Introduction
Type II collagen-induced arthritis (CIA) is a
typical animal model of rheumatoid arthritis (RA), a systemic
autoimmune disease characterized by chronic inflammation of the
synovium and hyperplasia of synovial fibroblasts; this inflammation
can erode adjacent cartilage and bone and cause subsequent joint
destruction (1). This destructive
process is at least partly mediated by fibroblast-like synoviocytes
(FLS) from the synovium. Indeed, FLS from RA patients were shown to
attach to and invade normal cartilage in a SCID mouse
co-implementation model (2).
Furthermore, FLS are implicated in all aspects of the pathogenesis
of RA (3). Thus, FLS in the
rheumatoid synovium are known to be aggressive and highly
proliferative, and may attack the cartilage, possessing
characteristics similar to those of transformed cells (3), including anchorage-independent growth
(4), insensitivity to apoptosis
and enhanced proliferation, and are able to invade the cartilage
(5). The CIA model exhibits
numerous clinical similarities with human RA (6).
Cultured FLS express high levels of proteinases,
which are able to degrade extracellular matrix components,
including collagens. One family of proteinases expressed by FLS are
the matrix metalloproteinases (MMPs). FLS express MMP-1, -2, -3, -9
and -10, and the expression levels of these MMPs are correlated
with their invasiveness (7).
However, MMPs are inactive precursors that must be processed by
pro-protein convertases (PCs), which cleave single basic or paired
basic residues of the pro-proteins to produce biologically active
proteins. To date, nine PCs have been identified: PC1/PC3, PC2,
pro-protein convertase subtilisin/kexin type 6 (PCSK6)/PACE4, PC4,
PC5/PC6, PC7/PC8/LPC, furin, PCSK8 and PCSK9.
Furin is highly expressed in the synovium of RA
patients and mice with CIA, and may protect against RA (8). PCSK6, however, has a major role in
promoting the progression of prostate tumors to a status of
increased aggressiveness (9). Of
note, tumor tissues and the synovium of rats with CIA or patients
with RA share common features, including excessive angiogenesis and
fibrin deposition, de-regulation of cell proliferation and high
coagulation activity (10). Thus,
PCSK6 was suggested to have an important role in CIA, which differs
from that of furin. Furthermore, a variant of PCSK6 was highly
associated with reduced pain in knee osteoarthritis, providing a
possible explanation as to why in the presence of an identical
structural damage, certain individuals developed chronic pain,
while others were protected. Studies on PCSK6-null mice also
implicated PCSK6 in pain (11).
However, a role for this protein in CIA or RA has not yet been
reported.
The present study investigation of the role of PCSK6
in synovitis of rats with CIA. For this, FLS were isolated from the
synovium of a rat model of CIA, and PCSK6 knockdown was performed
in isolated FLS to identify changes in their proliferation,
migratory and invasive capacity, and cell cycle progression.
Materials and methods
Induction of CIA
Ten male Wistar rats (age, 6–8 weeks; weight 100–120
g) susceptible to developing CIA were purchased from Vital River
Laboratories (Beijing, China). All rats were kept under controlled
environmental conditions with a mean temperature of 22±3°C, 12 h
dark-light cycle, relative humidity of 40% and access to food and
water ad libitum. The experimental protocol was approved by
the Ethics Committee for the use of animals of Shandong Academy of
Medical Science (Jinan, China). All efforts were made to minimize
discomfort and reduce the number of experimental animals used. All
procedures conformed to the ethical guidelines regarding the care
and use of laboratory animals, published by the International
Association for the Study of Pain and the National Institutes of
Health (12). After
acclimatization for one week in their cages, the rats received a
sub-cutaneous injection of 100 µg native bovine type II
collagen (Chondrex, Inc., Redmond, WA, USA) emulsified in Freund's
complete adjuvant (Chondrex, Inc.) into the base of the tail. A
second sub-cutaneous boost of 100 µg type II collagen in
Freund's complete adjuvant was given 21 days later. The animals
were anesthetized followed by a lethal dose of sodium pentobarbital
(Euthasol; Virbac USA, Fort Worth, TX, USA) 20 days after the
second immunization when the paws were notably swollen.
