampus and cortex, suggesting its beneficial role in
reducing neuroinflammation, which differs to earlier reports that
selective COX-II inhibitors fail to demonstrate beneficial effects
in patients with neurodegenerative disease (7,55).
Therefore, these findings suggested that depressive symptoms of
disease may be treated using celecoxib.
It has already been accepted and established that
oxidative stress is one of the hallmarks of several neurological
disorders, particularly AD (56).
In the present study the AlCl3-treated model exhibited
increased oxidative stress in the brain tissue, compared with the
control, which was concordant with an earlier study, confirming the
role of Al in producing oxidative damage in brain tissues (57). The ex-vivo anti-oxidant
activity of piroxicam and celecoxib exhibited increased free
radical inhibition in the hippocampus, compared with the
AlCl3-treated group. In the cortex, the two drugs
equally decreased oxidative stress, indicating their therapeutic
potential in neurodegenerative disorders.
In the present study, comparison of piroxicam and
celecoxib in reference to Al-induced neurodegeneration was
performed for the first time. The ability of piroxicam to improve
organizational behavior and sociability are significant findings,
suggesting the role of piroxicam in various neurodegenerative
disorders. Celecoxib treatment markedly improved cognitive
functions, including learning, memory and anxious behavior. Its
effect on social activity was also examined, which exhibited
positive effects as a novel finding. The two drugs also improved
AlCl3-induced neuroinflammation and decreased oxidative
stress, which demonstrates their potential for use in
neurodegenerative diseases. These results suggested that COX
enzymes are important in neuropathology and have potential as drug
targets in neurodegeneration. This investigation can be broadened
to further investigate the possible molecular mechanisms of these
drugs in other neurodegenerative conditions.
Acknowledgments
This study was supported by the Atta-ur-Rahman
School of Applied Biosciences, National University of Sciences and
Technology and the Small Research Project Grants 2012, College of
Medicine, Alfaisal University (grant. no. 313090202133).
References
|
1
|
Choi SH, Aid S, Choi U and Bosetti F:
Cyclooxygenases-1 and -2 differentially modulate leukocyte
recruitment into the inflamed brain. Pharmacogenomics J.
10:448–457. 2010. View Article : Google Scholar
|
|
2
|
McGeer PL, Itagaki S, Tago H and McGeer
EG: Reactive microglia in patients with senile dementia of the
Alzheimer type are positive for the histocompatibility glycoprotein
HLA-DR. Neurosci Lett. 79:195–200. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haga S, Akai K and Ishii T: Demonstration
of microglial cells in and around senile (neuritic) plaques in the
Alzheimer brain. An immunohistochemical study using a novel
monoclonal antibody. Acta Neuropathol. 77:569–575. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wisniewski HM, Wegiel J, Wang KC and Lach
B: Ultrastructural studies of the cells forming amyloid in the
cortical vessel wall in Alzheimer's disease. Acta Neuropathol.
84:117–127. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGeer PL, Schulzer M and McGeer EG:
Arthritis and anti-inflammatory agents as possible protective
factors for Alzheimer's disease: A review of 17 epidemiologic
studies. Neurology. 47:425–432. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
in t'Veld BA, Ruitenberg A, Hofman A,
Launer LJ, van Duijn CM, Stijnen T, Breteler MM and Stricker BH:
Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's
disease. N Engl J Med. 345:1515–1521. 2001. View Article : Google Scholar
|
|
7
|
McGeer PL and McGeer EG: NSAIDs and
Alzheimer disease: Epidemiological, animal model and clinical
studies. Neurobiol Aging. 28:639–647. 2007. View Article : Google Scholar
|
|
8
|
Lim GP, Yang F, Chu T, Chen P, Beech W,
Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA and Cole GM:
Ibuprofen suppresses plaque pathology and inflammation in a mouse
model for Alzheimer's disease. J Neurosci. 20:5709–5714.