Culture and identification of synovial
fibroblasts
The synovial tissues of rats with CIA was finely
chopped and incubated with type II collagenase (1 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) in Dulbecco's modified Eagle's
medium (DMEM, HyClone, Thermo Fisher Scientific, Waltham, MA, USA)
for 6 h in an incubator at 37°C with 5% CO2. The tissue
was treated with 0.25% trypsin (Solabio, Beijing, China) in
phosphate-buffered saline (PBS), in a volume equivalent to that of
DMEM. Cells were filtered and cultured overnight in DMEM
supplemented with 10% fetal bovine serum (FBS; HyClone) as well as
penicillin (100 IU/ml) and streptomycin (100 µg/ml)
(Gibco-BRL, Invitrogen Life Technologies, Carlsbad, CA, USA) for
three passages. FLS of rats with CIA at passage 4–6 were used for
the present study, which were negative for CD14, CD3, CD19 and CD56
expression as identified by flow cytometric analysis using a
Coulter Epics XL flow cytometer (Beckman Coulter, Brea, CA, USA).
Phycoerythrin (PE)-conjugated CD14 (cat. no. 12-0149), fluorescein
isothiocyanate (FITC)-conjugated CD3 (cat. no. 11-0039),
FITC-conjugated CD19 (cat. no. 11-0199) and PE-conjugated CD56
(cat. no. 12-0567) antibodies were obtained from eBio-science Inc.
(San Diego, CA, USA) and used at a 1:50 dilution.
Inhibition of PCSK6 expression with small
interfering (si)RNAs
siRNA targeting PCSK6 (target mRNA sequence:
5′-GCAGAGAAGAAUGUAUUCATT-3′) was designed and synthesized by
GenePharma Co. Ltd (Shanghai, China). Cultured FLS were transfected
with siRNA at 160 nmol/l using a HiPerFect transfection reagent
(Qiagen, Hilden, Germany) according to the manufacturer's
instructions. The cells were harvested for analysis at 24 h
following the transfection. A negative siRNA (sequence:
5′-UUCUCCGAACGUGUCACGUTT-3′) was designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). was used as the
negative control; treatment with transfection reagent only was used
as the Mock group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells and
the human tissue using a Total RNA kit (R6834; Omega Bio-Tek, Inc.,
Norcross, GA, USA) and reverse-transcribed using a ReverTra Ace
qPCR RT kit (FSQ-101; Toyobo, Osaka, Japan) according to the
manufacturer's instructions. qPCR was performed using the
LightCycler 480 (4887352001; Roche Diagnostics, Basel, Switzerland)
using the following amplification protocol: Denaturation at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 10
sec followed by annealing at 60°C for 1 min and extension at 72°C
for 1 sec. The comparative threshold cycle (Ct) method was used to
analyze the relative expression of mRNA (13). The relative target gene expression
was normalized to GAPDH mRNA levels. Primers for qPCR were also
designed according to the consensus sequence as determined using
AlignX (Vector NTI Advance 11.0; Invitrogen Life Technologies). The
primers for the amplification of PCSK6 were as follows: Forward,
5′-ACTCCAGAAGAAGAGGAAGAGTA-3′ and reverse,
5′-ACCATCGCAGCCTTTATCA-3′. The primers for the amplification of
GAPDH were as follows: Forward, 5′-TGAACGGGAAGCTCACT-3′ and
reverse, 5′-CATGTCAGATCCACAACGGATA-3′. All primers are synthesized
by Bio-Asia Diagnostics Co., Ltd. (Shanghai, China). For all PCRs,
standard curves, dissociation curves and migration of PCR products
on acrylamide gels were performed to confirm the specificity of the
products. The specificity of the qPCR assay was evaluated by
melting curve analysis, which showed that the PCSK6 amplification
product generated a melting peak at 81.20±0.34°C without
primer-dimers or non-specific products.
Cell proliferation assay
The CIA FLS were seeded onto 96-well culture plates
and cultured at 37°C to 80% confluence. The cultures were treated
with PCSK6 siRNA (160 nmol/l). After incubation for 24, 48 or 72 h,
20 µl 5 mg/ml MTT in PBS was added to each well, and
cultures were incubated for 4 h at 37°C in the incubator. MTT
solution was removed and 150 µl dimethyl sulfoxide was added
to extract the MTT-formazan products at room temperature for 10
min. The absorbance was measured in triplicate at 490 nm using a
spectrophotometer (DNM-9602G; Prolong Group, Beijing, China).
Cell invasion assay and migration
assays
The invasive ability of the cells was tested using
Transwell plates (BD Biosciences, Franklin Lakes, NJ, USA). FLS
were seeded into the upper chamber of the Transwell plate at a
density of 3×104 cells/well and incubated at 37°C with
160 nmol/l siRNA for 8 h. The upper and the lower chambers were
then filled with medium without FBS, followed by incubation for 12
h. Subsequently, the lower chamber was filled with 20% FBS in DMEM,
followed by a further incubation for 24 h. The non-invaded cells at
the upper surface of the membrane were removed with cotton swabs
and the invaded cells on the lower side were stained with Giemsa
(Solabio). The cell number of cells that had transgressed through
the filter was quantified in five random fields at x100
magnification and the average number was calculated. Cells were
observed using a XDS-1B microscope (Nikon, Tokyo, Japan)
A wound-healing assay was performed to test the
migration ability of FLS. Cells were plated onto 24-well plates and
cultured at 37°C until reaching 80% confluence. The cell monolayers
were scratched linearly in multiple areas with a cell scraper
(Corning Life Sciences, Tewksbury, MA, USA), and cells were
subsequently transfected with 160 nmol/l PCSK6 siRNA or control
siRNA. After 24 h of incubation, the number of cells migrated into
the scratched area was calculated using a method identical to that
described above.
Determination of interleukin (IL)-6,
IL-1α, IL-1β, IL-17 and tumor necrosis factor (TNF)-α levels by
ELISA
FLS were transfected with 160 nmol/l siRNA for 24 h,
and the culture medium was collected and centrifuged at 800 × g for
5 min at 4°C. 100 µl medium was added to a 96-well
microplate (Corning-Costar, Corning, NY, USA), which was stored
overnight at 4°C. After gently washing with PBS containing Tween 20
(Solabio, Beijing, China), 1% bovine serum albumin (Solabio) plus
5% sucrose (Solabio) was used for blocking for 1 h at 37°C.
Following three washes with PBS, antibodies against IL-1α, IL-1β,
IL-17 and TNF-α (all from Abcam, Cambridge, MA, USA; dilution,
1:1,000) were applied to the plate for overnight incubation. The
plate was washed, blocked and incubated with a 1:2,000 dilution of
horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
(ProteinTech, Chicago, IL, USA) for 3 h at 37°C. Staining was
developed using a TMB kit (CW0050; CWBIO, China). The absorbance at
450 nm was measured using a plate reader (Synergy HT; BioTek,
Winooski, VT, USA). FLS treated with transfection reagent only were
used as a control.
Cell cycle analysis
Cells were seeded onto six-well culture plates
(Corning-Costar) at 1.0×106 cells/well and the cells
were treated with siRNA as described above. After removal of the
culture medium, the cells were harvested at 24 h by trypsinization,
washed twice with ice-cold PBS and fixed overnight with 70% ethanol
at 4°C. Prior to analysis, the fixed cells were rinsed with PBS,
re-suspended in PBS and stained for 30 min at 37°C with 1 ml 0.05
mg/ml propidium iodide solution (Digguo Biotech, Beijing, China)
containing 10 µg/ml RNase (Sigma-Aldrich) for 30 min at
37°C. Cells were analyzed using a Coulter Epics XL flow cytometer
(Beckman Coulter, Brea, CA, USA) and the DNA content was determined
using EXPO32 software (Beckman Coulter).