2000.PubMed/NCBI
|
|
9
|
Lim GP, Yang F, Chu T, Gahtan E, Ubeda O,
Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA and Cole GM:
Ibuprofen effects on Alzheimer pathology and open field activity in
APPsw transgenic mice. Neurobiol Aging. 22:983–991. 2001.
View Article : Google Scholar
|
|
10
|
McKee AC, Carreras I, Hossain L, Ryu H,
Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW and Dedeoglu A:
Ibuprofen reduces Abeta, hyperphosphorylated tau and memory
deficits in Alzheimer mice. Brain Res. 1207:225–236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi SH, Aid S, Caracciolo L, Minami SS,
Niikura T, Matsuoka Y, Turner RS, Mattson MP and Bosetti F:
Cyclooxygenase-1 inhibition reduces amyloid pathology and improves
memory deficits in a mouse model of Alzheimer's disease. J
Neurochem. 124:59–68. 2013. View Article : Google Scholar :
|
|
12
|
Williams CS and DuBois RN: Prostaglandin
endoperoxide synthase: Why two isoforms? Am J Physiol.
270:G393–G400. 1996.PubMed/NCBI
|
|
13
|
Candelario-Jalil E: A role for
cyclooxygenase-1 in beta-amyloid-induced neuroinflammation. Aging
(Albany NY). 1:350–353. 2009.
|
|
14
|
Kelley KA, Ho L, Winger D, Freire-Moar J,
Borelli CB, Aisen PS and Pasinetti GM: Potentiation of
excitotoxicity in transgenic mice overexpressing neuronal
cyclooxygenase-2. Am J Pathol. 155:995–1004. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoozemans JJ, Rozemuller AJ, Janssen I, De
Groot CJ, Veerhuis R and Eikelenboom P: Cyclooxygenase expression
in microglia and neurons in Alzheimer's disease and control brain.
Acta Neuropathol. 101:2–8. 2001.PubMed/NCBI
|
|
16
|
Li XB, Zheng H, Zhang ZR, Li M, Huang ZY,
Schluesener HJ, Li YY and Xu SQ: Glia activation induced by
peripheral administration of aluminum oxide nanoparticles in rat
brains. Nanomedicine. 5:473–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcia-Bueno B, Serrats J and Sawchenko
PE: Cerebrovascular cyclooxygenase-1 expression, regulation and
role in hypothalamic-pituitary-adrenal axis activation by
inflammatory stimuli. J Neurosci. 29:12970–12981. 2009. View Article : Google Scholar
|
|
18
|
Matousek SB, Hein AM, Shaftel SS,
Olschowka JA, Kyrkanides S and O'Banion MK: Cyclooxygenase-1
mediates prostaglandin E(2) elevation and contextual memory
impairment in a model of sustained hippocampal interleukin-1beta
expression. J Neurochem. 114:247–258. 2010.PubMed/NCBI
|
|
19
|
Choi SH and Bosetti F: Cyclooxygenase-1
null mice show reduced neuroinflammation in response to
beta-amyloid. Aging (Albany NY). 1:234–244. 2009.
|
|
20
|
Choi SH, Langenbach R and Bosetti F:
Genetic deletion or pharmacological inhibition of cyclooxygenase-1
attenuate lipopolysaccharide-induced inflammatory response and
brain injury. FASEB J. 22:1491–1501. 2008. View Article : Google Scholar :
|
|
21
|
Candelario-Jalil E, de Oliveira AC, Gräf
S, Bhatia HS, Hüll M, Muñoz E and Fiebich BL: Resveratrol potently
reduces prostaglandin E2 production and free radical formation in
lipopolysaccharide-activated primary rat microglia. J
Neuroinflammation. 4:252007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Candelario-Jalil E, Taheri S, Yang Y, Sood
R, Grossetete M, Estrada EY, Fiebich BL and Rosenberg GA:
Cyclooxygenase inhibition limits blood-brain barrier disruption
following intracerebral injection of tumor necrosis factor-alpha in
the rat. J Pharmacol Exp Ther. 323:488–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed T and Gilani AH: A comparative study
of curcuminoids to measure their effect on inflammatory and
apoptotic gene expression in an Aβ plus ibotenic acid-infused rat
model of Alzheimer's disease. Brain Res. 1400:1–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masferrer JL, Zweifel BS, Seibert K and
Needleman P: Selective regulation of cellular cyclooxygenase by
dexamethasone and endotoxin in mice. J Clin Invest. 86:1375–1379.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi SH, Aid S and Bosetti F: The distinct
roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications
for translational research. Trends Pharmacol Sci. 30:174–181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoozemans JJ, Rozemuller JM, van Haastert
ES, Veerhuis R and Eikelenboom P: Cyclooxygenase-1 and -2 in the
different stages of Alzheimer's disease pathology. Curr Pharm Des.
14:1419–1427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niwa K, Araki E, Morham SG, Ross ME and
Iadecola C: Cyclooxygenase-2 contributes to functional hyperemia in
whisker-barrel cortex. J Neurosci. 20:763–770. 2000.PubMed/NCBI
|
|
28
|
Yang H and Chen C: Cyclooxygenase-2 in
synaptic signaling. Curr Pharm Des. 14:1443–1451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pasinetti GM and Aisen PS:
Cyclooxygenase-2 expression is increased in frontal cortex of
Alzheimer's disease brain. Neuroscience. 87:319–324. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujimi K, Noda K, Sasaki K, Wakisaka Y,
Tanizaki Y, Iida M, Kiyohara Y, Kanba S and Iwaki T: Altered
expression of COX-2 in subdivisions of the hippocampus during aging
and in Alzheimer's disease: The Hisayama study. Dement Geriatr Cogn
Disord. 23:423–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yermakova AV and O'Banion MK:
Downregulation of neuronal cyclooxygenase-2 expression in end stage
Alzheimer's disease. Neurobiol Aging. 22:823–836. 2001. View Article : Google Scholar
|
|
32
|
Tocco G, Freire-Moar J, Schreiber SS,
Sakhi SH, Aisen PS and Pasinetti GM: Maturational regulation and
regional induction of cyclooxygenase-2 in rat brain: Implications
for Alzheimer's disease. Exp Neurol. 144:339–349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miettinen S, Fusco FR, Yrjünheikki J,
Keinänen R, Hirvonen T, Roivainen R, Närhi M, Hökfelt T and
Koistinaho J: Spreading depression and focal brain ischemia induce
cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic
acid-receptors and phospholipase A2. Proc Natl Acad Sci USA.
94:6500–6505. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greenberg ER and Baron JA: Aspirin and
other nonsteroid anti-inflammatory drugs as cancer-preventive
agents. IARC Sci Publ. 91–98. 1996.PubMed/NCBI
|
|
35
|
Abd-Elhady RM, Elsheikh AM and Khalifa AE:
Anti-amnestic properties of Ginkgo biloba extract on impaired
memory function induced by aluminum in rats. Int J Dev Neurosci.
31:598–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Belaid-Nouira Y, Bakhta H, Bouaziz M,
Flehi-Slim I, Haouas Z and Ben Cheikh H: Study of lipid profile and
parieto-temporal lipid peroxidation in AlCl3 mediated
neurotoxicity. Modulatory effect of fenugreek seeds. Lipids Health
Dis. 11:162012. View Article : Google Scholar
|
|
37
|
Meyer-Baron M, Schäper M, Knapp G and van
Thriel C: Occupational aluminum exposure: Evidence in support of
its neurobehavioral impact. Neurotoxicology. 28:1068–1078. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hashmi AN, Yaqinuddin A and Ahmed T:
Pharmacological effects of Ibuprofen on learning and memory,
muscarinic receptors genes expression and APP isoforms levels in
Pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J
Neurosci. 125:277–287. 2015. View Article : Google Scholar
|
|
39
|
Bondy SC: The neurotoxicity of
environmental aluminum is still an issue. Neurotoxicology.