Statistical analysis
All results were confirmed in at least three
independent experiments and were expressed as the mean ± standard
deviation. Multiple comparisons were performed using one-way
analysis of variance. All statistical analyses were performed using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Knockdown of PCSK6 affects the
proliferation, migration, and invasion of FLS from rats with CIA in
vitro
Since the present study hypothesized that PCSK6 may
be involved in the progression of RA, RNA interference was used to
knockdown PCSK6 expression in order to assess the resulting effects
on FLS from rats with CIA. PCSK6 expression was determined by
RT-qPCR 24 h after transfection with either control siRNA or PCSK6
siRNA. The mRNA expression of PCSK6 was significantly decreased
following transfection with PCSK6 siRNA (P=0.0001) (Fig. 1A), demonstrating effective
knockdown. To evaluate the roles of PCSK6 in the proliferation,
migration and invasion of FLS in RA, MTT, wound healing and
Transwell assays were performed after PCSK6 knockdown. Transfection
with siRNA-PCSK6 reduced the proliferation of FLS from rats with
CIA compared to that of the control- or Mock-transfected cells with
normal PCSK6 expression (P=0.0001) (Fig. 1B). In the cell migration assay,
monolayers of FLS were scratched and subsequently incubated with
siRNA-PCSK6, control siRNA or transfection reagent only for 24 h.
Significantly fewer FLS were present in the wounded area when PCSK6
was knocked down (P=0.0001) (Fig.
1C). Furthermore, the invasion assay showed that significantly
fewer FLS transgressed through the Transwell filter when PCSK6 was
knocked down (P=0.0001) (Fig.
1D).
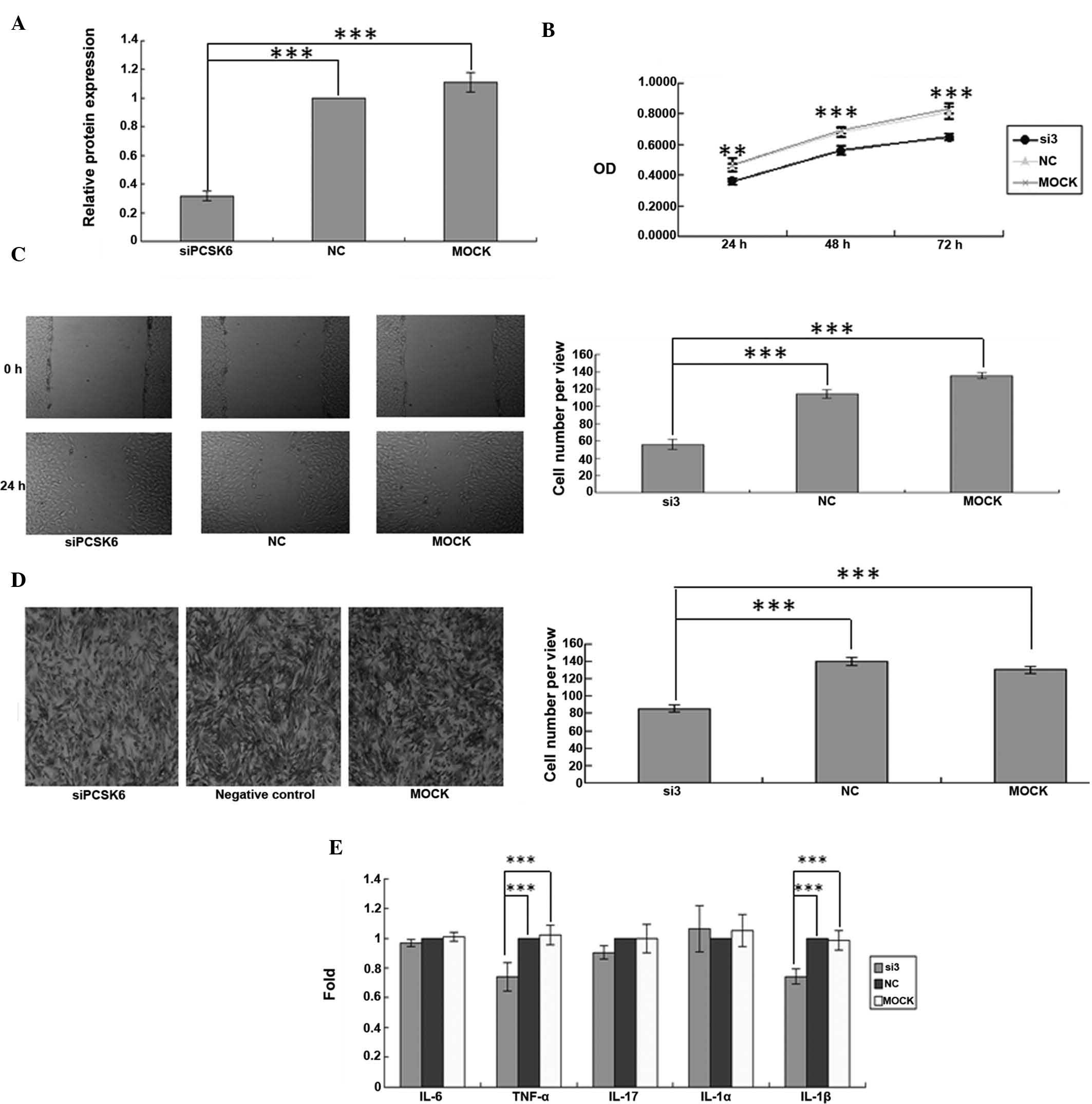 | Figure 1Synovial fibroblasts of a rat model of
collagen-induced arthritis were transiently transfected with siRNA
targeting PCSK6. (A) Knockdown of PCSK6 was confirmed by
reverse-transcription polymerase chain reaction. (B) Cell
proliferation was assessed using an MTT assay. (C) A wound healing
assay was used to assess cell migration (magnification, ×100). (D)
The invasive ability was assessed using a Transwell assay and the
average number of cells invaded through the filter following 24 h
of incubation was quantified (magnification, ×100). (E) Cytokine
levels of IL-6, IL-1α, IL-1β, IL-17 and TNF-α were assessed using