31:575–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andrasi E, Páli N, Molnár Z and Kösel S:
Brain aluminum, magnesium and phosphorus contents of control and
Alzheimer-diseased patients. J Alzheimers Dis. 7:273–284.
2005.PubMed/NCBI
|
|
41
|
National Research Council: Guide for the
care and use of laboratory animals. Washington, D.C: National
Academy Press; 1996
|
|
42
|
Thirunavukkarasu SV, Venkataraman S, Raja
S and Upadhyay L: Neuroprotective effect of Manasamitra vatakam
against aluminium induced cognitive impairment and oxidative damage
in the cortex and hippocampus of rat brain. Drug Chem Toxicol.
35:104–115. 2012. View Article : Google Scholar
|
|
43
|
Ahmed T and Gilani AH: Inhibitory effect
of curcuminoids on acetylcholinesterase activity and attenuation of
scopolamine-induced amnesia may explain medicinal use of turmeric
in Alzheimer's disease. Pharmacol Biochem Behav. 91:554–559. 2009.
View Article : Google Scholar
|
|
44
|
Meeker HC, Chadman KK, Heaney AT and Carp
RI: Assessment of social interaction and anxiety-like behavior in
senescence-accelerated-prone and -resistant mice. Physiol Behav.
118:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deacon R: Assessing burrowing, nest
construction and hoarding in mice. J Vis Exp. e26072012.
|
|
46
|
Ahmed T, Enam SA and Gilani AH:
Curcuminoids enhance memory in an amyloid-infused rat model of
Alzheimer's disease. Neuroscience. 169:1296–1306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Habila N, Agbaji AS, Ladan Z, Bello IA,
Haruna E, Dakare MA and Atolagbe TO: Evaluation of in vitro
activity of essential oils against trypanosoma brucei brucei and
trypanosoma evansi. J Parasitol Res. 2010:2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kotilinek LA, Westerman MA, Wang Q,
Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH,
Younkin SG, et al: Cyclooxygenase-2 inhibition improves
amyloid-beta-mediated suppression of memory and synaptic
plasticity. Brain. 131:651–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bickart KC, Hollenbeck MC, Barrett LF and
Dickerson BC: Intrinsic amygdala-cortical functional connectivity
predicts social network size in humans. J Neurosci. 32:14729–14741.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao
BS and Luo F: Chronic treatment with celecoxib reverses chronic
unpredictable stress-induced depressive-like behavior via reducing
cyclo-oxygenase-2 expression in rat brain. Eur J Pharmacol.
612:54–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Min Z, Wang S, Wu J and Zhang S:
Impairment of nesting behavior in APPswe/PS1dE9 mice. Life Sci J.
10:1942–1945. 2013.
|
|
52
|
Deacon RM, Croucher A and Rawlins JN:
Hippocampal cytotoxic lesion effects on species-typical behaviours
in mice. Behav Brain Res. 132:203–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Holson RR and Walker C: Mesial prefrontal
cortical lesions and timidity in rats II Reactivity to novel
stimuli. Physiol Behav. 37:231–238. 1986. View Article : Google Scholar
|
|
54
|
Rogers J, Kirby LC, Hempelman SR, Berry
DL, McGeer PL, Kaszniak AW, Zalinski J, Cofield M, Mansukhani L and
Willson P: Clinical trial of indomethacin in Alzheimer's disease.
Neurology. 43:1609–1611. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reines SA, Block GA, Morris JC, Liu G,
Nessly ML, Lines CR, Norman BA and Baranak CC; Rofecoxib Protocol
091 Study Group: Rofecoxib: No effect on Alzheimer's disease in a
1-year, randomized, blinded, controlled study. Neurology. 62:66–71.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Smith MA, Rottkamp CA, Nunomura A, Raina
AK and Perry G: Oxidative stress in Alzheimer's disease. Biochim
Biophys Acta. 1502:139–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chazot G and Broussolle E: Alterations in
trace elements during brain aging and in Alzheimer's dementia. Prog
Clin Biol Res. 380:269–281. 1993.PubMed/NCBI
|