ELISA. Values are expressed as the mean ± standard deviation. Three
independent experiments were performed for all of the above assays.
***P<0.05. NC, negative control; MOCK, control
treated with transfection reagent only; si3, group transfected with
anti-PCSK6 siRNA. Three independent experiments were conducted for
each assay. IL, interleukin; siRNA, small interfering RNA; PCSK6,
pro-protein convertase subtilisin/kexin type 6; TNF, tumor necrosis
factor; NC, negative control; OD, optical density. |
Knockdown of PCSK6 decreases TNF-α
secretion in FLS from rats with CIA
As RA is a chronic inflammatory condition,
pro-inflammatory cytokines have prominent roles in the disease. In
particular, TNF-α, IL-1β and IL-17 are important pro-inflammatory
cytokines associated with synovitis and joint destruction (5). TNF-α, IL-1 α, IL-1β and IL-17 levels
were compared in the supernatant of FLS following treatment with
160 nmol/l PCSK6 siRNA or control siRNA. The secretion of IL-6,
TNF-α and IL-1β by siRNA-PCSK6-transfected FLS was significantly
lower as compared with that in the controls (P=0.0001) (Fig. 1E).
Cell cycle arrest in FLS from rats with
CIA following PCSK6 knockdown
To further study the effects of PCSK6 on the
proliferation of FLS from rats with CIA, flow cytometric cell cycle
analysis was performed. The ratio of
G0/G1-phase cells was significantly higher in
FLS with PCSK6 knockdown as compared with that in the controls
after 12, 48 and 72 h (Fig. 2A),
which suggested that downregulation of PCSK6 inhibited the cell
cycle in FLS of mice with CIA.
Knockdown of PCSK6 induces downregulation
of genes associated with proliferation, invasion, migration and
inflammation
To gain further insight into the role of PCSK6 in
the pathology of RA, the expression levels of genes associated with
proliferation, invasion, migration and inflammation were detected
by RT-qPCR. A total of eight genes were found to be down-regulated
after PCSK6 knockdown (Fig. 3).
Among these genes, IGF-2 was associated with cell proliferation,
while CXCL9 was associated with inflammation. Other downregulated
genes including, NOSTRIN, MMP2 and MMP9, were associated with
angiogenesis, while MPZL2 is involved in cell adhesion. Of note,
HIF-1α and PADI4, which are closely associated with hypoxia, the
main contributor to RA and CIA (14,15),
were also downregulated after PCSK6 knockdown.
The present study also examined the expression of
Furin, which has a protective role in immune response-induced
arthritis (8), following PCSK6
knockdown. However, silencing of PCSK6 had no significant effect on
the expression of Furin.
Discussion
PCSK6 is one of the neuroendocrine-specific
mammalian subtilisin-associated endoproteases (16), which contains a C-terminal
cysteine-rich region (17), and
which is thought to function in the secretory pathway.
Hyperplasia of synovial fibroblasts contributes to
the pathogenesis of RA and is capable of eroding adjacent cartilage
and bone and causing subsequent joint destruction. As PCSK6 had
been indicated to have a critical role in tumor progression
(7), cell proliferation, migration
and invasion, the present study attempted to determine the function
of PCSK6 in FLS of rats with CIA. Of note, significant decreases in
the proliferation as well as in the migratory and invasive
capacities were observed in FLS from rats with CIA after PCSK6
silencing. This suggested that PCSK6 has an important role in the
hyperplasia and erosion capacity of FLS.
CIA is a typical model of RA, which is characterized
as a chronic inflammatory disease. IL-6, TNF-α, IL-1β and IL-17 are
important pro-inflammatory cytokines in CIA, which are associated
with synovitis and joint destruction. In the present study,
knockdown of endogenous PCSK6 led to reduced TNFα and IL-1β
expression in FLS from rats with. TNF-α is known to promote
fibrosis of synovial tissues as well as immune responses (18). It has an important role in the
growth and differentiation of numerous types of normal cell and
forms a complex immune network with other cytokines during the
inflammatory process (19).
Joosten et al (20)
reported that in murine collagen-induced arthritis, inflammation
and cartilage degradation were inhibited by blocking IL-1β. The
present study found that PCSK6 knockdown led to decreases in IL-1β
and TNF-α levels in FLS of rats with CIA, which suggested that
PCSK6 exacerbates inflammation by regulating the expression or
secretion of cytokines, including IL-1β and TNF-α, in FLS during
RA.
To elucidate the roles of PCSK6 in FLS during RA,
the present study analyzed the expression levels of several genes
which are known to be associated with inflammation, proliferation,
invasion, migration and hypoxia after knockdown of PCSK6 in FLS
from rats with CIA. Among them, CXCL9 is a sensitive marker for
disease activity in patients with RA (21). In the present study, downregulation
of CXCL9 expression was observed in FLS of rats with CIA following
knockdown of PCSK6. This result confirmed that PCSK6 regulates the
proliferation and migration of FLS through chemokines such as
CXCL9.
MMPs are secreted or membrane-anchored
zinc-dependent endopeptidases, which have been shown participate in
the initiation of cell movement in the extracellular matrix and to
be able to degrade most extracellular matrix proteins, particularly
type IV collagen, the major component of basement membranes
(7). Re-modeling of the
extracellular matrix by MMPs is important in angiogenesis. As
abnormal angiogenesis in the synovium is a key characteristic of
CIA, factors which stimulate MMP expression may have a role in CIA.
In the present study, a marked decrease of MMP-2 and MMP-9
expression was observed in FLS of mice with CIA after PCSK6
knockdown, which is consistent to the function of PCSK6 in
processing pre-mature MMP-2/9 to mature types a reported by
Mahloogi et al (15). MMPs
participate in angiogenesis by degrading and remodelling the
extracellular matrix and basement membranes, allowing activated
endothelial cells to proliferate and migrate, as well as releasing
extracellular matrix-bound growth factors, including fibroblast
growth factor-2, vascular endothelial growth factor or insulin-like
growth factor-1 (22). NOSTRIN is
another type of protein with an important role in developmental
angiogenesis (23). In the present
study, NOSTRIN mRNA was detected to be downregulated after PCSK6
knockdown in FLS of mice with CIA. This suggested that PCSK6 may
have an important role in angiogenesis during CIA through its
impact on NOSTRIN and MMP-9.
As mentioned above, hypoxia of synovial tissues in
inflamed joints is one of the most important characteristics of
RA/CIA. Hypoxia-inducible factor 1α (HIF-1α), the key
transcriptional factor in the hypoxic response, is upregulated in
RA (24). RA is thought to
decrease the oxygen supply, leading to synovial hypoxia and
hypo-perfusion (25). The present
study we found that HIF-1α expression was regulated, either
directly or indirectly, by PCSK6. Therefore, it was hypothesized
that PCSK6 may be involved in the regulation of the hypoxic
synovial microenvironment.
Another important member of the pro-protein
convertase family, furin, has been previously reported to have
protective roles in immune response-induced arthritis (8); therefore, the present study also
tested the expression of furin after PCSK6 knockdown. However, the
results showed that knockdown of PCSK6 did not affect the
expression of furin. According to the in vitro results of
the present study, the role of PCSK6 in CIA is opposite to that
reported for furin.
In conclusion, the present study demonstrated that
PCSK6 may have important roles in the proliferation, migration,
invasion and angiogenesis of FLS during RA/CIA. In addition, PCSK6
contributes to the secretion of pro-inflammatory cytokines, hypoxia
of FLS and deregulation of the cell cycle of FLS in RA/CIA. The
results of the present study indicated that inhibition of PCSK6 may
have a protective role against synovitis in RA.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (NSFC) (no. 81102275), the
Natural Science Foundation of Shandong Province (no. ZR2011CQ028),
the National Basic Research Program of China (no. 2010CB529105) and
the Shandong Science and Technology Research Program (no.
2012GSF12115).
Abbreviations:
|
PCSK6
|
pro-protein convertase
subtilisin/kexin type 6
|
|
RA
|
rheumatoid arthritis
|
|
CIA
|
collagen-induced arthritis
|
|
FLS
|
fibroblast-like synoviocytes
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
siRNA
|
small interfering RNA
|
|
NOSTRIN
|
nitric oxide synthase trafficking
|
|
MMP9
|
matrix metallopeptidase 9
|
|
CXCL9
|
chemokine (C-X-C motif) ligand 9
|
|
HIF-1α
|
hypoxia inducible factor 1, α
subunit
|
|
IGF-2
|
insulin-like growth factor 2
|
|
MPZL2
|
myelin protein zero-like 2
|
|
PADI4
|
peptidyl arginine deiminase, type
IV
|
References
|
1
|
Sokka T: Work disability in early
rheumatoid arthritis. Clin Exp Rheumatol. 21(5 Suppl 31): S71–S74.
2003.
|
|
2
|
Muller-Ladner U, Kriegsmann J, Franklin
BN, Matsumoto S, Geiler T, Gay RE and Gay S: Synovial fibroblasts
of patients with rheumatoid arthritis attach to and invade normal
human cartilage when engrafted into SCID mice. Am J Pathol.
149:1607–1615. 1996.PubMed/NCBI
|
|
3
|
Tolboom TC, van der Helm-van Mil AH,
Nelissen RG, Breedveld FC, Toes RE and Huizinga TW: Invasiveness of
fibroblast-like synoviocytes is an individual patient
characteristic associated with the rate of joint destruction in
patients with rheumatoid arthritis. Arthritis Rheum. 52:1999–2002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lafyatis R, Remmers EF, Roberts AB, Yocum
DE, Sporn MB and Wilder RL: Anchorage-independent growth of
synoviocytes from arthritic and normal joints: Stimulation by
exogenous platelet-derived growth factor and inhibition by
transforming growth factor-and retinoids. J Clin Invest.
83:1267–1276. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holmdahl R, Mo J, Nordling C, Larsson P,
Jansson L, Goldschmidt T, Andersson M and Klareskog L: Collagen
induced arthritis: An experimental model for rheumatoid arthritis
with involvement of both DTH and immune complex mediated
mechanisms. Clin Exp Rheumatol. 7(Suppl 3): S51–S55.
1989.PubMed/NCBI
|
|
7
|
Tolboom TC, Pieterman E, van der Laan WH,
Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC and Huizinga TW:
Invasive properties of fibroblast-like synoviocytes: Correlation
with growth characteristics and expression of MMP-1, MMP-3, and
MMP-10. Ann Rheum Dis. 61:975–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin H, Ah Kioon MD, Lalou C, Larghero J,
Launay JM, Khatib AM and Cohen-Solal M: Protective role of systemic
furin in immune response-induced arthritis. Arthritis Rheum.
64:2878–2886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Anjou F, Routhier S, Perreault JP, Latil
A, Bonnel D, Fournier I, Salzet M and Day R: Molecular validation
of PACE4 as a target in prostate cancer. Transl Oncol. 4:157–172.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang X and Han J: Expression of
peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol
Carcinog. 45:183–196. 2006. View
Article : Google Scholar
|
|
11
|
Malfait AM, Seymour AB, Gao F, Tortorella
MD, Le Graverand-Gastineau MP, Wood LS, Doherty M, Doherty S, Zhang
W and Arden NK: A role for PACE4 in osteoarthritis pain: Evidence
from human genetic association and null mutant phenotype. Ann Rheum
Dis. 71:1042–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press; Washington, DC: 2011
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta c(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH,
Cho SY, Yang HI, Yoo MC and Kim KS: Adiponectin may contribute to
synovitis and joint destruction in rheumatoid arthritis by
stimulating vascular endothelial growth factor, matrix
metalloproteinase-1, and matrix metalloproteinase-13 expression in
fibroblast-like synoviocytes more than proinflammatory mediators.
Arthritis Res Ther. 11:R1612009. View
Article : Google Scholar
|
|
15
|
Mahloogi H, Bassi DE and Klein-Szanto AJ:
Malignant conversion of non-tumorigenic murine skin keratinocytes
over-expressing PACE4. Carcinogenesis. 23:565–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mains RE, Berard CA, Denault JB, Zhou A,
Johnson RC and Leduc R: PACE4: A subtilisin-like endoprotease with
unique properties. Biochem J. 321:587–593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seidah NG, Chrétien M and Day R: The
family of subtilisin/kexin like pro-protein and pro-hormone
convertases: Divergent or shared functions. Biochimie. 76:197–209.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee A, Qiao Y, Grigoriev G, Chen J,
Park-Min KH, Park SH, Ivashkiv LB and Kalliolias GD: Tumor necrosis
factor alpha induces sustained signaling and a prolonged and
unremitting inflammatory response in rheumatoid arthritis synovial
fibroblasts. Arthritis Rheum. 65:928–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feldmann M and Maini RN: Lasker clinical
medical research award: TNF defined as a therapeutic target for
rheumatoid arthritis and other autoimmune diseases. Nat Med.
9:1245–1250. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joosten LA, Helsen MM, Van deLoo FA and
Van deBerg WB: Anticytokine treatment of established type II
collagen-induced arthritis in DBA/1 mice. A comparative study using
anti-TNF alpha, anti-IL-1 alpha/beta and IL-1Ra. Arthritis Rheum.
39:797–809. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuan WP, Tam LS, Wong CK, Ko FW, Li T, Zhu
T and Li EK: CXCL 9 and CXCL 10 as sensitive markers of disease
activity in patients with rheumatoid arthritis. J Rheumatol.
37:257–264. 2010. View Article : Google Scholar
|
|
22
|
Kovacevic I, Hu J, Siehoff-Icking A, Opitz
N, Griffin A, Perkins AC, Munn AL, Müller-Esterl W, Popp R, Fleming
I, et al: The F-BAR protein NOSTRIN participates in FGF signal
transduction and vascular development. EMBO J. 31:3309–3322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pardo A and Selman M: Matrix
metalloproteases in aberrant fibrotic tissue remodeling. Proc Am
Thorac Soc. 3:383–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hollander AP, Corke KP, Freemont AJ and
Lewis CE: Expression of hypoxia-inducible factor 1alpha by
macrophages in the rheumatoid synovium: Implications for targeting
of therapeutic genes to the inflamed joint. Arthritis Rheum.
44:1540–1544. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muz B, Khan MN, Kiriakidis S and Paleolog
EM: Hypoxia. The role of hypoxia and HIF-dependent signaling events
in rheumatoid arthritis. Arthritis Res Ther. 11:2012009. View Article : Google Scholar
|

















